Abstract
The aim of this meta-analysis was to evaluate the effect of peritoneal dialysis (PD) and hemodialysis (HD) on renal anemia (RA) in renal disease patients by a meta-analysis. Relevant studies published before June 2015 were searched. Pooled odds ratio (OR) with 95% confidence interval (CI) was used to evaluate the effect of HD and PD on RA based on five indexes: hemoglobin, ferritin, transferrin saturation index, serum albumin, and parathyroid hormone. Sensitivity analysis and publication bias assessment were conducted to evaluate the stability and reliability of our results. A total of fourteen eligible studies with 1103 cases underwent HD and 625 cases underwent PD were used for this meta-analysis. There were no significant difference for levels of hemoglobin (SMD = −0.23, 95% CI: −0.74 to 0.28), ferritin (SMD = 0.01, 95% CI: −0.59 to 0.62), parathyroid hormone (SMD = 0.11, 95% CI: −1.53 to 1.75) and transferrin saturation index (SMD = −0.06, 95% CI: −0.67 to 0.56) between HD and PD group. However, the content of serum albumin in HD group was much more than that in PD group (SMD = 1.58, 95% CI: 0.35 to 2.81). Neither of the included studies could reverse the pooled side effect and Egger’s test demonstrated no publication bias. Both of the two dialysis strategies have a similar effect on RA in renal disease patients.
Introduction
Anemia is a clinical manifestation that commonly happened in patients with renal disease.Citation1 Untreated anemia is associated with several physiological abnormalities which will significantly reduce patients’ life quality.Citation2,Citation3 The management of anemia with erythropoiesis-stimulating agents (ESAs) is an important treatment for chronic kidney disease (CKD) patients with anemia (namely renal anemia [RA]) who receiving dialysis programs.Citation4
Recently, a number of factors were recognized to be associated with dialysis modes, such as the iron deficiency, severe hyperparathyroidism, vitamin deficiency, aluminum toxicity, inflammation, as well as inadequate dialysis.Citation5,Citation6 However, little evidence has been made with regard to the contribution of inflammation to RA observed in different dialysis patients. Inflammatory stimuli will cause the release of several cytokines and results in a series of systemic changes. The serum albumin, hemoglobin, ferritin, and transferrin saturation index were reported to be involved in the presence of inflammation.Citation7 In secondary hyperparathyroidism of CKD patients, the high level of parathyroid hormone is suggested to be of multiple biological effects, including a negative influence on the RA patients.Citation8,Citation9
The most common treatment modalities for CKD are HD (hemodialysis) and PD (peritoneal dialysis). However, the function and outcomes between these two dialysis techniques are controversial. A recent clinical study has demonstrated that the serum albumin could predict the mortality of both PD and HD patients while the mortality was different in different dialysis modalities.Citation10,Citation11 However, the most recent cohort studies suggested that patients treated with either PD or HD in end-stage renal disease showed remarkably similar outcomes.Citation12,Citation13
Therefore, in the present work, we performed a meta-analysis to compare the effect of PD and HD on RA in patients with renal disease. The data of hemoglobin, ferritin, transferrin saturation index, serum albumin, and parathyroid hormone indexes between HD and PD groups were pooled respectively. Our study aimed at finding the effect of dialysis modalities on RA and provide a basis for clinical application.
Materials and methods
Search strategy
Related clinical studies about the effect of PD and HD on RA were systematically searched from Pubmed (http://www.ncbi.nlm.nih.gov/pubmed) and Embase (http://www.embase.com) databases, with the retrieval deadline of June 2015. Keywords for all searches were (“renal anemia” or “renal anemia” or “anemia of renal failure”) in combination with (“hemodialysis” or “hemodialysis”) and (“peritoneal dialysis”).
Inclusion and exclusion criteria
Studies included in this meta-analysis should meet the following criteria: (1) they reported clinical research about the effects of PD and HD on RA; (2) the number of patients in both PD and HD groups should be provided; (3) at least one of the following indexes should be provided, including hemoglobin (g/dL), ferritin (ng/mL), transferrin saturation index (%), serum albumin (g/dL), and parathyroid hormone.
Studies should be excluded if they (1) were reviews, reports, letters, and comments; (2) had not available data to be extracted.
