Abstract
Objective: To investigate plasma-free carnitine (Fc), acylcarnitine (Ac), and total carnitine (Tc) levels in patients undergoing hemodialysis (HD), and to explore their clinical significance.
Methods: A total of 20 subjects were in the normal control group and 133 patients undergoing HD were divided into medicated (received carnitine treatment) and non-medicated groups. The medicated group was further divided into three subgroups according to Fc level: Fc = 80–199, 200–299, and ≥ 300 μmol/L. We used non-derivative tandem mass spectrometry to determine carnitine levels, and clinical symptoms such as weakness, hypotension, and muscle cramps were recorded during dialysis.
Results: Fc and Tc levels were significantly lower in the non-medicated group than in the control group, whereas Fc, Ac, and Tc levels were higher in the medicated than non-medicated group (p< .05). The medicated group had fewer symptoms during dialysis than the non-medicated group such as weakness, hypotension, and muscle cramps (p< .05). An additional comparison showed that the incidence rates of hypotension and muscle cramps in the Fc < 80–199 μmol/L group were significantly lower than those in the Fc ≥ 300 μmol/L medicated and non-medicated groups.
Conclusions: Patients undergoing HD have low carnitine levels. l-Carnitine can effectively increase Fc concentration and improve clinical symptoms; however, only the proper Fc range can reduce complications caused by dialysis. Thus, this range needs to be determined.
Introduction
Carnitine is an amino acid derivative that helps produce energy for muscle and cell metabolism by mediating the mitochondrial β-oxidation of fatty acids. It is mainly derived from the diet and by endogenous biosynthesis, primarily by the liver and kidney. Healthy people rarely lack carnitine, but carnitine homeostasis can be disturbed in patients receiving long-term hemodialysis (HD).Citation1 These patients typically have concomitant carnitine insufficiency in plasma and tissues due to impaired carnitine biosynthesis, reduced protein uptake, and increased removal of carnitine by dialysis, resulting in reduced carnitine activity.Citation2,Citation3 Carnitine deficiency worsens in patients undergoing long-term dialysis and can cause energy metabolic disorders and symptoms such as weakness, fatigue, dialysis hypotension, and muscle cramps in the short term; and angina, arrhythmia, heart failure, and limited erythropoietin effects during long-term deficiency. These symptoms are easily confused with uremia caused by retained toxins, but determining plasma carnitine levels is diagnostic.
In this study, we compared plasma carnitine levels in normal subjects and patients undergoing HD to determine its clinical significance and to provide a basis for carnitine supplementation in patients undergoing HD.
Subjects and methods
Patients
A total of 20 patients (our hospital healthcare workers) were in the normal control group; thus, 20 plasma samples were collected. A total of 133 patients were in the maintenance HD (MHD) group. These patients received MHD in the blood purification center of the China-Japan Friendship Hospital during October 2014, and were divided into medicated (received carnitine treatment) and non-medicated groups. HD conditions: performed three times/week, 4 h/once; HD unit area ≥ 1.5 m2; dialysate flow, 500 mL/min; blood flow, 200–300 mL/min.
General information records
Basic patient information including age, sex, weight, primary disease, and date the patients accepted MHD treatment were recorded. The patients’ clinical symptoms such as weakness after dialysis, low blood pressure, and muscle cramps during dialysis were also recorded.
Reagents
The NeoBaseTM Non-derivatized MSMS Kit (PerkinElmer, Waltham, MA) contains mobile phase solvents, extract liquid, 96-well microtiter plates, microtiter plate covers, high-low quality product, and one bottle of acylcarnitine (Ac) stable isotopes containing 13 kinds of Ac stable isotopic internal standards: 2H9-free carnitine, 2H3-acetylcarnitine, 2H3-propionyl carnitine, 2H3-butyryl carnitine, 2H9-isovaleryl carnitine, 2H6-glutaryl carnitine, 2H3-caproyl carnitine, 2H3-capryloyl carnitine, 2H3-decanoyl carnitine, 2H3-dodecanoyl carnitine, 2H3-myristoyl carnitine, 2H3-palmitoyl carnitine, and 2H3-stearyl carnitine.
Instruments
Acquity UPLC ultra performance liquid chromatography (Waters Co, Billerica, MA); Quattro Premier XE three-level series of four-pole mass spectrometer with ESI (Waters) and the Masslynx 4.1 data processing system (Waters); and the MB 100-4A Micro hole plate constant temperature oscillator (Hangzhou Allsheng Instruments Co., Ltd, Zhejiang, China) were used for the analysis.
