Abstract
Aim: MicroRNAs (miR) are important diagnostic and treatment targets due to their different tissue expressions and their central position in the regulation of gene expressions. miR studies might pioneer emerging of new diagnostic tools and treatment goals in kidney diseases. Captopril (CAP) and telmisartan (TEL) were shown to be effective in ischemia reperfusion (IR) injury. There is not any study about the effect of TEL and CAP over miR-21-320-146a. Our aim was to study the effects of CAP and TEL over miR on renal IR model.
Methods: We used 12–16 weeks-old Wistar-Albino rats that weigh 300–350 g. Rats (n, 6) were randomized into four groups (Control, IR, IR + CAP, IR + TEL). Urea, creatinine, total antioxidant status (TAS), total oxidant status (TOS), oxidative stress index (OSI), super oxide dismutase (SOD), and miRs were analyzed.
Results: Urea, creatinine, TOS, OSI levels of IR + CAP, and IR + TEL groups were lower comparing to IR group. TAS and SOD levels were higher in IR group than IR + TEL group. miR-21-320-146a showed increase in renal IR injury. miR-320, 146a showed significant decrease in IR + CAP and IR + TEL groups comparing to IR group. We showed histopathological recovery and decreased apoptosis in IR + CAP and IR + T groups than IR group.
Conclusion: We, for the first time in the literature, showed that miR-320 is increased in IR injury. miR-320 might be a novel diagnosis and treatment target in renal ischemic reperfusion injury. Also, for the first time, we showed that CAP and TEL cause functional and histopathological recovery and lower miR-146a and miR-320.
Introduction
Acute renal failure (ARF) is a frequently encountered problem in clinical nephrology and has a high mortality rate.Citation1 It is also one of the main causes of permanent and progressive kidney failure and it also increases acute mortality rate.Citation2 Ischemia reperfusion (IR) injury is the central event in ARF. Even with progresses in diagnosis and treatment methods, ischemic ARF is a major and common clinical problem.Citation3
MicroRNAs (miR) are type of single-stranded RNA molecule, approximately 21–23 nucleotide long and have vital role in the regulation of gene expression. Tissue expression differences of miRs and their central role on regulation of gene expressions make them an important target on diagnosis and treatment of diseases. Recently, researchers showed that miRs have an important role in prognosis of kidney diseases.Citation4 miR studies might pioneer the emergence of novel diagnostic tools and treatment targets in kidney diseases.
miR-21 and miR-146a were shown to have a role in renal IR injury.Citation5,Citation6 It was also shown that miR-320 is upregulated in gentamicin toxicity of kidney.Citation7 We hypostatized that miR-320 might be a potential biomarker in ischemic ARF because miRs are specific to tissues.
Angiotensin II (A2) and renin–angiotensin–aldosterone system (RAAS) might impair kidney functions by increasing systemic vascular resistance and inflammatory process. Several studies showed that angiotensin activity or inhibition of A2 products prevent renal injury in case of ischemic ARF.Citation8,Citation9 Captopril (CAP) is an angiotensin-converting enzyme (ACE)-inhibitor. It lowers A2 level in circulation. CAP has a potent antioxidant feature due to the presence of thiol compound. It aggregates different types of reactive oxygen radicals and prevents lipid peroxidation.Citation10,Citation11 It has been shown that CAP is preventive against tissue damage induced by oxidative stress and inflammation in various experimental models.Citation12,Citation13
Telmisartan (TEL) is a highly-selective angiotensin I (AT1) receptor blocker. Protective role of TEL was shown in rat models of nephrectomy,Citation14 cyclosporine-induced nephrotoxicity,Citation15 and IR.Citation16 Also, TEL has antioxidant and anti-inflammatory effect independently of AT1 receptor blockageCitation17,Citation18. CAP and TEL were shown to be effective in IR injury. However, the mechanism of action is unknown.
There is no reported study that shows the effect of CAP and TEL on miR-21, -320 and -146a. Our aim was to evaluate the effect of CAP and TEL on miRs in renal IR model.
Materials and methods
This study was done in accordance with the Care and Use of Laboratory Animals published by the Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council after obtaining local ethics committee approval (date 04 January 2016, approval number: 2016.01.05).
Animals
We used 12–16 weeks old Wistar-Albino male rats that weigh 300–350 g for the study. Six rats were randomized to each group. All rats were kept in transparent polycarbonate cages, where they can reach fresh water and food, under 12 h of darkness and light cycles.