Data extraction and quality evaluation
Wan-ning Wang and Wen-long Zhang reviewed and screened the articles, respectively based on the inclusion and exclusion criteria. Afterward, information about the publications and the patients from the included studies were extracted, including the first author’s name, year of publication, country, demographic information of patients in both PD and HD groups (age, gender, weight/BMI, time on dialysis, and the levels of hemoglobin, ferritin, transferrin saturation index, serum albumin, and parathyroid hormone). Any discrepancies during data extraction were resolved by discussion with Tao Sun.
The quality assessment of included trials was conducted by Wan-ning Wang and Wen-long Zhang independently according to the evaluation criterion tool proposed by AHRQ (Agency for Healthcare Research and Quality).Citation14 Any disagreement was resolved by inviting Zhong-gao Xu for discussion. The tool evaluates the study quality through eleven items, each of which was responded with “yes” or “no” or “not clear”.
Statistical analysis
Meta-analysis was conducted by the R 3.12 software (R Foundation for Statistical Computing, Vienna, Austria). The effect size was assessed by standardized mean difference (SMD) and 95% confidence interval (CI). A random-effects model was used for heterogeneous outcomes (p < .05 and I2 > 50%), and a fixed-effect model was used for the homogeneous outcomes (p > .05 and I2 < 50%).Citation15 Publication bias was evaluated by Egger’s test.Citation16 Finally, the sensitivity analysis was also conducted to calculate the results by omitting one study at one time to measure its effect on the pooled SMD.
Results
Trail flow
The flow chart of the literature search and study selection was presented in . A total of 1636 potentially relevant studies were searched from PubMed (n = 1020) and Embase (n = 616). Afterward, 528 duplicates and 987 obvious irrelevant articles were removed. After title and abstract evaluation, 41 were excluded because they were letters (n = 8), case series (n = 7), and literature reviews (n = 26). The left 80 publications underwent further review while 66 of them (23 were unable to be data-extracted and 43 were not reported RA disease) were removed. Finally, a total of fourteen studies were included in this meta-analysis.Citation17–30
Characteristics of included studies
The characteristics of the fourteen studies and basic information of the subjects were listed in . These studies distributed from Europe (Spain, UK, Portugal, Ireland, and Germany), Africa (South Africa), America (Canada and USA) and Asia (Turkey and Kuwait) were published from 1991 to 2014. Among them, 11 studies reported the comparison of hemoglobin between HD and PD, ten reported the comparison of ferritin between HD and PD, eight reported the transferrin saturation index in both HD and PD groups, nine reported the serum albumin in both HD and PD groups, and six studies reported the parathyroid hormone in both HD and PD groups. A total of 1103 cases underwent HD and 625 cases underwent PD were used for this meta-analysis. There were more males than females in these patients. There was no significant difference in weight or BMI between the patients who underwent HD and PD.
Table 1. Characteristics of 14 included studies in this meta-analysis.
Quality evaluation
As shown in Supplementary Table 1, the quality of all the studies was relatively high. Almost all the included studies described more than four items according to AHRQ.
Meta-analysis
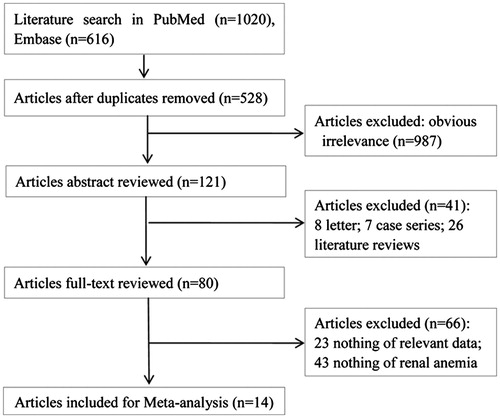
Comparison of hemoglobin, ferritin, transferrin saturation index, serum albumin, and parathyroid hormone indexes between HD and PD groups were shown in .
Figure 2. Forest plot. (a) Forest plot for the comparison of hemoglobin between HD and PD groups. (b) Forest plot for the comparison of ferritin between HD and PD groups. (c) Forest plot for the comparison of transferrin saturation index between HD and PD groups. (d) Forest plot for the comparison of serum albumin between HD and PD groups. (e) Forest plot for the comparison of parathyroid hormone between HD and PD groups.
