Abstract
Purpose: To evaluate the association of Chronic hepatitis B virus (HBV) infection and chronic kidney disease (CKD).
Methods: We searched Embase, Grateful Med, Ovid, PubMed, and the China Biological Medicine Database. A meta-analysis was performed to assess whether HBV infection plays an independent impact on the development of CKD in the general population. Relative risks of CKD (defined as reduced glomerular filtration rate or proteinuria) according to HBsAg serologic status were studied.
Results: Six eligible clinical studies (189,709 individuals in total) were included in the analysis. There was no association between HBsAg seropositive status and prevalence of CKD, the summary estimate for adjusted relative risk (RR) was 1.16 (95% confidence interval (CI), 0.78, 1.71; p = .46) according to the random-effects model, and between studies heterogeneity was noted (p values by Q test <0.001). Also, there were no significant associations between positive HBV serologic status and low eGFR (adjusted relative risk, 0.95; 95% CI, 0.72, 1.26; p = .72) or proteinuria (adjusted relative risk, 1.00; 95% CI, 0.83, 1.20; p = .99).
Conclusions: This meta-analysis shows that there was no association between exposure to HBV and the risk of developing CKD in Asian populations.
Introduction
Chronic kidney disease (CKD) is an increasingly serious public health problem due to its increased prevalence and incidence and progressive nature to end-stage renal disease (ESRD).Citation1,Citation2 According to a number of population-based studies, the prevalence of CKD, defined by reducing glomerular filtration rate and/or increased proteinuria, more than 10% of the general population.Citation3 Therefore, it is critical to determine the major risk factors associated with CKD. Traditional risk factors for CKD such as age, hypertension, diabetes, and metabolic syndrome cannot fully explain the frequency of CKD in the general population of developed world.
Hepatitis B affects approximately 350 million people all over the world, and patients with chronic hepatitis B virus (HBV) infection are at risk of progression to cirrhosis and hepatocellular carcinoma.Citation4,Citation5 In addition to its impact on the liver, chronic HBV infection can have serious consequences for other organ systems. Several studies have highlighted the involvement of the kidney.Citation4,Citation6,Citation7 The HBV is associated with the pathogenesis of kidney diseases, such as polyarteritis nodosa, membranoproliferative glomerulonephritis and membranous nephropathy.Citation8 However, several studies have failed to find an association between HBV and CKD.Citation9,Citation10
The purpose of this study was to investigate the available evidence on the relationship between HBV infection and CKD in the general population by performing a meta-analysis of clinical observational studies.
Methods
Literature search
Embase, Grateful Med, Ovid, PubMed, and the China Biological Medicine Database were searched for all relevant cohort studies up to November 2015, without language limitation. The key words “kidney disease,” “proteinuria,” “hepatitis B,” “glomerular filtration rate,” and their synonyms were used. We included all randomized controlled trials, cohort trials, and prospective, controlled, non-randomized trials. In order to maximize data acquisition, we contacted authors whose articles contained insufficient information. Studies were compared to eliminate duplicate reports for the same patients, which included contact with investigators when necessary. Eligibility and exclusion criteria were prespecified.
Inclusion and exclusion criteria
The following inclusion criteria were used: (1) a randomized control trial-, cohort- or case-control-based study designs; (2) a description of details of incidence or prevalence of low-estimated GFR (eGFR) or frequency of proteinuria according to HBsAg serologic status in the general populationCitation11; (3) HBV infection was defined by testing for HBsAg in serum and/or HBV DNA detection by PCR; studies based on the ICD-9 were also considered.Citation12,Citation13 Information on HBsAg status was registered at the time of enrollment. If similar data were reported in more than one study, the recent study was involved in the analysis of results. Data were excluded in the meta-analysis if we could not gain sufficient statistical information on the association between HBsAg seropositivity status and CKD.