Chemicals
Both CAP and TEL were obtained from Santa Cruz Biotechnology (Santa Cruz Biotechnology, Santa Cruz, CA). CAP was dissolved in sterile saline. Tween-80 was bought from Merck Corporation (Merck, Darmstadt, Germany). TEL was dissolved in 1% Tween-80 in sterile saline.Citation19 All chemicals were freshly prepared just before being used in the experiment.Citation20,Citation21
Experimental study design
Study animals were stratified into four groups by randomization (n, 6); Group C: all animals were subjected to laparotomy without IR; Group IR: rats were subjected to 60 min of ischemia followed by 120 min of reperfusion; Group IR + CAP: Rats were subjected to 60 min of ischemia followed by 120 min of reperfusion, 1 ml of 50 mg/kg CAP was administered 2 h before IR by oral gavage; Group IR + TEL: Rats were subjected to 60 min of ischemia followed by 120 min of reperfusion, 1 mL of 3 mg/kg TEL was administered 3 h before IR by oral gavage.Citation20,Citation21
Surgical procedures
Rats were anesthetized by 10 mg/kg xylazine hydrochloride (Rompun, Bayer, Istanbul, Turkey) and 70 mg/kg ketamine (Ketalar, Pfizer, Istanbul, Turkey). After administering enough anesthesia, abdominal walls of rats were shaved and sterilized with povidone iodine on homoeothermic table to keep body temperature stabile at 37.1 °C. Midline incision was applied and right and left renal arteries and veins were revealed. Both renal peduncles were clamped for 60 min with atraumatic vascular clamp (Vascu Stop Bulldog Clamp, Istanbul, Turkey). Clamps were loosening after 60 min of ischemia. Hundred and twenty minutes of reperfusion was allowed. After 120 min, blood samples were taken from abdominal artery. Following, animals received bilateral nephrectomy under anesthesia and killed via decapitation. Blood samples were centrifuged at +4 °C, 1500 g for 10 min and kept at −80 °C temperature. Renal tissue samples were washed with cold-heparinized phosphate-buffered saline, and cleaned from blood cells and clots. Tissues were fixated with 10% formalin for histopathological analysis. In addition, a part of the tissues was put into Eppendorf tubes and stored at −80 C.
Biochemical analysis
Preparation of renal tissue homogenates: renal tissue was mixed with cold study solution (50 mmol/L phosphate buffer, pH 7.40) and centrifuged at 10,000 g for 15 min at +4 °C after homogenization using a mechanic homogenizer.
Measurement of serum urea and creatinine values
Serum urea and creatinine values were measured using Beckman Coulter AU680 analyzer (Beckman Coulter, Miami, FL). Serum urea values were expressed as mg/dL.
Measurement of tissue TAS, TOS and OSI values
Tissue total antioxidant status (TAS) and total oxidant status (TOS) values were measured with Beckman Coulter AU680 analyzer (Beckman Coulter) using commercial reagents (Rel Assay Diagnostic, Gaziantep, Turkey).Citation22 TAS values were expressed as TroloxEq/mg protein. TOS values were expressed as μmolH2O2 Eq/mg protein. Oxidative stress index (OSI) was measured as percentage ratio of TOS level to TAS level after mmol value of TAS test unit was converted to μmol as in TOS test.Citation23 Tissue super oxide dismutase (SOD) activities were measured on a Beckman Coulter AU680 analyzer (Beckman Coulter) using the Ransod kit (Randox Laboratories, Crumlin, UK). SOD activities were expressed as U/mg of protein.
miR analysis
Total RNA isolation and real-time PCR
The kidney was sectionalized into smaller pieces using a lancet on ice in trizol solution. We isolated total RNA from kidney tissues of rats of all groups with TRIzol reagent (Invitrogen Thermo Scientific, Waltham, MA) according to the manufacturer’s protocol and quantitated with a Nanodrop™ spectrophotometer (Thermo Scientific). miR cDNA was synthesized by TaqMan® MicroRNA Reverse Transcription kit according to manufacturer’s procedure (ThermoFischer Scientific). We used Step One real-time PCR with miR-21- and miR146a-specific primers to evaluate the miR expression. Analysis of relative quantification of miR-320, miR-21, and miR-146a were done with RT-PCR using TaqMan® Universal Master Mix II, noUNG, miR qPCR profiling kits. U6 was used as rat endogenous controls.
