Abstract
Background: Current vancomycin dosing guidelines recommend targeting trough concentrations of 15–20 mg/L in complicated infections to avoid treatment failure and resistance. How to accomplish this in the intermittent hemodialysis (IHD) population has not been adequately described. A weight-based vancomycin dosing protocol for IHD patients was developed to provide standardization of vancomycin dosing for this patient population. Prior to implementation of this protocol, clinical pharmacists used their individual judgment for dosing and monitoring.
Objective: Compare achievement of goal (15–20 mg/L) pre-IHD vancomycin levels between a group of patients dosed prior to implementation of this weight-based vancomycin dosing protocol and a group dosed after implementation.
Methods: This retrospective study evaluated hospitalized IHD patients who received vancomycin and had an appropriate pre-IHD vancomycin level. Any patients with acute kidney injury or who required continuous renal replacement therapy or peritoneal dialysis were excluded.
Results: A total of 145 vancomycin courses (94 pre-protocol and 51 post-protocol) were included in this study. The post-protocol group had an increased percentage of patients who achieved a pre-IHD vancomycin level of 15–20 mg/L. We also found improvement in pre-IHD vancomycin levels attained in patients weighing less than 75 kg and the need for additional study in patients weighing more than 105 kg.
Conclusion: Simplifying and standardizing vancomycin dosing for hospitalized IHD patients based on weight resulted in 37% of patients achieving goal pre-IHD vancomycin level of 15–20 mg/L with zero patients having a pre-IHD vancomycin level <10 mg/L.
Introduction
Infection remains a critical issue for patients who require intermittent hemodialysis (IHD), especially infections due to gram positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA).Citation1 Despite its introduction in the 1950s, vancomycin remains a key antibiotic in the treatment of MRSA infections.Citation2 Recent guidelines recommend vancomycin dosing based upon actual body weight, maintaining trough levels >10 mg/L to reduce emergence of vancomycin resistance in S. aureus, and goal trough levels of 15–20 mg/L for complicated infections to ensure that the area under the curve to minimum inhibitory concentration (AUC/MIC) ratio remains ≥400.Citation2,Citation3
With the increase in goal vancomycin trough levels of 15–20 mg/L, several retrospective studies have reported nephrotoxicity rates of 12 to 42.7%.Citation1 While many clinicians consider nephrotoxicity a negligible concern in patients with end stage renal disease on IHD, repeated studies have shown that residual kidney function in IHD patients is associated with greater survival.Citation4 Residual kidney function allows IHD patients to maintain fluid and metabolic homeostasis, reduce mineral abnormalities, and optimize uremic toxin clearance.Citation4 Even for IHD patients, maintenance of pre-IHD vancomycin levels above 10 mg/L is important for bacteria eradication and reduction of resistance, and avoiding pre-IHD vancomycin levels greater than 20 mg/L helps maintain residual kidney function by reducing the risk of nephrotoxicity. With this improved understanding of the goal pre-IHD vancomycin levels, there are no published guidelines for vancomycin dosing in the IHD population. Additionally, a recently published review by Crew et al. concluded that the single best method of vancomycin administration for IHD patients remains unclear.Citation1,Citation5
Prior to the introduction of high-flux (polysulfone) dialysis membranes in the late 1990s, vancomycin was generally dosed weekly due to the poor removal of vancomycin with low-flux (cuprophane and cellulose acetate) membranes.Citation6 Initial studies with high-flux membranes showed vancomycin removal of 30–46% which generally necessitates dosing after each IHD session.Citation6–8 Early dosing regimens with high-flux dialyzer membranes recommended a loading dose (LD) of 1000 mg or 20 mg/kg dry weight followed by maintenance doses (MD) of either 500 mg or 1000 mg.Citation6,Citation8,Citation9 All of these studies were published prior to the most recent vancomycin dosing guidelinesCitation2 and used a goal pre-IHD vancomycin level of 5–20 mg/L with 84–97%Citation6,Citation8,Citation9 of these levels being within the goal range. This study investigated the efficacy of a weight-based vancomycin dosing protocol for IHD patients by comparing the percentage of patients with pre-IHD vancomycin levels within goal range of 15–20 mg/L before and after implementation of this protocol.