Efficacy measures
The primary efficacy end points were unadjusted or adjusted estimates of the risk (and 95% CIs) of low eGFR, proteinuria, and the prevalence of CKD according to HBsAg serologic status. Staging of CKD was categorized according to the Kidney Disease Outcomes Quality Initiative (K/DOQI) definition, and estimated glomerular filtration rate was calculated using the four-variable MDRD equation.Citation14 After adjustment for potential confounders (e.g., age, gender, and diabetes mellitus), the effect of HBsAg status on the frequency of CKD was estimated independently by multivariate analysis. The adjusted relative risk (RR) of CKD in the patients with HBV infection was identified in each study.
Data extraction
Two authors (Cai and Zhao) independently extracted data from studies retrieved on the basis of the inclusion criteria; another author (Shi) checked the data. The information extracted was: (1) number of patients in the study; (2) details of the study design; (3) patient characteristics; and (4) outcome measures, as defined above. Discrepancies were resolved with the assistance of an arbiter (Shi) when necessary.
Study quality
The quality of each study was independently assessed by the same two reviewers (Cai and Zhao) by use of a four-point scale based on the Newcastle–Ottawa scale specifically developed for this study.Citation15 The assessment of each study was based on following criteria: (1) the diagnose of HBV infection were obtained through PCR or serologic testing; (2) CKD were diagnosed by standardized laboratory procedures; (3) results were adjusted for potential confounders; and (4) people in the control group were recruited from the same source population. When there was disagreement between the two reviewers, a third author (Shi) was consulted.
Statistical analyses
Meta-analysis was performed by using fixed effect or random effect methods, depending on the absence or presence of significant heterogeneity.Citation16 Statistical heterogeneity between trials was evaluated by the chi-square and I-square (I2) tests.Citation17 When heterogeneity was confirmed, the random-effect method was used. In the absence of statistically significant heterogeneity, the fixed-effect method was used to combine the results.Citation18 We used the RR of the main dichotomous outcomes as the measure of efficacy. The 95% confidence interval (CI) for the combined RR was also provided. Publication bias was assessed by use of funnel plots. Meta-analysis was performed using Stata Statistical Software version12.0 (StataCorp., College Station, TX).
Results
Search results and characteristics
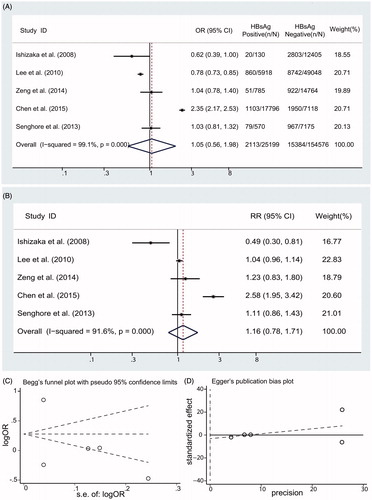
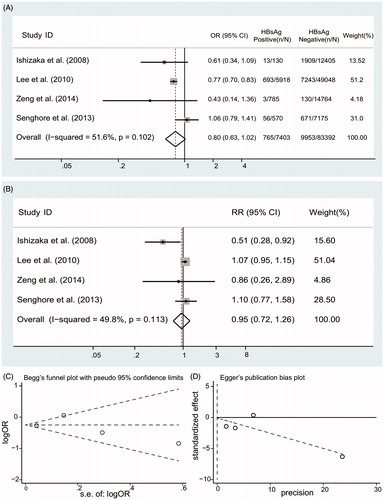
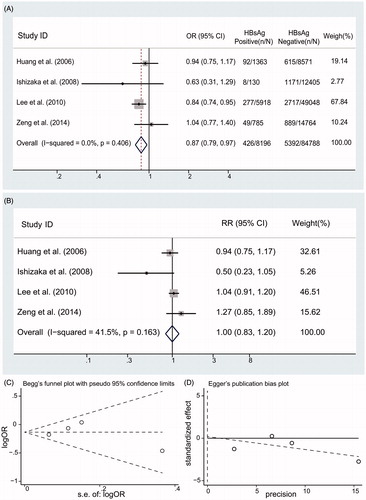
We identified 1267 citations from the literature by using the strategy described above (). After screening the title or abstract, on the basis of the inclusion criteria, 18 studies were retrieved and subjected to detailed evaluation. Eventually, 6 studies,Citation9,Citation10,Citation19–22 all of which were performed, were selected for inclusion in the meta-analysis. Four surveys (90,795 individuals) provided data on the prevalence of low eGFR in HBV-infected patients ().Citation10,Citation19,Citation20,Citation22 Five studies (179,775 individuals) gave the risk of CKD in HBV patients,Citation10,Citation19–22 and four reports (92,984 individuals) addressed information on the effect of HBV on proteinuria.Citation9,Citation10,Citation19,Citation20 A total of 189,709 individuals in total were included in the meta-analysis. The basic characteristics of each of the studies included in our meta-analysis are listed in .
