Abstract
Introduction and aim: To study the protective, preventive effect of luteolin from colistin-induced nephrotoxicity.
Material and method: Four different treatment options were tested on rats: colistin, luteolin, and a combination of colistin and luteolin, intraperitoneally as two doses a day, for seven days. Another group of rats were used as the control and treated with sterile saline. Serum creatinine levels were measured before and after treatment. Histological changes and colistin-induced apoptosis (Insitu BrdU-red DNA Fragmentation Assay Kit) of the renal tissues were examined after the scarification procedure.
Results: In the Colistin Group, post-treatment creatinine levels were statistically higher than the pretreatment levels (p = .001). In the remaining groups, no significant changes were observed. Cells that undergo apoptosis were counted and it was shown that all groups except the colistin-treated group had a similar number of apoptotic cells, whereas the colistin-treated group had statistically higher number of apoptotic cells compared to other groups (p = .0001). Renal histological damage was also measured and the score of the colistin treated group was higher as compared to other groups.
Conclusion: The results obtained from this study demonstrated us that luteolin was capable of preventing colistin-induced nephrotoxicity and that this effect was significant at histopathological level.
Keywords:
Introduction
Colistin is a very important antibacterial agent that should be used in the treatment of Gram-negative bacterial infections who are resistant to many other drugs (MDR); in spite its high ratio of nephrotoxicity effect.Citation1 This drug has become a part of the most effective antibiotic combinations against infections that developed with multiple resistive microorganisms in clinical environments.Citation2–4 However, nowadays colistin resistance trends to widespread and this may be considered as a scary development.Citation5 The nephrotoxicity ratio monitored in recommended colistin dosage is approximately 50% range, but there are some studies reporting that even at such dosage, clinical success may not be adequate.Citation6–8 The resistance that rapidly develops against the drug together with inefficient treatment has become another significant problem. Reducing the risk of nephrotoxicity due to high dose was considered as a factor that directly affects the morbidity and mortality of the patient.Citation9 Oxidative damage induced by free oxygen radicals and apoptosis are the very first phases of colistin nephrotoxicity.Citation10 Nevertheless, there are some findings that antioxidant substances such as ascorbic acid, vitamin E, melatonin, and astaxanthin may reduce colistin nephrotoxicity in experimental studies.Citation11–13
In a prospective clinical study carried out by Dalfino et al., where 70 intensive care patients who used colistin were assessed, based on the results obtained from this experimental study, it was proven that ascorbic acid made colistin administration safer regarding nephrotoxicity.Citation14
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a common flavonoid found in many vegetative drugs, vegetables, and fruits.Citation15 Flavonoids especially perform their function directly by the means of NADPH oxidase, xanthine oxidase enzyme inhibition, enzyme inhibition, and indirectly by increasing the number of the antioxidant enzymes in cells and protecting lyophilic antioxidants. In a study carried out by Domitrović et al., it was shown that oxidative stress phases of cisplatin nephrotoxicity were avoided by using luteolin while the tubular accumulation of the drug was prohibited and apoptosis was immobilized.Citation16 However, there are no studies in the literature where the effect of luteolin on colistin-induced nephrotoxicity was investigated. Therefore, our study can be considered as the first one that investigated the effect of luteolin on histological renal damage due to colistin administration. The purpose of the present study is to determine if acute renal damage induced by colistin in rats varied due to the effect of luteolin in consideration of biochemical and immune histochemical data.
Material and method
Animals
Male Wistar rats (n = 28, body weight 280 ± 25 g, 24 weeks old) obtained from experimental animals laboratory of Experimental Research and Skill Development Center (BADABEM), Bagcilar Training and Research Hospital were included in the study. The animals were kept in rooms with a fixed temperature (21 ± 1 °C) and moisture (75 ± 5%), and 12 h of brightness/darkness cycle. Two days before animals were transferred to a metabolic cage, to measure basal creatinine levels, 1 cm3 blood was collected from each animal from their jugular veins, under general anesthesia. Animals were fed with a standard laboratory fodder in polyethylene cages for a period of two days. Animals were allowed to reach water and food freely and the experiment was initiated after they were followed for three days in metabolic cages.