Sample processing
In accordance with the method in the NeoBaseTM Non-derivatized MSMS Kit (PerkinElmer, Waltham, MA), we collected morning fasting venous blood samples from the control group and fasting venous blood before HD from the HD group. The medicated group was given 1 g l-carnitine after each HD session. Venous blood was dropped on 10 mm diameter filter paper, allowed to dry at room temperature, and stored at −20 °C.
Testing method
The filter paper containing dried blood (equivalent to 3.2 μL whole blood) was placed in a 96-well microtiter plate. Then 100 μL Ac isotopic internal standard working solution was added and the sample was subjected to constant temperature oscillator 700 rpm at 45 °C for 45 min. A 75 μL aliquot of the extracted liquid was removed, placed on a heat-resistant plate, and sealed with aluminum foil for testing.
Instrument settings
The following kit conditions were used: velocity gradient, 0–0.22 min at 0.15 mL/min; 0.22–1.0 min at 0.023 mL/min; and 1.0–1.5 min at 0.60 mL/min. The electrospray cation scan mode and multiple reaction monitoring mode were used for data collection. Each sample was measured for 1.5 min and the injection volume was 10 μL.
Statistical analysis
The mass spectrum response values were processed using Masslynx V4.1. software. Ac levels were quantified by calculating ionic strength divided by the ionic strength of the isotopic internal standard. SPSS 19.0 software (SPSS Inc., Chicago, IL) was used to analyze the differences in spectrometry parameters. Measurement data are expressed as mean ± standard deviation. The two groups were compared using the t-test and the count data were analyzed with the χ2 test. A p value <.05 was considered statistically significant.
Results
General data analysis
Normal control group: The mean age of the 20 healthcare workers (nine males and 11 females) was 29.65 ± 4.80 years (range, 22–43 years) and the mean weight was 58.48 ± 9.95 kg.
HD group: One-hundred and thirty-three patients (68 females and 65 males) who received MHD in the department of blood purification center of China-Japan Friendship Hospital in March 2015. The mean age was 61.24 ± 13.45 years (range, 25–86 years) and the mean weight was 65.85 ± 16.24 kg. A total of 38 (28.57%) cases had chronic glomerulonephritis, 35 (26.32%) had diabetic nephropathy, 21 (15.79%) had hypertensive renal damage, 12 (9.02%) had tubulointerstitial nephropathy, seven (5.26%) had polycystic kidney, two (1.50%) had renal tumors, and 18 (13.53%) had other kidney diseases. The patients were divided into medicated and non-medicated groups. A total of 66 non-medicated cases had been undergoing MHD for 62.16 ± 69.70 months (range, 1–264 months); 25 (34.85%) of the 66 patients had <1 year dialysis history, 11 (16.67%) had a dialysis history of 1–3 years, and 32 (48.48%) had >3 years of dialysis history. A total of 67 medicated cases received 1 g l-carnitine intravenously after each dialysis session. The mean MHD history of the medicated group was 80.06 ± 69.22 months (range, 4–324 months) and their medication history was >2 months.
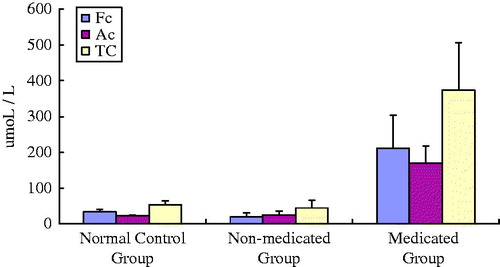
Carnitine levels in the two groups
The results are shown in . The plasma-free carnitine (Fc) and total carnitine (Tc) levels in the non-medicated group were lower than those in the normal control group (p< .05). The Ac level in the non-medicated group tended to be higher than that in the control group. Fc, Ac, and Tc levels in the medicated group were higher than those in the non-medicated group (p< .05).
Clinical symptoms in the HD group
Dialysis hypotension refers to a decrease in systolic blood pressure >20 mmHg during dialysis or a decrease in mean arterial pressure >10 mmHg with symptoms of low blood pressure.Citation4 We divided the medicated group into three subgroups based on Fc level: Fc = 80–199; 200–299 and Fc ≥300 μmol/L. The detailed data are shown in . The incidence of weakness increased with Fc level after dialysis. The incidence of weakness in the Fc ≥ 300 μmol/L group tended to be higher than that in the non-medicated group. Similarly, the incidence rates of hypotension and muscle cramps also increased during dialysis as Fc level increased. The Fc ≥ 300 μmol/L group had more cases of hypotension and muscle cramps than those in the non-medicated group (χ2 test; p< .05). The incidence rates of hypotension and muscle cramps in the subgroups were significantly different (by χ2 test). We used the Bonferroni method to compare the pairs of subgroups; the significance level was adjusted to a = 2a/k (k − 1) = 0.008, where k is the number of groups (four in this study). The significant data were marked in the 2 × 2 chi-square test. The Fc 80–199 μmol/L group (17.14%) had a lower incidence of hypotension than the Fc ≥ 300 μmol/L (57.14%) and non-medicated groups (50.00%) (χ2 test; p< .05). No other comparisons were significant. The incidence of muscle cramps during dialysis in the Fc 80–199 μmol/L group of patients undergoing HD was lower than that in the Fc ≥ 300 μmol/L (64.28%) and non-medicated groups (59.09%) (χ2 test; p< .05). No other comparisons were significant.