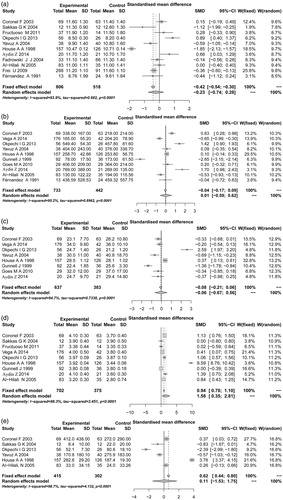
Figure 3. Sensitivity analysis of the included studies. (a) hemoglobin; (b) ferritin; (c) transferrin saturation index; (d) serum albumin; (e) parathyroid hormone.
There were eleven studies reported the content of hemoglobin between the HD and PD groups (). The random-effect model was used to evaluate the effect because a significant heterogeneity existed among these studies (I2 = 93.9%, p < .0001). Meta-analysis showed no significant difference in the hemoglobin content between patients suffered from HD and PD (SMD = −0.23, 95% CI: −0.74 to 0.28).
Totally ten studies reported the comparison of ferritin between HD and PD (). There was significant heterogeneity among these studies (I2 = 95.2%, p < .0001), therefore, the random-effect model was used to evaluate the effect size. No significant difference of ferritin was found between the HD and PD groups (SMD = 0.01, 95% CI: −0.59 to 0.62).
Eight publications reported the transferrin saturation index in both HD and PD groups (). As well, significant heterogeneity was detected (I2 = 94.7%, p < .0001) and the random-effect model was used for meta-analysis. As a result, the transferrin saturation index in both HD and PD groups showed no significant difference (SMD = −0.06, 95% CI: −0.67 to 0.56).
With regard to the serum albumin, nine studies were included in the meta-analysis (). The random-effect model was chosen because of the high heterogeneity (I2 = 98.3%, p < .0001). The content of serum albumin in HD group was much more than that in PD group (SMD = 1.58, 95% CI: 0.35 to 2.81), indicating that the HD dialysis may be superior to PD dialysis.
There were six studies reported the compassion of parathyroid hormone in HD and PD groups (). High heterogeneity was found among these studies (I2 = 98.7%, p < .0001). Therefore, the random-effect model was used to evaluate the effect. However, no significant difference was found for parathyroid hormone between the two dialysis strategies (SMD = 0.11, 95% CI: −1.53 to 1.75).
Sensitivity analysis and publication bias
In order to evaluate the effect of each study on the pooled SMD value, the sensitivity analysis was performed (). Neither of the included studies could reverse the pooled side effect in each meta-analysis of the five indexes, indicating the stable results of our meta-analysis.
The results of the Egger’s test were presented in Supplementary Table 2. The studies included in all the meta-analyses demonstrated no publication bias, suggesting that our results were reliable.
Discussion
Recently, both PD and HD are the most comment methods for clinical treatment of nephropathy. RA is one of the most common complications of CKD that mainly caused by nephropathy. In the present study, we evaluated the effect of PD and HD on RA, and the results demonstrated that there was no significant difference in hemoglobin, ferritin, transferrin saturation index, and parathyroid hormone between HD and PD groups. However, the content of serum albumin in HD group was much more than that in PD group.
During dialysis, several factors were recognized to be associated with postoperative outcomes of RA patients, such as hemoglobin, ferritin, transferrin saturation index, and parathyroid hormone. ESAs are recommended to correct RA, as maintenance of hemoglobin within a suitable range can play an important role in patients outcomes.Citation31 However, the issue remains controversial, because recent prospective randomized trials have suggested that maintaining hemoglobin to normal or near normal levels in CKD patients dose not confer any survival benefit.Citation31,Citation32 In our study, the hemoglobin levels showed no significant difference in both PD and HD groups, indicating that the impact of hemoglobin variability may produce a similar clinical outcome between patients treated with PD and HD. One of the factors involved in dialysis is the blood loss, which makes patients prone to develop iron deficiency.Citation19 However, there was no statically significant difference for ferritin and transferrin saturation index between PD and HD groups, which suggested that these two dialysis methods might present similar results for iron supply in patients with RA. The role of parathyroid hormone as a uremic toxin on erythropoiesis has been discussed in several studies. It was reported that high levels of serum parathyroid hormone are involved in decreasing survival of red blood cells and their progenitor number.Citation33 Middle-molecular-weight solutes were suggested to be responsible for the toxic effect of red blood cells.Citation34 Previous evidence has proved that ambulatory PD is more efficient in eliminating middle-molecular-weight substances and results in less anemia than HD.Citation35 However, a recent study indicated that no significant difference was found in red blood cell survival between HD and PD.Citation36 In our study, there was no significant difference in parathyroid hormone levels between HD and PD dialysis strategies, thus, the beneficial effect of PD and HD on anemia may be related to elevated erythropoiesis other than improved red blood cells survival.
