Abstract
Background: Acute kidney injury (AKI) is one of the major determinants of graft survival in kidney transplantation (KTx). Renal Transplant recipients are more vulnerable to develop AKI than general population. AKI in the transplant recipient differs from community acquired, in terms of risk factors, etiology and outcome. Our aim was to study the incidence, risk factors, etiology, outcome and the impact of AKI on graft survival.
Methods: A retrospective analysis of 219 renal transplant recipients (both live and deceased donor) was done.
Results: AKI was observed in 112 (51.14%) recipients, with mean age of 41.5 ± 11.2 years during follow-up of 43.2 ± 12.5 months. Etiologies of AKI were infection (47.32%), rejection (26.78%), calcineurin inhibitor (CNI) toxicity (13.39%), and recurrence of native kidney disease (NKD) (4.46%). New Onset Diabetes After Transplant (NODAT) and deceased donor transplant were the significant risk factors for AKI. During follow-up 70.53% (p = .004) of AKI recipients progressed to chronic kidney disease (CKD) in contrast to only 11.21% (p = .342) of non AKI recipients. Risk factors for CKD were AKI within first year of transplant (HR: 7.32, 95%CI: 4.37–15.32, p = .007), multiple episodes of AKI (HR: 6.92, 95%CI: 3.92–9.63, p = .008), infection (HR: 3.62, 95%CI: 2.8–5.75, p = .03) and rejection (HR: 9.92 95%CI: 5.56–12.36, p = .001).
Conclusion: Renal transplant recipients have high risk for AKI and it hampers long-term graft survival.
Introduction
Epidemiologic studies have documented AKI as a significant risk factor for mortality as well as progression to CKD. Recovery of AKI in non-transplant setting was reported to vary from 31 to 93%.Citation1,Citation2 Further the incidence and etiology of AKI in general population vary in different countries as it is influenced by prevailing climatic differences and infections.Citation3 AKI in the renal transplant setting differs from the community acquired, as the denervated allograft is susceptible for hemodynamic instability, use of nephrotoxic drugs especially calcineurin inhibitors, immune-mediated injury and predisposition to opportunistic infections. Studies on AKI in the renal transplant and its impact on long-term graft survival are very scarce. In this study we analyzed the incidence of AKI complying with the definition of KDIGO, etiological factors, immediate outcome and its impact on long-term graft survival in renal transplant recipients.
Subjects and methods
We retrospectively analyzed 219 renal transplant recipients of live and deceased donor transplantation performed in our center between the year 2009 and 2014. The patients who had stable graft function at three months after transplant were included in the study.
Immunosuppressive agents
Induction agents were given for high-risk transplantation. We used basiliximab induction with 20 mg on day 0 and day 4 for HLA mismatched spouse and sibling transplantation and rabbit anti thymocyte globulin, 50 mg on day 0 (single dose) for all deceased donor transplantation and second transplant recipients. We used triple immunosupression for maintenance, with cyclosporine (CsA) or tacrolimus (Tac), mycophenolate mofetil (MMF) and steroids. CsA and Tac trough level were maintained as follows: at 1 month (CsA: 200–250 ng/ml, tac: 8–10 ng/ml), 1–6 months (CsA: 150–200 ng/ml, Tac 5–8 ng/ml) and after 6 months (CsA: 100–150 ng/ml, Tac around 5 ng/ml), respectively.
Definitions
AKI was defined as per KDIGO 2012 guidelines as increase in serum creatinine by ≥0.3 mg/dl within 48 h or increase in serum creatinine to 1.5 times from baseline which is known or presumed to have occurred within the prior 7 days or urine volume <0.5 ml/kg/h for 6 h.
CKD as estimated GFR (eGFR) < 60 ml/min/1.73m2 for >3 months, ESRD as eGFR of 15 ml/min/1.73m2 and graft loss as either ESRD or requirement of dialysis for more than three months.Citation4,Citation5 The graft function was assessed by eGFR calculation using creatinine based CKD EPI (chronic kidney disease epidemiology collaboration) equation. The severity of AKI was categorized by both AKIN and RIFLE criteria.
Acute rejection, recurrence of native kidney disease and de novo glomerulonephritis was diagnosed by graft biopsy. Acute CNI toxicity was considered if elevated trough level with graft dysfunction and or graft biopsy.