Histopathological examinations
Renal tissue samples were embedded into paraffin blocks after fixation using 10% formalin. Paraffin blocks were sectioned (4 lm) and stained with hematoxylin eosin. All samples were evaluated by a pathologist who was blind to all study groups with a light microscope (BX51; Olympus, Tokyo, Japan). The Jablonski grading scale (0–4) was used for the assessment of I-R-induced study groups.Citation24
In situ tunnel was used to evaluate renal apoptosis. Formalin-fixated sections were deparaffinized in xylene and rehydrated in graded concentrations of ethanol in water. Apoptotic DNA fragmentations were identified with commercial kit (ApopTag Peroxidase In Situ Apoptosis Detection Kit, Milli pore, Billerica, MA) according to the manufacturer’s protocol. Sections were examined using a light microscope (Olympus BX51, Olympus Optical, Tokyo, Japan). We counted tunnel positive cells among 100 cells in randomly chosen areas. Apoptotic index was measured as percentage of apoptotic cell.
Statistical analysis
We used ΔΔCT method to analyze the miR expression and quantified with a computer program. Groups were compared with “Volcano Plot” analysis. Volcano plot analysis is a part of “RT2 Profiles TMPCR Array Data Analysis” and uses “Student’s t-test” for statistical analysis. Spike frequency and amplitude values were measured with repeated measures analysis of variance (ANOVA) and post hoc Tukey’s test. The spike frequency and amplitude values of different dose groups were compared using one-way ANOVA and post hoc Tukey’s tests. p < .05 was considered significant.
Results
Mean urea and creatinine values of IR + CAP and IR + TEL groups were significantly lower comparing to IR group (<.001). Urea and creatinine values were not significantly different between IR + CAP and IR + TEL groups. TAS was significantly higher in IR + TEL group than IR group, while it showed a trend to statistical significance in IR + CAP group comparing to IR group. We identified significant decrease of OSI in IR + CAP and IR + TEL groups comparing to IR group (p = .001). However, no difference was identified between OSI IR + CAP and IR + TEL. We identified a significant increase of TAS in IR + CAP and IR + TEL groups comparing to IR group (p < .001) ().
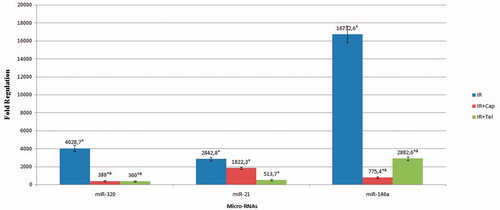
miR 320 showed significant increase in IR group comparing to rat endogenous. miR-320 was significantly decreased in IR + CAP and IR + TEL groups than IR group (p < .05). miR-21 showed a significant increase in IR group (p = .01), in IR + CAP group, in IR + TEL group comparing to endogenous rat controls (p < .05). miR-21 didn’t show any significant difference in IR + CAP and IR + TEL groups comparing to IR group. miR-146a was significantly increased in IR group (p = .01), in IR + CAP group (p = .027), in IR + TEL group comparing to endogenous rat controls. miR-146a was significantly decreased in IR + CAP and IR + TEL groups than IR group (p < .05) ().
Table 1. Comparison of biochemical and histological data among experimental groups.
Histopathological evaluation
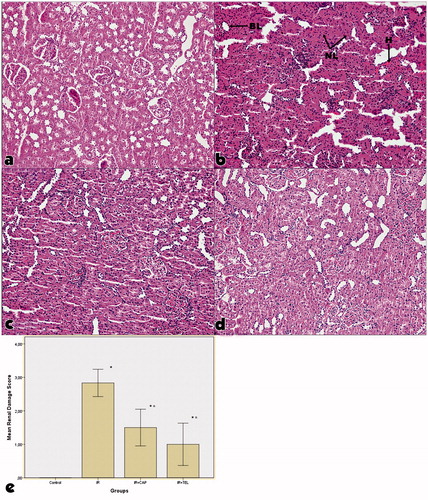
We observed significant degeneration in tubular structure, necrosis in proximal convoluted tubule, loss of brush border and nuclei and mild capillary congestion in glomerulus () in IR group. In CAP and TEL groups, we also observed mild cellular necrosis and tubular dilatation and recovery of most of the morphological changes which were observed in IR group. Renal injury score was significantly lower in IR + CAP and IR + TEL groups comparing to IR group (p < .01). We didn’t find any significant difference between IR + CAP and IR + TEL groups in terms of renal damage scores.