Severe RA tended to be associated with manifestations of fluid overload and other risk factors. In our study, the content of serum albumin in HD group was much more than that in PD group, which may in part be explained by fluid overload resulting in dilution of albumin in PD group. Besides, the PD itself, using peritoneum as the semipermeable membrane, can lead to albumin loss. The serum proteins in PD patients averages 5 g per 24 h, of which, 4 g is albumin.Citation17 However, we cannot get the conclusion from the serum albumin level in the two-dialysis ways that HD was more efficient than PD in the treatment of RA. For one thing, we did not know if the difference in serum albumin level causes by ESAs treatment or dialysis strategies. For the other, it is unclear that if the reduced serum albumin level in PD group leads to anemia-related inflammatory conditions. Therefore, the association between albumin and dialysis strategies needs further clinical studies to clarify.
In our meta-analysis, high levels of heterogeneity were detected in hemoglobin, ferritin, transferrin saturation index, parathyroid hormone, and albumin between HD and PD groups, thus, the random-effected model was used for effect size. The reason for the high heterogeneity may be explained by different detection methods of these indexes, ethnic and lifestyle differences. Other confounding factors such as gender and age may also contribute to the heterogeneity. In order to detect if the time span or changing guidelines the hemoglobin targets, we performed a cumulative meta-analysis, and the results showed that during 1991 and 2014, the results were not changed significantly. Therefore, neither the time span nor the changing guidelines attributed to the heterogeneity of this study.
Our meta-analysis is the first one to evaluate the effect of PD and HD on RA. Both the sensitivity analysis and the publication bias analysis suggested that our results were stable and reliable. However, several limitations should be pointed out in this study. Firstly, there were significant heterogeneities across the included studies in the meta-analysis of the five indexes. As discussed above, the heterogeneities may associate with many factors. We did not perform the subgroup meta-analysis because of the inconsistency caused by unavailable or incomplete data of some studies. For instance, the treatment of ESAs should be considered as an important factor that is associated with the five indexes analyzed in our study. However, some of the included patients received ESAs before dialysisCitation25,Citation26 and others were not.Citation23,Citation24 So the effects ESAs were not analyzed in our study. Besides, we did not correct the concomitant variables such as age, gender and other confounding factors that may affect the results of this analysis. Therefore, it is necessary to develop more studies with larger sample size to assess the commonality of our results.
In conclusion, in our study, though the albumin in HD group was much more than in PD group, the hemoglobin, ferritin, transferrin saturation index, and parathyroid hormone were similar in the two groups. These results suggest that both of the two-dialysis strategies have a similar effect on RA in patients with renal diseases.
Supplementary Tables
Download PDF (243.2 KB)Disclosure statement
All authors declare that they have no conflict of interests to state.
References
- Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39:1780–1786.
- Karl S, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med Overseas Ed. 2013;368:1210–1219.
- Macdougall IC, Robert P, Amit S, et al. Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. N Engl J Med Overseas Ed. 2013;368:320–332.
- Eschbach JW, Adamson JW. Recombinant human erythropoietin: Implications for nephrology. Am J Kidney Dis. 1988;11:203–209.
- Macdougall IC. Poor response to erythropoietin: Practical guidelines on investigation and management. Nephrol Dial Transplant. 1995;10:607–614.
- Casadevall N, Dupuy E, Molho-Sabatier P, Tobelem G, Varet B, Mayeux P. Autoantibodies against erythropoietin in a patient with pure red-cell aplasia. N Engl J Med Overseas Ed. 1996;334:630–633.