Statistical analysis was done with Pearson chi square test and relative risk of graft dysfunction was assessed with multivariate Cox regression model. Graft survival assessed by Kaplan–Meier analysis.
Results
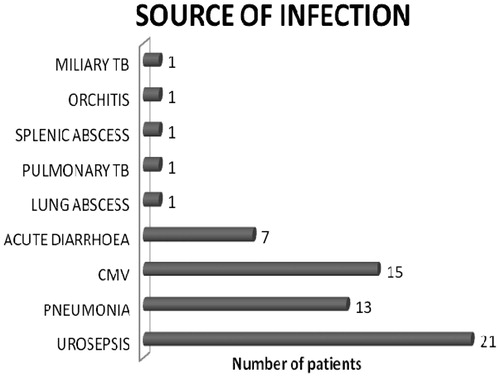
Of the 219 kidney transplant recipients 73.6% (n = 161) and 26.4% (n = 58) received live and deceased donor kidneys, respectively. Male:female ratio was 2.6:1. Mean duration of the follow-up was 47.5 ± 12.5 months. Incidence of AKI was 51.14% (n = 112). A total of 220 AKI episodes occurred cumulatively in 112 recipients, of which 70 episodes were single and 52 were multiple. Majority of the recipients developed AKI within first year of transplant (n = 61, 54.4%). Infection was the most common etiology (55%, n = 61) followed by rejection (24.1%, n = 26), CNI toxicity (11.2%, n = 13), recurrence of native kidney disease (4.4%, n = 5) and others (5.3%, n = 7) which included acute cholecystitis, gastric perforation, NODAT complicated as diabetic ketoacidosis. Graft pyelonephritis was the commonest source of sepsis 34.4% (n = 21) followed by bacterial pneumonia and others as shown in (). Biopsy was done in 48 out of 219 recipients. All rejections were biopsy proven (n = 40, 18.26%) with antibody mediated rejection contributed 20% (n = 8), remaining biopsies with rejection showed acute cell mediated rejection with borderline rejection in 20 recipients (50%), Banff-1a in 6 recipients (15%), 1b in 2(5%) and 2a in 4 recipients (10%).
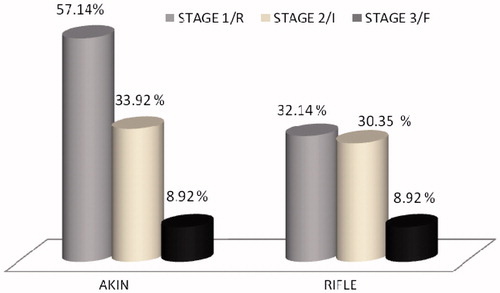
We analyzed severity of AKI by both AKIN and RIFLE criteria. AKIN identified almost all AKI episodes but RIFLE criteria identified only 72% of AKI episodes in stage 1, 33.92% were present in stage 2 and 8.92% were in stage 3 of AKIN respectively ().
On analyzing, NODAT (HR: 3.62, 95%CI: 1.23–6.62, p = .004) and deceased donor transplant (HR: 2.73, 95%CI: 3.52–5.78, p = .008) were found to be significant risk factors for the development of AKI (). Further, multiple AKI episodes (HR: 6.92, 95%CI: 3.92–9.63, p = .008), infection (HR: 3.62, 95%CI: 2.8–5.15, p = .03), rejection (HR: 9.92, 95%CI: 5.56–12.36, p = .001), AKI within first year of kidney transplant (HR: 7.32, 95%CI: 4.37–15.32, p = .007) were found to be significant risk factors for CKD progression ().
Table 1. Analysis of risk factors for AKI.
Table 2. Risk factors for the progression to CKD.
Overall, of the 219 transplant recipients 57.5% (n = 126) were progressed to CKD and 41.65% (n = 91) had normal graft function during the follow-up. Among the AKI group 70.53% (n = 78) of recipients progressed to CKD compared to non-AKI group (11.25%, n = 13) (p = .044). Two (0.9%) recipients succumbed to death in the AKI group.