Figure 2. Representative histological photographs of PAS-stained kidney tissue in the experimental groups. (a) Control group, (b) ischemia-reperfusion group, (c) captopril + ischemia-reperfusion group, (d) telmisartan + ischemia-reperfusion group, (e) hematoxylin and eosin staining (H&E 9 100) showed that there was no change in control rats. (IR) IR: ischemia-reperfusion group, IR + CAP: captopril + ischemia-reperfusion group, IR + TEL: telmisartan + ischemia-reperfusion group. The “BL” denotes brush border loss, “H” denotes hemorrhage, and “NL” denotes nuclei loss. In IR + CAP and IR + TEL groups, we also observed mild cellular necrosis and tubular dilatation and recovery of most of the morphological changes which were observed in IR group. p shows the differences between all groups (one-way ANOVA test). *< .001, compared to the control group; α< .001, compared to the IR group (post hoc Tukey’s test).
Evaluation with tunnel method
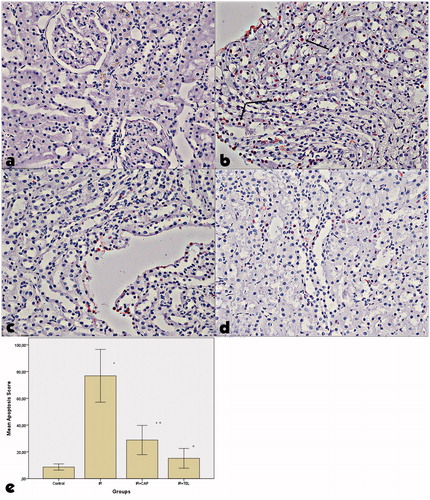
TUNEL + cell count was increased in IR group comparing to control group. TUNEL + cell count was significantly lower in IR + CAP and IR + T groups comparing to IR group. Apoptosis scores were significantly increased in IR group than control group (p < .01). Apoptosis scores of IR + CAP and IR + T groups were significantly lower than IR group (p < .01). There was no significant difference between IR + CAP and IR + T groups ().
Figure 3. Examination of tunel + cells in kidney tissues in the experimental groups. The positive TUNEL reaction is visible as a dark brown apoptosis in control rats. (a) control group, (b) ischemia-reperfusion group, (c) captopril + ischemia-reperfusion group, (d) telmisartan + ischemia-reperfusion group. Significant positive cell count was identified in IR injury comparing to control. TUNEL + cell count was increased in IR group comparing to control group. TUNEL + cell count was significantly lower in IR + CAP and IR + TEL groups comparing to IR group. p shows the differences between all groups (one-way ANOVA test). *<.001, compared to the control group; α<.001, compared to the IR group (post hoc Tukey’s test).
Discussion
Kidney tissue, especially renal tubule, is sensitive to hypoxic ischemic injury due to its demand for high energy. Importance of miR-21 in IR injury grew recently. In accordance with our study, Godwin et al.Citation6 and Wei et al.Citation25 also observed that miR-21 increases in IR. It has been shown that miR-21 has an important role in myocardial IR injury.Citation26,Citation27 Mechanism of IR injury is quite complex; on the other hand, a substantial part of damage is related to reactive oxygen radicals during reperfusion.Citation28 In accordance with previous studies, we showed that TOS and OSI activities, which are indicators of reactive oxygen radicals, were significantly increased in IR group. It has been shown that Hif-1 alpha increases with increase in ROS, while miR-21 activation also increases with HIF-1 alpha.Citation29,Citation30 miR-21 increase in our study might be caused by the increase in HIF-1 alpha which is due to ROS increase.
In our study, for the first time, we showed that miR-320 increases in IR injury. Lorenzen et al. showed that miR-320 downregulates in critically ill patients with ARF who were admitted to the intensive care unit.Citation31 However, common factors seen in intensive care unit patients such as infection, frequent drug administration and comorbidities might affect miR levels, but we excluded these factors in our study and showed that miR-320 significantly increases in renal IR injury. Cell- and tissue-specific expressions are the major characteristics of the miR expression. Identification of miR-320 upregulation in renal tubular injury caused by gentamicin toxicity (7) revealed the importance of miR-320 in renal tissues. Increases in cell death and apoptosis due to miR-320 overexpression were shown in invitro study. Also, larger myocardial infarction areas were identified in transgenic rats with miR-320 overexpression after IR. In addition to this, infarction area was decreased after inhibition of miR-320.Citation32 These studies are in accordance with the role of miR-320 in IR injury and apoptosis. We also found that apoptosis was significantly higher in IR group, which had significantly higher levels of miR-320. In our study, we identified decreases in apoptosis and miR-320 levels due to CAP and TEL treatment. This finding supports the relation between miR-320 and apoptosis. MiR-320 was shown to inhibit Hsp20 expression. Increased Hsp20 expression was shown to be protective and to lower apoptosis rate against IR injury in neuroblastoma cells.Citation33 In our study, higher levels of miR-320 in IR group might have increased renal injury and apoptosis rate by decreasing HSP20.