Methods
Vancomycin dosing protocol
For patients at the study institution, all vancomycin is dosed by clinical pharmacists. Prior to the implementation of the IHD weight-based dosing protocol, there was no standardization for dosing IHD patients. Each pharmacist independently determined the vancomycin LD and MD and the frequency of monitoring of pre-IHD vancomycin levels based upon their individual experience and clinical judgment. With this individualized approach to vancomycin dosing for IHD patients, training of PGY-1 pharmacy residents and new clinical pharmacists was especially difficult. Additionally, the efficacy of vancomycin dosing, determined by achievement of guideline recommended trough levels of 15–20 mg/L, was unknown. In October 2012, a weight-based vancomycin dosing protocol for IHD patients was instituted in order to standardize dosing and monitoring. The goal pre-IHD vancomycin level for this protocol was 15–20 mg/L which complies with current guideline recommendations for empiric therapy. Each patient received a 20 mg/kg LD followed by post-IHD MD per their total body weight (). Per hospital policy, no antibiotics are administered or labs drawn in the dialysis unit. With the estimated vancomycin half-life in anuric patients being 100–200 hCitation10 and the post-dialysis rebound effect occurring 2–6 h after IHDCitation11, the researchers determined that the difference in the random vancomycin level drawn the morning following an IHD session and the morning prior to the next IHD session is minimal. Thus the first of any vancomycin levels drawn after three vancomycin doses was included in the data analysis. If the first vancomycin level was >20 mg/L, the MD was reduced to the next lower protocol MD (i.e. 1000 mg decreased to 750 mg). If the first vancomycin level was <15 mg/L, the MD was increased to the next higher protocol MD.
Table 1. Weight-based vancomycin dosing for IHD patients.
Study design
This study was conducted at a community teaching hospital located in an urban setting in the southeast. Both pre-protocol and post-protocol data were obtained via retrospective chart review as approved by the Institutional Review Board. Patients were identified through disease codes from the 9th revision of the International Classification of Diseases (ICD-9), pharmacy consult lists, and dialysis records. Patients were included in the study if they required IHD, received at least three doses of vancomycin, and had at least one appropriately drawn pre-IHD vancomycin level. An individual patient could be included in the study multiple times as long as they received independent courses of vancomycin. Patients were excluded if they had acute kidney injury or received continuous renal replacement therapy or peritoneal dialysis at any point in their stay. Due to the lack of standardization in dosing and monitoring of pre-IHD vancomycin levels prior to the initiation of the weight-based dosing protocol, the researchers determined that a longer pre-protocol time period was required to obtain a similar number of pre-protocol and post-protocol patients with pre-protocol patients identified from November 2009 through October 2012 and post-protocol patients identified from November 2012 through May 2013.
Each dialysis prescription was ordered by the attending nephrologist. The study institution exclusively uses the high-flux dialyzer membrane, Optiflux F160NR made by Fresenius (polysulfone, surface area 1.5 m2, ultrafiltration coefficient KUF = 45), for all patients receiving IHD. Individual dialysis prescriptions were not collected in this study, but the primary prescription used is a blood flow rate of 300–400 mL/min with a dialysate rate of 600 mL/min for 3–5 h. The majority of patients receive IHD 3–4 times per week. As the weight-based protocol gives a MD after each IHD session and pre-IHD vancomycin levels are drawn after 3 doses are given, the number of IHD sessions per week was not expected to affect the overall results.
Outcome measures
The primary outcome was the percentage of pre-IHD vancomycin levels between 15 and 20 mg/L. Secondary outcomes include the percentage of pre-IHD vancomycin levels less than 10 mg/L and greater than 20 mg/L. A post-hoc analysis investigated percentage of pre-IHD vancomycin levels less than 10, between 15 and 20 mg/L, and greater than 20 mg/L by the four weight ranges used in the protocol and compared the pre- and post-protocol mean pre-IHD vancomycin levels for the entire population and for each weight range.
Statistics
Continuous baseline characteristics were described using mean with standard deviation. For categorical data, the chi-square test was used to evaluate differences between the pre-protocol and post-protocol groups. For continuous data, the Student’s t-test was used to evaluate normally distributed data. An alpha value of less than 0.05 is considered significant.
Results
A total of 2262 patients were screened for inclusion in this study. Ninety-four vancomycin courses were included in the pre-protocol data set with 51 included in the post-protocol data set. Baseline characteristics () show similar mean age and weight between the two groups. All post-protocol patients received a LD indicating adherence to the protocol.
Table 2. Baseline characteristics.
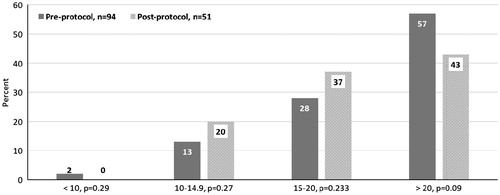
For the primary outcome, 28% (26/94) of patients in the pre-protocol group had a pre-IHD vancomycin level between 15 and 20 mg/L versus 37% (19/51) in the post-protocol group (p = .233). compares the pre-IHD vancomycin levels for the pre- and post-protocol groups by category: less than 10 mg/L, 10–20 mg/L, 15–20 mg/L, and greater than 20 mg/L. The post-protocol group had a higher percentage of patients in the 10–20 mg/L range and fewer in the >30 mg/L range when compared to the pre-protocol group, but these differences were not statistically significant.
divides the pre-IHD vancomycin levels for both the pre- and post-protocol groups into the four weight classes used in the protocol. For patients weighing <75 kg, 57% of pre-IHD vancomycin levels in the post-protocol group were in the goal range of 15–20 mg/L (34% vs. 57%, p = .1) and there were statistically fewer pre-IHD vancomycin levels >20 mg/L in the post-protocol group (50% vs. 19%, p = .022). While the total number of vancomycin courses were small in the group weighing >130 kg, all of the post-protocol pre-IHD vancomycin levels and 80% of the pre-protocol were >20 mg/L.