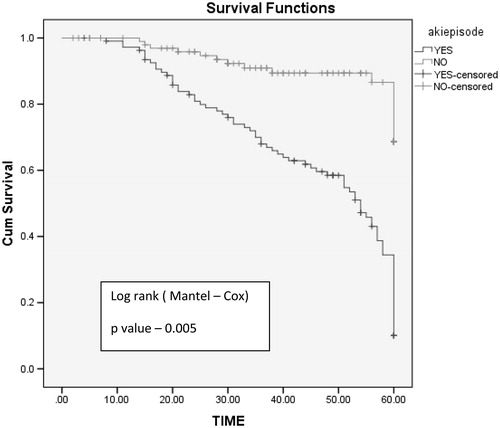
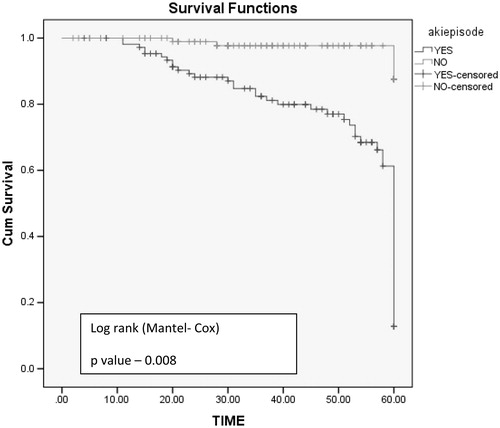
Five-year graft survival was better in non-AKI group with mean graft survival of 56 months compared to AKI group with mean graft survival of 22 months (log rank test, p values: .005) (). Graft loss was significant in AKI group (n = 22, 20.5%, p = .03) compared to non-AKI group (n = 4, 3.8%). Mean time required for doubling of serum creatinine is shorter in AKI group (29 months) compared to non AKI group (54 months) log rank test p = .008 ().
Discussion
Kidney transplant recipients are vulnerable to develop AKI secondary to ischemic reperfusion injury, infection, immune mediated injury, CNI toxicity, graft vessel thrombosis, ureter obstruction and by various surgical complications.Citation6 The real incidence of post-transplant AKI was not known since only scarce data are available in literature. Mehrotra et al.Citation7 published the incidence of AKI in transplant recipient from USRDS database as 11.3%. This was apparently low compared to other studies because they defined AKI by ICD-9-CN code (serum creatinine increase by 100% from baseline), with a low sensitivity of 35.4% leading to underestimation of the actual incidence.
Filiponi et al.Citation8 recently shown the incidence as 80% from 458 hospitalized transplant recipients and defined the AKI by AKIN criteria. Nakamura et al.Citation6 from Japan observed 20.45% of AKI by both AKIN and RIFLE criteria from his 289 transplant recipients. One major obstacle in transplant recipients was the absence of consensus definition for AKI. AKIN and RIFLE criteria have been validated in various studies to correlate outcomes like hospitalization, dialysis and mortality.Citation9,Citation10 KDIGO 2012 guidelineCitation4 has merged the AKIN and RIFLE criteria, to further increase the sensitivity to diagnose AKI, hence in our study we used KDIGO 2012 guideline to define AKI. Both AKIN and KDIGO criteria identified all AKI patients equally (100%), compared to RIFLE which identified only 72% of AKI in stage 1. Compared to population-based studies incidence of AKI in post renal transplant is 40 to 50 fold higher than the community acquired one.Citation1,Citation6–8,Citation11,Citation12
In our study, NODAT and deceased donor transplant were found to be significant risk factor for development of AKI. The delayed graft function in deceased donor and increased susceptibility to infections in NODAT may be the reason for higher incidence of AKI. In one study deceased donor, elderly age, diabetic nephropathy and long duration of pre transplant dialysis were found to be risk factors. Several recent studies showed CKD is one of the risk factor for AKI.Citation6,Citation13,Citation14 In our study, baseline serum creatinine and GFR did not influence the AKI incidence.