Experimental protocol
In this project, a total number of 24 animals were used, six rats per group. Group 1 was the Control Group who received isotonic sodium chloride while Group 2 received 480,000 IU/kg/day intraperitoneal colistin and Group 3 received 10 mg/kg luteolin and Group 4 received 480,000 IU/kg/day colistin and 10 mg/kg luteolin for seven days by the intraperitoneal route, 4 h before colistin. The daily drug volume of drug concentrations was adjusted equally in every drug group. At day 7 of colistin therapy, animals were anesthetized (with 90 mg/kg ketamine and 10 mg/kg of xylazine) 16 h after the final dosage was administered and their left and right kidneys were extracted; right after these procedure animals were sacrificed by exsanguination. The right kidneys of the animals were placed in 10% neutral buffered formalin for further histologic assessment by light microscopy and the left kidney was stored at −80 °C in dry ice for apoptosis assessment. The blood obtained by exsanguination was used to measure serum creatinine levels at the end of therapy.
Biochemical measurements
Blood samples of animals obtained before therapy and during exsanguination were centrifuged for 10 min at 3000 cycle/min. Creatinine levels were measured by an Enzyme-Linked Immunosorbent Assay (ELISA) method using separated serum samples.
Light microscopy study
One of the resected rat kidneys was sent for pathological study in a 10% buffered formaldehyde solution. Tissues were fixed for a period of 24 h in a 10% buffered formaldehyde solution.
The entire tissue was sampled. After routine tissue follow-up procedures, tissue was embedded in paraffin blocks. Cross-sections at 4-μm thickness were obtained. An Insitu BrdU-Red DNA Fragmentation (TUNEL) Assay Kit was applied to tissues that were transferred to polylysine-plated slides, to carry out an immunofluorescent study. All tissues were studied under positive control. Preparations were studied under fluorescent and light microscopy (Leica DFC310 FX) regarding histopathological study and signal intensities. The same pathologist who did not know which group the samples belonged to studied the tissue samples of rats in a blind manner and in accordance with the scale below.
The lesion that formed in the kidney was assessed pathologically at three degrees: Grade 1, tubular dilation, unveiled nucleuses unveiled nucleuses and a mild tubular damage that consist of a few number of pale tubular cast; Grade 2, multiple numbers of tubular cast’s and severe tubular damage induced by the necrosis of the tubular epithelium; and Grade 3, acute cortical necrosis without or with papillary necrosis.
Renal histological variations are scored according to grading as the following:
Grade 1 lesion 1 point; grade 2 lesion 4 points and grade 3 lesion 10 points.
The following points were granted according to the percentage of the effective area of the kidney:
<1% = 0 points; 1–4% = 1 points; 5–9% = 2 points; 10–19% = 3 points; 20–29% = 4 points; 30–39%= 5 points; ≥ 40% = 6 points.
Total points were calculated according to adding grades and the percentage of the effective area. Semi-quantitative score (SQS) for renal histological variations was calculated as follows:
SQS +1, mild damage (total points 1–15); SQS +2, mild–moderate damage (total points 15–30); SQS +3, moderate damage (total points 30–45); SQS +4, moderate–severe damage (total points 30–45) ve SQS +5, severe damage (total points 60).
Assessment of apoptosis
To stain apoptotic cells, samples were dyed with a standard TUNEL kit using a terminal Insitu BrdU-Red DNA Fragmentation (TUNEL) Assay Kit (ab66110, Abcam, Cambridge, UK). Apoptotic cells that display DNA fragmentation were studied under orange, red and blue color filtered immunofluorescent. Apoptotic cells were painted in bright colors while the nucleuses of other cells were painted with a pale color. Tubular cells painted with brilliant colors were magnified 400 times and then counted by the aid of a light microscope. The number of apoptotic cells was calculated at each 400 magnification of the light microscope. Apoptotic cells obtained from the cortex and medulla from each subject were counted under a 5-microscope area and then their average was calculated.