Figure 1. Flow diagram of studies of hepatitis B virus-related kidney disease considered for inclusion.
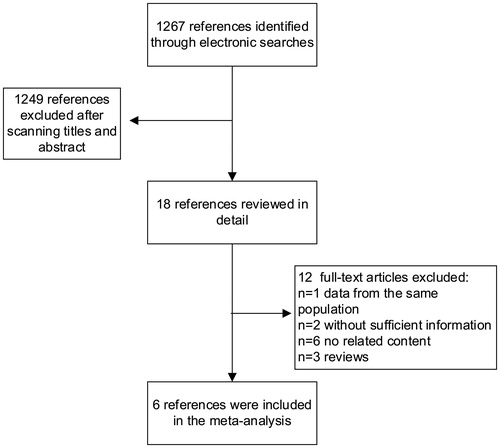
Table 1. Characteristics and outcomes of studies included in the meta-analysis.
Prevalence of CKD in HBV-infected patients
Among five studies,Citation10,Citation19–22 significant heterogeneity was identified (I2 = 91.6%; p < .001). A summary estimate of unadjusted OR of CKD using a random-effects approach was 1.05 (95% CI, 0.56, 1.98; p = .868) in HBsAg-positive individuals compared with HBsAg-negative individuals in . After adjustment for various covariates, the pooled adjusted RR of CKD was 1.16 with a 95% CI of 0.78, 1.71 (p= .991) (). There were no significant associations between HBV status and CKD. No asymmetry are found in our funnel plot (Begg’s test, p = .806; Egger’s test, p = .750) ().
Prevalence of low eGFR in HBV-infected patients
The unadjusted ORs for eGFR in HBsAg-positive patients are listed in . Moderate heterogeneity between studies (I2 = 49.8%; p values by Q test, 0.113) is observed, the summary estimate of OR using a random-effects model was 0.80 (95% CI, 0.63, 1.02; p = .075). After adjustment for various covariates, a summary estimate of RR of low eGFR in HBsAg-positive patients was 0.95 with a 95% CI of 0.72, 1.26 (p = .72) (). We did not find asymmetry in our funnel plot (Begg’s test, p = 1.000; Egger’s test, p = .952) ().
Prevalence of proteinuria in HBV-infected patients
Four reports addressed information on the relationship between proteinuria and HBsAg serologic status in .Citation9,Citation10,Citation19,Citation20 The unadjusted ORs in are showed for proteinuria according to HBsAg serologic status; significant differences in heterogeneity were observed (I2 = 41.5%; p values by Q test, 0.163), a summary estimate of OR suggest that no significant associations between HBV status and proteinuria (OR, 0.87; 95% CI, 0.79, 0.97). After adjustment for various covariates, the pooled RR of proteinuria was 1.00 with a 95% CI of 0.83, 1.20 in HBsAg-positive patients compared with HBsAg-negative patients (). There were no asymmetry in our funnel plot (Begg’s test, p = .734; Egger’s test, p = .891) ().