In our study post-transplant infection (55%) was the commonest etiology of AKI and urinary tract infection was the most common infection, which was consistent with the recent studies.Citation6,Citation15 Immunocompromised state along with reflux from the neocystoureterostomy might have contributed to the high incidence of UTI after transplant.Citation15
AKI is major contributor to CKD, by up regulating various inflammatory and fibrotic pathways like NFKB, MAP-K which was proven in various human and animal studies.Citation16–18 In our study, 70.53% of AKI recipients progressed to CKD compared to 27.67% of non-AKI group at 5 years. Patients with more than one episode of AKI, requirement of dialysis, AKI within first year of transplant and AKI due to infection and rejection had high risk of progression to CKD. Further, severity of AKI proportionately increases the risk of graft failure which was evidenced in our study with four recipients out of 64 in stage 1, 8 out of 38 recipients in stage 2 and all the 10 recipients of stage 3 of AKIN criteria progressed to CKD which was comparable to the study of Nakamura et al.Citation6 Overall, AKI in the transplant setting differs from community acquired AKI by high risk of progression to CKD.Citation1–3
Graft loss defined as ESRD or second transplant or death varied among different studies from 5.8% to 35%.Citation6–8 In our study cohort 20.5% of AKI recipients lost their graft either by ESRD or death. Mean graft survival in our AKI patients was 22 months compared to 10 months by the retrospective study conducted in Japan.Citation6
Limitations of our study were single center, retrospective study with small number of recipients. Further, in our study we have not evaluated the influence of delayed graft function, hypertension, proteinuria and nephrotoxic drugs other than calcineurin inhibitors.
To conclude, we observed that AKI was a relatively common complication of renal transplantation with significant risk of graft failure. Because the infections were the most common etiology of AKI after transplant, prevention and early detection of infection might improve the patient and graft survival.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298.
- Jha V, Malhotra HS, Sakhuja V, Chugh KS. Spectrum of hospital-acquired acute renal failure in the developing countries: Chandigarh study. Q J Med. 1992;83:497–505.
- Jayakumar M, Prabahar MR, Fernando EM, Manorajan R, Venkatraman R, Balaraman V. Epidemiologic trend changes in acute renal failure: A tertiary center experience from South India. Ren Fail. 2006;28:405–410.
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline for acute kidney injury. March 2012; Section 2, chapter 2.1, volume 2, issue1. Available at: http:/www.kidneyinternational.org.
- KDIGO 2012. Clinical practice guideline for the evaluation and management of chronic kidney disease. January 2013; volume 3, issue 1, chapter 1. Available at: http://www.kidney-international.org.
- Nakamura M, Seki G, Iwadoh K, et al. Acute kidney injury as defined by the RIFLE criteria is a risk factor for kidney transplant graft failure. Clin Transplant. 2012;26:520–528.
- Mehrotra A, Rose C, Pannu N, Gill J, Tonelli M, Gill JS. Incidence and consequences of acute kidney injury in kidney transplant recipients. Am J Kidney Dis. 2012;59:558–565.
- Filiponi TC, Requião-Moura LR, Tonato EJ, et al. Admission following acute kidney injury in kidney transplant recipients is associated with a negative impact on graft function after 1-year. PLoS One. 2015;10:e0138944.
- Bagshaw S. Acute kidney injury: Diagnosis and classification of AKI: AKIN or RIFLE? Nat Rev Nephrol. 2010;6:71–73.
- Fujii T, Uchino S, Takinami M, Bellomo R. Validation of the kidney disease improving global outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin J Am Soc Nephrol. 2014;9:848–854.
- Cooper JE, Wiseman AC. Acute kidney injury in kidney transplantation. Curr Opin Nephrol Hypertens. 2013;22:698–703.
- Srivastava RN, Bagga A, Moudgil A. Acute renal failure in North Indian children. Indian J Med Res. 1990;92:404–408.
- Okusa M, Chertow G, Portilla D. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol. 2009;4:520–522.
- Hsu C, Ordoñ ez J, Chertow G, Fan D, McCulloch C, Go A. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107.
- Snyder J, Israni A, Peng Y, Zhang L, Simon T, Kasiske B. Rates of first infection following kidney transplant in the United States. Kidney Int. 2009;75:317–326.
- Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899.
- Forbes JM, Hewitson TD, Becker GJ, Jones CL. Ischemic acute renal failure: Long-term histology of cell and matrix changes in the rat. Kidney Int. 2000;57:2375–2385.
- Forbes JM, Hewitson TD, Becker GJ, Jones CL. Simultaneous blockade of endothelin A and B receptors in ischemic acute renal failure is detrimental to long-term kidney function. Kidney Int. 2001;59:1333–1341.