Statistical analysis
An SPSS-17 (SPSS Inc., Chicago, IL) program was used for statistical analysis.
Categorical variables, normally distributed continuous variables and non-normally distributed continuous variables were referred to as a number of cases and percentage, mean ± standard deviation and median (minimum–maximum) values, respectively. One-way ANOVA [Tukey’s honestly significant difference (HSD) test for comparison of two groups] and Kruskal–Wallis (Mann–Whitney U test with the Bonferroni correction in comparison of two groups) tests were used for the comparison of normally distributed variables and non-normally distributed variables between more than two groups, respectively. One-way ANOVA test and paired-samples t-test were used for the comparison of repetitive measurements in a group. p < .05 value was considered as statistically significant.
Results
A statistically significant increase in the level of post-treatment creatinine was observed in the Colistin Group in comparison to pretreatment creatinine level (p = .028). No significant difference in the level of creatinine between pretreatment and post-treatment among all groups was observed, as illustrated in (Luteolin, Sterile Saline, Colistin + Luteolin Groups) (p > .05).
Table 1. Comparison of pretreatment and post-treatment serum creatinine levels.
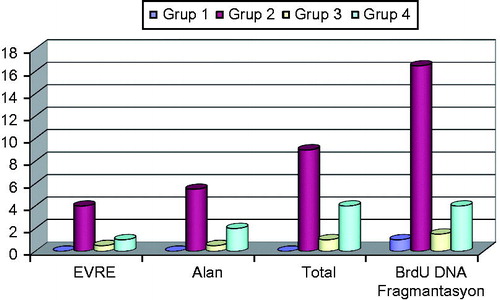
The rate of apoptosis was observed to be significantly higher in Colistin Group as compared to other experimental groups (p < .0001). No statistical significant difference was observed among groups other than the Colistin Group, as illustrated in and . Renal histological damage score was found to be higher in Colistin Group in comparison to other experimental groups. Renal histological damage score of colistin + luteolin group was greater than the Control Group of normal saline (). Renal histological changes in all experimental groups are presented in , and . Immuno-fluorescent findings of Insitu BrdU DNA fragmentation are illustrated in and .
Figure 2. Histopathologic findings. (A, H&E; ×200) This picture shows degenere-necrotic tubules (arrows) in Kolistin group. (B, H&E; ×400) High magnification, vacuolar degeneration (arrowhead) and tubular dilatation in tubules. (C, H&E; ×600) High magnification, necrotic tubules with prominent nucleoli. (D, H&E; ×400) This picture displays extensive cortical necrosis (grade 3) without glomerular damage in Kolistin group. (E, H&E; ×400) Decreased tubular damage (arrows) seen in Kolistin and Luteolin group. (F, H&E; ×200) Improved toxicity with minimal tubular damage seen in Luteolin group (compare to D).
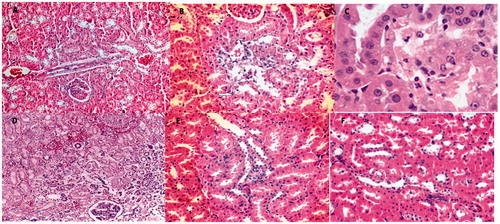
Figure 3. Immuno-fluorescent findings: Insitu BrdU DNA fragmentation; (A) scarce apoptotic cells at the tubulus epithelium in the Control Group (orange; ×200). (B) (red; ×200). (C) (blue; ×200). (D) Apoptotic cells that display significant signal density at the tubulus epithelium in the Colistin Group (orange; ×200). (E) (red; ×200). (F) (blue; ×200). (G) Apoptotic cells that display sparse signal density at the tubulus epithelium in the Luteolin group (orange; ×200). (H) (red; ×200). (I) (blue; ×200). (J) Apoptotic cells that display sporadic intensive signal density at the tubulus epithelium in the Colistin Group + Luteolin Group (orange; ×200). (K) (red; ×200). (L) (blue; ×200).