Figure 4. Summary estimate for RR of proteinuria according to HBsAg serologic status (A) Summary estimate for OR of proteinuria according to HBsAg serologic status; (B) Summary estimate for adjusted RR of proteinuria according to HBsAg serologic status; (C) Begg’s funnel plot; (D) Egger’s funnel plot.
Discussion
There is continuing debate about whether HBV infection promotes the development and progression of CKD. The results in the meta-analysis indicate that there is a higher likelihood of CKD among people with HBV infection compared to people without HBV infection. However, the difference was not statistically significant. In addition, there were no significant associations between HBsAg serologic status and low eGFR or proteinuria. Remarkably, all included studies were conducted in Asian populations and that findings may not apply to other racial groups.
However, HBV infection associated with membranous nephropathy is well established, and there also have been reports of HBV association with proliferate glomerulonephritis or membranoproliferative glomerulonephritis.Citation8,Citation23 Glomerular deposition of HBV antigens has been observed in long-term HBV carriers with different glomerulonephritis entities, although the etiologic role of HBV is uncertain.Citation24 The absence of any association between HBV exposure and CKD in this study is unsurprising for two reasons. Firstly, HBV is hepatotropic viruses, and it has been documented that HBV infection have clinically and histologically more severe liver disease and higher risk of HCC and cirrhosis.Citation25,Citation26 The possible explanation why HBV infection did not correlate with CKD, proteinuria, or low eGFR may be related to the competing risk of death. Secondly, the immune response to HBV is present only in patients with active hepatitis B, and this response plays a role in both hepatitis B and HBV associated nephropathy. HBV replication may accelerate apoptosis of renal tubular cells and subsequently contribute to deterioration in renal function.Citation27,Citation28
This meta-analysis is potentially subject to several limitations. Firstly, most of the clinical studies included in this meta-analysis were cross-sectional, a design that does not allow for causal inference. When restricted to cross-sectional studies, the available data are of limited nature due to the lack of controlled studies that provide baseline data, and sequential follow-up, including kidney histology, in patients with initially HBV and normal renal function. There is then some evidence that the findings may be impacted by study design. In our meta-analysis, the only cohort study in the group found a large and statistically significant association between HBV and CKD, while the other five cross-sectional studies found no association. For us, we cannot give a reasonable explanation and can only look forward to more quality researches to explain their differences. Secondly, the studies in this meta-analysis might give incomplete information on potential unmeasured confounders that could introduce bias into the analysis. A magnitude of insensitive codes or missing data for the diagnosis of the combined disease is inherent to clinical databases as opposed to research databases. For example, information on HBV DNA and smoking was incomplete. Finally, individual data in each study was not available; therefore, it was not possible to perform the adjustments by us. On the basis of the RR in each study, we calculated pooled estimate for RR of low eGFR (or proteinuria) with HBsAg across the studies. However, this approach takes into account differential distribution of covariates in order to isolate the effect of HBsAg seropositive status per se.
In conclusion, there was no association between exposure to HBV and the risk of developing CKD in the general population. However, it is possible that the combined effects of risk factors, such as hypertension and diabetes might increase susceptibility to chronic inflammation, DNA damage, and development of CKD. Health care providers still should be aware of these possible risks and future studies should better investigate the potential association to allow for targeted interventions in susceptible patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
Tong-Dong Shi conceived the study, provided funding support, and revised the manuscript critically for important intellectual content. Qing-Chun Cai made substantial contributions to the design of the study and to acquisition, analysis, and interpretation of the data. Qing-Chun Cai, Shu-Qi Zhao, Tong-Dong Shi, and Hong Ren participated in the design of the study and in acquisition, analysis, and interpretation of data. All authors read and approved the final manuscript. This work was supported by National Nature Science Foundation of China [grant No. 81172804].
References
- Khedmat H, Taheri S. Hepatitis B virus-associated nephropathy: An International Data Analysis. Iran J Kidney Dis. 2010;4:101–105.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107.
- Balinsky W. Pediatric end-stage renal disease: Incidence, management, and prevention. J Pediatr Health Care. 2000;14:304–308.