In accordance with our study, it has been shown that miR-146a increases in renal IR injury.Citation6 Also, miR-146a had shown to be upregulated by ROS-producing metal sulfates in human astroglial cells.Citation34 These studies prove that ROS increase is closely related to miR-146a increase. In our study, miR-146a might be increased due to ROS increase in IR injury. Also, it has been shown that increased miR-146a downregulates SOD.Citation35 In accordance with the literature, we observed an increased miR-146a and a decreased SOD activity in our study.
In line with our study, it has been shown that CAP and TEL were protective against IR injury.Citation20,Citation36,Citation37 However, its mechanism of action cannot be explained clearly. MiR-146a was decreased in TEL and CAP treatment groups in our study. MiR-146a lowering effect of CAP and TEL treatment might be due to their lowering effect on NF-κB activity. It has been shown that TEL and CAP treatments inhibit NF-κB.Citation38,Citation39 Also, NF-κB activity increases miR-146 gene transcription.Citation34
In conclusion, we, for the first time, showed that miR-320 increases in IR injury. MiR-320 might be a novel diagnostic and therapeutic target in renal IR injury. Also, we for the first time showed that CAP and TEL therapies decrease miR-146a and -320. Recovery effects of CAP and TEL in renal IR injury might be over miR-146a and miR-320.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Varela CF, Greloni G, Schreck C, et al. Assessment of fractional excretion of urea for early diagnosis of cardiac surgery associated acute kidney injury. Ren Fail. 2015;37:327–331.
- Wu HC, Lee LC, Wang WJ. Incidence and mortality of postoperative acute kidney injury in non-dialysis patients: Comparison between the AKIN and KDIGO criteria. Ren Fail. 2016;38:330–339.
- Kline J, Rachoin JS. Acute kidney injury and chronic kidney disease: It's a two-way street. Ren Fail. 2013;35:452–455.
- Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol. 2009;4:1255–1266.
- Hu H, Jiang W, Xi X, Zou C, Ye Z. MicroRNA-21 attenuates renal ischemia reperfusion injury via targeting caspase signaling in mice. Am J Nephrol. 2014;40:215–223.
- Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci USA. 2010;107:14339–14344.
- Nassirpour R, Mathur S, Gosink MM, et al. Identification of tubular injury microRNA biomarkers in urine: Comparison of next-generation sequencing and qPCR-based profiling platforms. BMC Genomics. 2014;15:485.
- Altunoluk B, Soylemez H, Oguz F, Turkmen E, Fadillioglu E. An Angiotensin-converting enzyme inhibitor, zofenopril, prevents renal ischemia/reperfusion injury in rats. Ann Clin Lab Sci. 2006;36:326–332.
- Vargas AV, Robinson AV, Schulak JA. Captopril amelioration of renal reperfusion injury. J Surg Res. 1994;57:28–32.
- Scribner AW, Loscalzo J, Napoli C. The effect of angiotensin-converting enzyme inhibition on endothelial function and oxidant stress. Eur J Pharmacol. 2003;482:95–99.
- Liu YH, You Y, Song T, Wu SJ, Liu LY. Impairment of endothelium-dependent relaxation of rat aortas by homocysteine thiolactone and attenuation by captopril. J Cardiovasc Pharmacol. 2007;50:155–161.
- Ibrahim MA, Ashour OM, Ibrahim YF, El-Bitar HI, Gomaa W, Abdel-Rahim SR. Angiotensin-converting enzyme inhibition and angiotensin AT(1)-receptor antagonism equally improve doxorubicin-induced cardiotoxicity and nephrotoxicity. Pharmacol Res. 2009;60:373–381.