Table 1. Clinical symptoms in the groups.
Discussion
Most carnitine is obtained from dietary sources in humans and approximately 25% comes from de novo biosynthesis. Carnitine is obtained by consuming animal-based products including red meats, poultry, fish, and dairy products, whereas negligible quantities are available from plant-derived foods. On the other hand, carnitine is produced from lysine and methionine by a variety of enzymes in the liver, kidneys, and brain. The kidney is the main carnitine excretory organ, and a healthy person excretes 100–400 μmol/Lol daily in the urine. However, to reduce l-carnitine loss and maintain adequate endogenous concentrations, >95% of filtered l-carnitine is reabsorbed by the proximal tubules and returned to the bloodstream.
Tandem mass spectrometry is an efficient, rapid, and accurate technique to detect substances; thus, it was used to analyze the amino acid, organic acid, carnitine, and fatty acid levels in dried blood on filter paper. Plasma carnitine levels slightly differed among studies, as carnitine level can change due to diet, race, age, and method of determination.
Carnitine deficiency is common in patients with end-stage renal disease receiving HD treatment.Citation5 We found that Fc and Tc levels in non-medicated patients undergoing HD were lower than those in the control group. Patients may have low Tc and Fc levels due to lower protein and meat intake, which results in lower carnitine intake; less absorption by the gastrointestinal tract; reduced carnitine synthesis in the kidneys; low renal tubular reabsorption; carnitine transport disorder; and the fact that it is highly dialyzable because of its low molecular weight and lack of protein binding in the plasma. Plasma carnitine levels generally increase in patients with chronic renal failure who do not undergo HD, but decrease in patients who accept HD treatment. In fact, a single HD session can decrease the plasma carnitine concentration by as much as 75%.Citation6 We examined 75 non-medicated patients undergoing HD, 25 (20%) of whom had been undergoing HD for <1 year, with a minimum of one month.
Carnitine is a water-soluble zwitterionic quaternary amine (beta-hydroxy-gamma trimethyl aminobutyric acid; molecular weight, 161.2 Da) that exists as Fc and Ac in serum. Fc accounts for 85.2% of total carnitine, and Fc and Ac together constitute Tc. Carnitine exists as d- and l-carnitine enantiomers; however, only the l-isomer is physiologically active. l-carnitine is a naturally occurring compound found in all mammals. The most important biological function of l-carnitine is transport of fatty acids into the mitochondria for β-oxidation.Citation7 As l-carnitine plays an important role in biological metabolism, it is frequently used in clinical treatment. Administering carnitine is beneficial to patients with angina, ischemia-induced cardiac insufficiency, cardiogenic shock, cardiomyopathy, and myocardial infarction.Citation8 A hyperbolic increase in plasma carnitine concentration is observed after completing a dialysis session, as it is released from tissue stores. Repeated dialysis treatment over an extended period results in a deficiency of tissue carnitine.Citation9 Carnitine insufficiency in patients on long-term HD affects dialysis-related symptoms such as skeletal myopathy, cardiomyopathy, poor exercise performance, anemia, and dialytic complications, such as hypotension, cramps, weakness, and fatigue,Citation10,Citation11 which decrease dialysis tolerance and reduce the long-term survival rate. Consequently, carnitine therapy has been used and extensively studied in patients with ESRD. Although a large number of clinical studies have shown that administering carnitine can alleviate many dialysis-related symptoms in patients undergoing HD, controversy regarding its use persists because of conflicting results. These different results may be related to a lack of randomization and control groups, dose heterogeneity, duration and method of carnitine administration, nonstandard measures of symptom improvement, different laboratory or clinical endpoints, variation in the patient population, or patient response. However, it has been suggested that l-carnitine may have to be administered at doses that achieve supra-physiological concentrations in plasma and target tissues to fully exploit its pharmacologic potential.Citation12,Citation13
This study found that Fc and Tc levels in non-medicated patients undergoing HD were lower than those in the control group. The Fc, Ac, and Tc levels of patients increased significantly after 1 g l-carnitine was administered intravenously. Plasma carnitine levels decreased after HD therapy but significantly increased after administering l-carnitine, demonstrating that l-carnitine can effectively make up for the loss of carnitine in patients undergoing HD.