Table 3. Percentage of pre-IHD vancomycin levels by weight range.
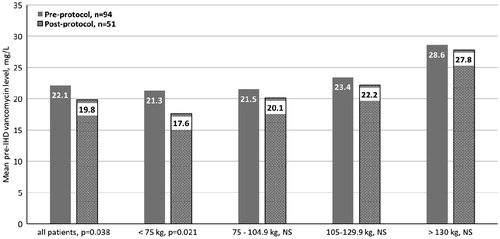
compares the mean pre-IHD vancomycin levels for the pre- and post-protocol groups for the entire study population and per weight group. For all patients, the post-protocol mean pre-IHD vancomycin level was statistically smaller than the pre-protocol group (22.1 mg/L vs. 19.8 mg/L, p = .038). This statistical significance remained true for the patients weighing <75 kg (21.3 mg/L vs. 19.8 mg/L, p = .021). For all remaining weight ranges, the post-protocol mean pre-IHD vancomycin level was lower than the pre-protocol mean pre-IHD vancomycin level but they were not statistically different. For those patients weighing >130 kg, the mean pre-IHD vancomycin level was highest at 28.6 mg/L pre-protocol and 27.8 mg/L post-protocol implementation.
Discussion
The study results show that hospitalized patients requiring IHD can benefit from a standardized weight-based vancomycin dosing protocol. The protocol resulted in improved dosing as 100% of post-protocol patients received a 20 mg/kg LD, a similar percentage of patients obtained the goal pre-IHD vancomycin level of 15–20 mg/L, and the post-protocol group’s mean pre-IHD vancomycin level was statistically lower than the mean pre-IHD vancomycin level for the pre-protocol group. In the hospital, the number of IHD treatments per week and length of individual IHD treatments vary due to volume status, acute disease state, IHD access, and patient adherence to IHD. Despite these differences in IHD treatments, this study shows that this weight-based vancomycin dosing protocol is robust and effective at achieving pre-IHD vancomycin levels >10 mg/L despite inter-patient differences.
In the review by Crew et al., the authors recommend using repeated pre-IHD vancomycin levels to determine the optimal MD.Citation5 While effective,Citation18 repeated pre-IHD vancomycin levels are costly and time-consuming in the hospital setting. The study protocol only required one pre-IHD vancomycin level drawn after the third dose of vancomycin, making it simpler to implement in the busy acute care setting. summarizes the results from other trials of weight-based vancomycin dosing protocols used for IHD patients. All of the protocols differ in doses used, patient setting and vancomycin administration. Our results add to this body of literature and demonstrate that a weight-based protocol is a reasonable option for dosing vancomycin in IHD patients.
Table 4. Studies evaluating vancomycin dosing for IHD patients.
We showed the best results in the group of patients weighing <75 kg as this group had the highest percentage of pre-IHD vancomycin levels within goal range (34% pre vs. 57% post, p = .01) and a statistically lower mean pre-IHD vancomycin level compared to the pre-protocol group (21.3 mg/L pre vs. 19.8 mg/L post, p = .021). We found opposite results in the two highest weight categories of 105–129.9 kg and >130 kg where the highest percentage of pre-IHD vancomycin levels was >20 mg/L and the mean pre-IHD vancomycin levels for the post-protocol group were high (28.6 mg/L vs. 27.8 mg/L, NS).
Currently, the optimal vancomycin dose for the obese population is an area of uncertainty. Both vancomycin clearance and volume of distribution is increased in obese patients.Citation16 Thus, it has been theorized that as both increase with obesity, total body weight dosing is correct for all weight ranges. Recent retrospective studies of patients with obesity have questioned this assumption and are recommending reduced doses in obese patients.Citation16,17 To date, no data have been published on vancomycin dosing in obese patients on IHD. With the IHD patient population, the vancomycin clearance would be the same for all weights as it is primarily determined by IHD resulting in a greater effect from the altered volume of distribution. In this study, the highest mean pre-IHD vancomycin level occurred in the group of patients weighing >130 kg with 100% of this post-protocol group having a pre-IHD vancomycin level >20 mg/L. As this group also had the fewest number of vancomycin courses, additional study is needed including collection of body mass index data.