- Amirshahrokhi K, Ghazi-khansari M, Mohammadi-Farani A, Karimian G. Effect of captopril on TNF-α and IL-10 in the livers of bile duct ligated rats. Iran J Immunol. 2010;7:247–251.
- Tsunenari I, Ohmura T, Seidler R, et al. Renoprotective effects of telmisartan in the 5/6 nephrectomised rats. J Renin Angiotensin Aldosterone Syst. 2007;8:93–100.
- Cibulskyte D, Pedersen M, Hørlyck A, et al. Telmisartan attenuates chronic ciclosporin A nephrotoxicity in a pig model. Nephrol Dial Transplant. 2007;22:369–375.
- Fouad AA, Qureshi HA, Al-Sultan AI, Yacoubi MT, Al-Melhim WN. Nephroprotective effect of telmisartan in rats with ischemia/reperfusion renal injury. Pharmacology. 2010;85:158–167.
- Benson SC, Pershadsingh HA, Ho C, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity . Hypertension. 2004;43:993–1002.
- Cianchetti S, Del Fiorentino A, Colognato R, Di Stefano R, Franzoni F, Pedrinelli R. Antiinflammatory and anti-oxidant properties of telmisartan in cultured human umbilical vein endothelial cells. Atherosclerosis. 2008;198:22–28.
- Fouad AA, Jresat I. Captopril and telmisartan treatments attenuate cadmium-induced testicular toxicity in rats. Fundam Clin Pharmacol. 2013;27:152–160.
- Fouad AA, Al-Mulhim AS, Jresat I, Morsy MA. Protective effects of captopril in diabetic rats exposed to ischemia/reperfusion renal injury. J Pharm Pharmacol. 2013;65:243–252.
- Kusunoki H, Taniyama Y, Azuma J, et al. Telmisartan exerts renoprotective actions via peroxisome proliferator-activated receptor-γ/hepatocyte growth factor pathway independent of angiotensin II type 1 receptor blockade. Hypertension. 2012;59:308–316.
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119.
- Eren Y, Dirik E, Neselioglu S, Erel O. Oxidative stress and decreased thiol level in patients with migraine: Cross-sectional study. Acta Neurol Belg. 2015;115:643–649.
- Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204.
- Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21:756–761.
- Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics. 2011;43:534–542.
- Zhu H, Fan GC. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc Res. 2012;94:284–292.
- Degroot H, Rauen U. Ischemia-reperfusion injury: Processes in pathogenetic networks: A review. Transplant Proc. 2007;39:481–484.
- Kulshreshtha R, Ferracin M, Wojcik SE, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2006;27:1859–1867.
- Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15:667–671.
- Lorenzen JM, Kielstein JT, Hafer C. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2011;6:1540–1546.
- Ren XP, Wu J, Wang X, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366.
- Zeng L, Tan J, Hu Z, Lu W, Yang B. Hsp20 protects neuroblastoma cells from ischemia/reperfusion injury by inhibition of apoptosis via a mechanism that involves the mitochondrial pathways. Curr Neurovasc Res. 2010;7:281–287.
- Pogue AI, Percy ME, Cui JG, et al. Upregulation of NF-kB-sensitive miRNA-125b and miRNA-146a in metal sulfate stressed human astroglial (HAG) primary cell cultures. J Inorg Biochem. 2011;105:1434–1437. 2011.
- Ji G, Lv K, Chen H, et al. MiR-146a regulates SOD2 expression in H2O2 stimulated PC12 cells. PLoS One. 2013;8:e69351doi: 10.1371/journal.pone.0069351.
- Efrati S, Berman S, Hamad RA, et al. Effect of captopril treatment on recuperation from ischemia/reperfusion-induced acute renal injury. Nephrol Dial Transplant. 2012;27:136–145.
- Tawfik MK. Renoprotective activity of telmisartan versus pioglitazone on ischemia/reperfusion induced renal damage in diabetic rats. Eur Rev Med Pharmacol Sci. 2012;16:600–609.
- Prathab Balaji S, Vijay Chand C, Justin A, Ramanathan M. Telmisartan mediates anti-inflammatory and not cognitive function through PPAR-γ agonism via SARM and MyD88 signaling. Pharmacol Biochem Behav. 2015;137:60–68.
- Andersson P, Cederholm T, Johansson AS, Palmblad J. Captopril-impaired production of tumor necrosis factor-alpha-induced interleukin-1beta in human monocytes is associated with altered intracellular distribution of nuclear factor-kappaB. J Lab Clin Med. 2002;140:103–109.