- Bhimma R, Coovadia HM. Hepatitis B virus-associated nephropathy. Am J Nephrol. 2004;24:198–211.
- Levy M, Chen N. Worldwide perspective of hepatitis B-associated glomerulonephritis in the 80s. Kidney Int Suppl. 1991;35:S24–S33.
- Dreyer L. The frequency of hepatitis B surface antigen in membranous nephropathy in black and white South Africans. S Afr Med J. 1984;65:166–168.
- Gonzalo A, Mampaso F, Barcena R, Gallego N, Ortuno J. Membranous nephropathy associated with hepatitis B virus infection: Long-term clinical and histological outcome. Nephrol Dial Transplant. 1999;14:416–418.
- Johnson RJ, Couser WG. Hepatitis B infection and renal disease: Clinical, immunopathogenetic and therapeutic considerations. Kidney Int. 1990;37:663–676.
- Huang JF, Chuang WL, Dai CY, et al. Viral hepatitis and proteinuria in an area endemic for hepatitis B and C infections: Another chain of link? J Int Med. 2006;260:255–262.
- Lee JJ, Lin MY, Yang YH, Lu SN, Chen HC, Hwang SJ. Association of hepatitis C and B virus infection with CKD in an endemic area in Taiwan: A cross-sectional study. Am J Kidney Dis. 2010;56:23–31.
- Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147.
- Fabrizi F, Verdesca S, Messa P, Martin P. Hepatitis C virus infection increases the risk of developing chronic kidney disease: A systematic review and meta-analysis. Dig Dis Sci. 2015;60:3801–3813.
- Fabrizi F, Martin P, Dixit V, Messa P. Hepatitis C virus infection and kidney disease: A meta-analysis. Clin J Am Soc Nephrol. 2012;7:549–557.
- National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266.
- Hartling L, Milne A, Hamm MP, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol. 2013;66:982–993.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188.
- Takkouche B, Cadarso-Suarez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. 1999;150:206–215.
- Petitti DB. Approaches to heterogeneity in meta-analysis. Stat Med. 2001;20:3625–3633.
- Zeng Q, Gong Y, Dong S, Xiang H, Wu Q. Association between exposure to hepatitis B virus and chronic kidney disease in China. J Int Med Res. 2014;42:1178–1184.
- Ishizaka N, Ishizaka Y, Seki G, Nagai R, Yamakado M, Koike K. Association between hepatitis B/C viral infection, chronic kidney disease and insulin resistance in individuals undergoing general health screening. Hepatol Res. 2008;38:775–783.
- Chen YC, Su YC, Li CY, Hung SK. 13-year nationwide cohort study of chronic kidney disease risk among treatment-naïve patients with chronic hepatitis B in Taiwan. BMC Nephrol. 2015;16:110.
- Senghore T, Su F-H, Lin Y-S, Chu F-Y, Yeh C-C. Association between hepatitis B virus infection and chronic kidney disease in University students receiving physical checkups: A cross-sectional study. J Exp Clin Med. 2013;5:181–186.
- Lai KN, Li PK, Lui SF, et al. Membranous nephropathy related to hepatitis B virus in adults. N. Engl J Med. 1991;324:1457–1463.
- Lai KN, Ho RT, Tam JS, Lai FM. Detection of hepatitis B virus DNA and RNA in kidneys of HBV related glomerulonephritis. Kidney Int. 1996;50:1965–1977.
- Wu CF, Yu MW, Lin CL, et al. Long-term tracking of hepatitis B viral load and the relationship with risk for hepatocellular carcinoma in men. Carcinogenesis. 2008;29:106–112.
- Eun JR, Lee HJ, Kim TN, Lee KS. Risk assessment for the development of hepatocellular carcinoma: According to on-treatment viral response during long-term lamivudine therapy in hepatitis B virus-related liver disease. J Hepatol. 2010;53:118–125.
- Ganem D, Prince AM. Hepatitis B virus infection-natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129.
- McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–S55.