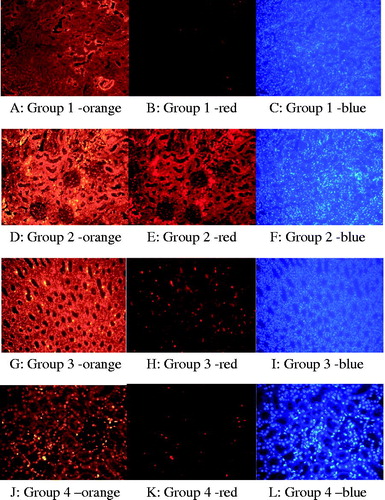
Table 2. TUNEL results and renal histological damage scoring in all experimental groups.
Discussion
Although the mechanism of the nephrotoxic effect of colistin not yet completely certain, it was observed that it may lead to multiple pro-inflammatory cytokine increases by enhancing the number of free oxygen radicals in both neural, and renal cells.Citation17 By the means of the activated intracellular paths, DNA damage that occurred alongside with increased inflammatory activity can stimulate apoptosis by triggering apoptotic factors known as p53, cytochrome c, Bax, Bcl 2, Fas, Fas-L, and the caspase family.Citation18 In the present study, a significant apoptosis was monitored in the Colistin Group while apoptosis was significantly reduced in the Luteolin + Colistin Group.
Studies carried out on rats during the recent years showed that oxidative stress phases played a role in colistin nephrotoxicity while there was a tendency that especially antioxidant drugs could eliminate this effect. It was shown in animal experiments that renal histological abnormalities that developed due to colistin improved when used in combination with antioxidant drugs such as ascorbic acid, vitamin E, melatonin, astaxanthin, and proanthocyanidins grapeseed extracts whereas levels of free oxygen radicals decreased and apoptosis reduced.Citation12,Citation13,Citation19–21 In our study, based on the study carried out by Domitrović et al., the main reason we selected luteolin as the active substance is because luteolin was capable of preventing cisplatin-induced nephrotoxic effect that has strongly abstained in oncology nephrotoxicity.Citation16
In the present study, investigators administered doses of 10 mg/kg and 20 mg/kg cisplatin and 10 mg/kg luteolin to six groups of animals that consisted of five animals in each group (together with Control Groups). The kidneys of the animals were comprehensively analyzed according to histopathologic, inflammatory variations, apoptosis ve biochemical renal damage. As a result, it was shown that luteolin avoided oxidative stress phases and prevented tubular accumulation of the drug and apoptosis. It is clear that luteolin possesses a preventive effect against most of the main mechanisms regarding renal damage as mentioned above. In our study, we established a significant level of nephrotoxicity by administering colistin for a long period that can be easily viewed by light microscopy and consequently showed that it was possible to improve renal damage with luteolin. Our study is also the first one in the literature where we showed that luteolin reduced the ratio of apoptotic cells that occurred due to nephrotoxicity induced by colistin. However, histopathological phase, area, apoptosis scoring of the colistin-luteolin group was found significantly lower in terms of statistics when compared with the Colistin Group. The present study makes us assume that a longer period of colistin exposure may be required to show biochemical kidney damage due to colistin-induced nephrotoxicity in rats. It is possible that the first phase of nephrotoxicity that may develop in rats is a nephrotoxicity that occurred at a histopathological level. We obtained findings that luteolin was effective at this level.
We assume that preventing nephrotoxicity at a histopathological level prior the development of a clinically significant nephrotoxicity is very important. The present study is the second study in the literature that indicates that nephrotoxicity can be prevented at the cellular level similar to the study where nephrotoxicity related with cisplatin was prevented as in the previous study with luteolin. However, there are many limitations regarding our study. First, this study was performed with a limited number of animals. According to our opinion, the reason to be unable to develop significant biochemical renal damage in groups is related to the exposure period to colistin. After all, the strength of our study is obviously revealed as we determined that histopathological renal damage had occurred and was decreased by the effect of luteolin. Consequently, this study demonstrates that luteolin is capable of avoiding colistin-induced nephrotoxicity and to prevent apoptosis in renal tubular cells.