Fewer clinical symptoms, such as weakness, hypotension, and muscle cramps, were observed after dialysis in patients undergoing HD in the medicated group compared to the non-medicated group. The incidence of weakness increased after dialysis as Fc level increased. The incidence of weakness in the Fc ≥ 300 μmol/L group tended to increase more than that in the non-medicated group (by χ2 test; p> .05). Similarly, the incidence rates of hypotension and muscle cramps also increased during dialysis as Fc levels increased. In this case, the incidence rates of hypotension and muscle cramps in the Fc ≥ 300 μmol/L group were also higher than those in the non-medicated group (by χ2 test; p< .05). The incidence of hypotension during dialysis in the Fc 80–199 μmol/L group (17.14%) was lower than that in the Fc ≥ 300 μmol/L (57.14%) and non-medicated groups (50.00%) (p< .05). No other comparisons were significant. The incidence of muscle cramps in the Fc 80–199 μmol/L group (20.00%) was lower than that in the Fc ≥ 300 μmol/L (64.28%) and non-medicated groups (59.09%) (p< .05). No other comparisons were significant. Our results showed that l-carnitine effectively improved hypotension and muscle cramp symptoms during dialysis in the Fc 80–199 μmol/L group. The Fc ≥ 300 μmol/L group had the highest incidence rates of hypotension and muscle cramp compared to those in the non-medicated group.
In conclusion, patients undergoing HD generally have low levels of carnitine. We determined Fc levels in patients before dialysis and found that their levels were lower than those in the control group. We speculate that reductions in carnitine would be more noticeable after dialysis, so we recommend that doctors monitor carnitine levels in patients undergoing HD. l-Carnitine is important in clinical medicine, and our results demonstrate that it effectively increases blood Fc concentrations and improves clinical symptoms. This comprehensive evaluation results suggest that the appropriate range of free carnitine can improve complications in patients undergoing dialysis; however, beyond a certain range its effects may be counterproductive. Thus, the appropriate Fc range requires further study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Hedayati S. Dialysis-related carnitine disorder. Semin Dial. 2006;19:323–328.
- Reuter SE, Faull RJ, Ranieri E, et al. Endogenous plasma carnitine pool composition and response to erythropoietin treatment in chronic hemodialysis patients. Nephrol Dial Transplant. 2009;24:990–996.
- Bene J, Csiky B, Komlosi K, Sulyok E, Melegh B. Dynamic adaptive changes of the serum carnitine esters during and after l-carnitine supplementation in patients with maintenance haemodialysis. Scand J Clin Lab Invest. 2011;71:280–286.
- Chen XM. Blood hemodialysis. In: Ding XQ, Liu FY, Liu ZH, Chen JH, Mei CL, editors. Purification Standard Operating Procedure (SOP). Chapter 10. Beijing, China: People’s Military Medical Press; 2012.
- Csiky B, Bene J, Wittmann I, Sulyok E, Melegh B. Effect of hemodialysis session on the dynamics of carnitine ester profile changes in l-carnitine pretreated end-stage renal disease patients. Int Urol Nephrol. 2013;45:847–855.
- Bene J, Csiky B, Wittmann I, Sulyok E, Melegh B. Dramatic decrease of carnitine esters after interruption of exogenous carnitine supply in hemodialysis patients. Ren Fail. 2012;34:555–558.
- Reuter SE, Evans AM. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet. 2012;51:553–572.
- Kudoh Y, Aoyama S, Torii T, et al. Hemodynamic stabilizing effects of l-carnitine in chronic hemodialysis patients. Cardiorenal Med. 2013;3:200–207.
- Evans AM, Faull RJ, Nation RL, et al. Impact of hemodialysis on endogenous plasma and muscle carnitine levels in patients with end-stage renal disease. Kidney Int. 2004;66:1527–1534.
- Schreiber BD. Debate forum: Levocarnitine therapy is rational and justified in selected dialysis patients. Blood Purif. 2006;24:128–134.
- Bellinghieri G, Santoro D, Calvani M, Mallamace A, Savica V. Carnitine and hemodialysis. Am J Kidney Dis. 2003;41:S116–S122.
- Bohomini M. Pharmacological use of l-carnitine in uremic anemia: Has its full potential been exploited? Pharmacol Res. 2011;63:157–164.
- Marcovina SM, Sirtori C, Peracino A, et al. Translating the basic knowledge of mitochondrial functions to metabolic therapy: Role of l-carnitine. Transl Res. 2013;161:73–84.