This study has several limitations including its retrospective nature. First, all included patients were receiving regular IHD but some could have been newly started during this hospitalization and residual renal function was not considered. Second, as this study was performed in a hospital setting where dry weight is not consistently measured, all weights used to determine the vancomycin MD were from the weight entered upon the patient’s arrival to the hospital which could have been significantly higher than the patient’s dry weight as IHD patients often present to the hospital with volume overload. Third, we did not capture the data necessary to calculate body mass index.
Conclusion
This study demonstrated that simplifying and standardizing vancomycin dosing for IHD patients using a weight-based protocol is effective at attaining pre-IHD vancomycin levels of 15–20 mg/L. For patients weighing <75 kg, the weight-based protocol was statistically better than pre-protocol. For the highest weight group, the protocol showed similar pre-IHD vancomycin levels to pre-protocol with both having unacceptably high pre-IHD vancomycin levels. Further study is needed in patients weighing greater than 105 kg to determine the optimal dosing regimen.
Acknowledgement
The authors would like to thank Andy Johnston, PharmD, BCPS for his assistance in developing this protocol.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Vandecasteele SJ, DeVriese AS. Recent changes in vancomycin use in renal failure. Kidney Int. 2010;77:760–764.
- Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325–327.
- Kullar RK, Leonard SN, Davis SL, et al. Validation of the effectiveness of a vancomycin nomogram in achieving target trough concentrations of 15–20 mg/L suggested by the Vancomycin Consensus Guidelines. Pharmacotherapy. 2011;31:441–448.
- Yoshitsugu O, et al. Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: A cohort study. Am J Kid Dis. 2016;68:256–265.
- Crew P, Heintz SJ, Heintz BH. Vancomycin dosing and monitoring for patients with end-stage renal disease receiving intermittent hemodialysis. Am J Health Syst Pharm. 2015;72:1856–1864.
- Pai AB, Pai MP. Vancomycin dosing in high flux hemodialysis: A limited-sampling algorithm. Am J Health Syst Pharm. 2004;61:1812–1816.
- Vandecasteele SJ, De Vriese AS. Vancomycin dosing in patients on intermittent hemodialysis. Semin Dial. 2011;24:50–55.
- Ariano RE, Fine A, Sitar DS, Rexrode S, Zelenitsky SA. Adequacy of a vancomycin dosing regimen in patients receiving high-flux hemodialysis. Am J Kidney Dis. 2005;46:681–687.
- Barth RH, DeVincenzo N. Use of vancomycin in high-flux hemodialysis: Experience with 130 courses of therapy. Kidney Int. 1996;50:929–936.
- Vandecasteele SJ, De Bacquer D, De Vriese AS. Implementation of a dose calculator for vancomycin to achieve target trough levels of 15–20 microg/mL in persons undergoing hemodialysis. Clin Infect Dis. 2011;53:124–129.
- Touchette MA, Patel RV, Anandan JV, Dumler F, Zarowitz BJ. Vancomycin removal by high-flux polysulfone hemodialysis membranes in critically ill patients with end-stage renal disease. Am J Kidney Dis. 1995;26:469–474.
- Zelenitsky SA, Ariano RE, McCrae ML, Vercaigne LM. Initial vancomycin dosing protocol to achieve therapeutic serum concentrations in patients undergoing hemodialysis. Clin Infect Dis. 2012;55:527–533.
- Panais R, Hirsch DJ, Dipchand C, Storsley L, Finkle SN. A protocolized approach to vancomycin dosing in conventional hemodialysis. J Nephrol. 2010;23:569–574.
- Taylor ME, Allon M. Practical vancomycin dosing in hemodialysis patients in the era of emerging vancomycin resistance: A single-center experience [Letter to the editor]. Am J Kidney Dis. 2010;5:1163–1165.
- Lin SY, Shen MC, Hwang SJ, Chen TC, Chiu YW, Lu PL. Evaluation of vancomycin dosing protocols to achieve therapeutic serum concentrations in patients receiving high-flux hemodialysis [Letter to the editor]. Int J Antimicrob Agents. 2014;43:384–385.
- Reynolds DC, Waite LH, Alexander DP, DeRyke CA. Performance of a vancomycin dosage regimen developed for obese patients. Am J Health Syst Pharm. 2012;69:944–950.
- Morrill HJ, Caffrey AR, Noh E, LaPlante KL. Vancomycin dosing considerations in a real-world cohort of obese and extremely obese patients. Pharmacotherapy. 2015;35:869–875.
- Jeremiah CJ, Wills C, Bayly A, et al. Vancomycin dosing nomogram for haemodialysis patients. Nephrology. 2014;19:513–516.