By the means of this study, an idea was formed that luteolin can be simultaneously administered in order to avoid the high possibility of a nephrotoxic status, together with colistin which was used as an ultimate option in the treatment of MDR pathogens that are hardly managed in clinics. However, it is explicitly essential that certain clinical studies must be performed to validate this effect.
Acknowledgements
This study was carried out after a permit was obtained from Bagcılar Training and Research Hospital, Local Ethical Board of Animal Testing and following the guide (NIH Publication No. 85-23, revised 1996) related to the care and use of experimental animals. Colistimethate sodium was provided from Koçak Farma Pharmaceutical Company free of charge.
Disclosure statement
The authors report no conflicts of interest.
References
- Falagas ME, Rafailidis PI. Nephrotoxicity of colistin: New insight into an old antibiotic. Clin Infect Dis off Publ Infect Dis Soc Am. 2009;48:1729–1731.
- Karli A, Paksu MS, Karadag A, et al. Colistin use in pediatric intensive care unit for severe nosocomial infections: Experience of an university hospital. Ann Clin Microbiol Antimicrob. 2013;12:32.
- Kift EV, Maartens G, Bamford C. Systematic review of the evidence for rational dosing of colistin. S Afr Med J. 2014;104:183–186.
- Michalopoulos A, Falagas ME. Colistin and polymyxin B in critical care. Crit Care Clin. 2008;24:377–391.
- Skov RL, Monnet DL. Plasmid-mediated colistin resistance (mcr-1 gene): Three months later, the story unfolds. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2016;21:pii=30155.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55:3284–3294.
- Mohamed AF, Karaiskos I, Plachouras D, et al. Application of a loading dose of colistin methanesulfonate in critically ill patients: Population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother. 2012;56:4241–4249.
- Plachouras D, Karvanen M, Friberg LE, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother. 2009;53:3430–3436.
- Balkan II, Dogan M, Durdu B, et al. Colistin nephrotoxicity increases with age. Scand J Infect Dis. 2014;46:678–685.
- Azad MAK, Akter J, Rogers KL, Nation RL, Velkov T, Li J. Major pathways of polymyxin-induced apoptosis in rat kidney proximal tubular cells. Antimicrob Agents Chemother. 2015;59:2136–2143.
- Ghlissi Z, Hakim A, Mnif H, et al. Evaluation of colistin nephrotoxicity administered at different doses in the rat model. Ren Fail. 2013;35:1130–1135.
- Liu Y, Dai C, Gao R, Li J. Ascorbic acid protects against colistin sulfate-induced neurotoxicity in PC12 cells. Toxicol Mech Methods. 2013;23:584–590.
- Yousef JM, Chen G, Hill PA, Nation RL, Li J. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother. 2012;67:452–459.
- Dalfino L, Puntillo F, Ondok MJM, et al. Colistin-associated acute kidney injury in severely ill patients: A step toward a better renal care? A prospective cohort study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015;61:1771–1777.
- Lin Y, Shi R, Wang X, Shen H-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–646.
- Domitrović R, Cvijanović O, Pugel EP, Zagorac GB, Mahmutefendić H, Škoda M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology. 2013;310:115–123.
- Ozkan G, Ulusoy S, Orem A, et al. How does colistin-induced nephropathy develop and can it be treated? Antimicrob Agents Chemother. 2013;57:3463–3469.
- Dai C, Li J, Tang S, Li J, Xiao X. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob Agents Chemother. 2014;58:4075–4085.
- An G, Wang X, Morris ME. Flavonoids are inhibitors of human organic anion transporter 1 (OAT1)-mediated transport. Drug Metab Dispos Biol Fate Chem. 2014;42:1357–1366.
- Dai C, Tang S, Deng S, et al. Lycopene attenuates colistin-induced nephrotoxicity in mice via activation of the Nrf2/HO-1 pathway. Antimicrob Agents Chemother. 2015;59:579–585.
- Ozyilmaz E, Ebinc FA, Derici U, et al. Could nephrotoxicity due to colistin be ameliorated with the use of N-acetylcysteine? Intensive Care Med. 2011;37:141–146.