- Mercadal L, Metzger M, Haymann JP, et al. A 3-marker index improves the identification of iron disorders in CKD anaemia. PLoS One. 2014;9:e84144.
- Conzo G, Perna A, Della Pietra C, et al. Role of parathyroidectomy on anemia control and erythropoiesis-stimulating agent need in Secondary Hyperparathyroidism of Chronic Kidney Disease. A retrospective study in 30 hemodialysis patients. Parathyroidectomy Ameliorates Anemia Ann Ital Chir. 2013;84:25–31.
- Di Iorio BR, Minutolo R, De Nicola L, et al. Supplemented very low protein diet ameliorates responsiveness to erythropoietin in chronic renal failure. Kidney Int. 2003;64:1822–1828.
- Mehrotra R, Duong U, Jiwakanon S, et al. Serum albumin as a predictor of mortality in peritoneal dialysis: Comparisons with hemodialysis. Am J Kidney Dis. 2011;58:418–428.
- Duong U, Kalantar-Zadeh K, Molnar MZ, et al. Mortality associated with dose response of erythropoiesis-stimulating agents in hemodialysis versus peritoneal dialysis patients. Am J Nephrol. 2012;35:198–208.
- Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med. 2011;171:110–118.
- Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant. 2012;27:3568–3575.
- Rostom A, Dubц C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) 3, Results. 2013.
- Feng R-N, Zhao C, Sun C-H, Li Y. Meta-analysis of TNF 308 G/A polymorphism and type 2 diabetes mellitus. PLoS One. 2011;6:e18480.
- Luo AJ, Wang FZ, Luo D, et al. Consumption of vegetables may reduce the risk of liver cancer: Results from a meta-analysis of case-control and cohort studies. Clin Res Hepatol Gastroenterol 2015;39:45–51.
- Al-Hilali N, Al-Humoud H, Ninan VT, Nampoory MR, Puliyclil MA, Johny KV. Does parathyroid hormone affect erythropoietin therapy in dialysis patients?. Med Princ Pract. 2007;16:63–67.
- Aydin Z, Gursu M, Karadag S, et al. The relationship of Prohepcidin levels with anemia and inflammatory markers in non-diabetic uremic patients: A controlled study. Ren Fail. 2014;36:1253–1257.
- Coronel F, Herrero JA, Montenegro J, et al. Erythropoietin requirements: A comparative multicenter study between peritoneal dialysis and hemodialysis. J Nephrol. 2003;16:697–707.
- Fadrowski JJ, Furth SL, Fivush BA. Anemia in pediatric dialysis patients in end-stage renal disease network 5. Pediatr Nephrol. 2004;19:1029–1034.
- Fernandez A, Hortal L, Rodriguez JC, Vega N, Plaza C, Palop L. Anemia in dialysis: Its relation to acquired cystic kidney disease and serum levels of erythropoietin. Am J Nephrol. 1991;11:12–15.
- Frei U, Kwan JT, Spinowitz BS. Epoetin Delta Study G. Anemia management with subcutaneous epoetin delta in patients with chronic kidney disease (predialysis, hemodialysis, peritoneal dialysis): Results of an open-label, 1-year study. BMC Nephrol. 2009;10:5.
- Fructuoso M, Castro R, Oliveira L, Prata C, Morgado T. Quality of life in chronic kidney disease. Nefrologia: Publicacion Oficial De La Sociedad Espanola Nefrologia. 2011;31:91–96.
- Goes M, Dalboni M, Manfredi S, et al. Serum-soluble Fas and serum levels of erythropoietin in chronic kidney disease. Clin Nephrol. 2010;73:7–13.
- Gunnell J, Yeun JY, Depner TA, Kaysen GA. Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 1999;33:63–72.
- House A, Pham B, Page D. Transfusion and recombinant human erythropoietin requirements differ between dialysis modalities. Nephrol Dial Transplant. 1998;13:1763–1769.
- Okpechi IG, Nthite T, Swanepoel CR. Health-related quality of life in patients on hemodialysis and peritoneal dialysis. Saudi J Kidney Dis Transplant. 2013;24:519.
- Sakkas GK, Ball D, Sargeant AJ, Mercer TH, Koufaki P, Naish PF. Skeletal muscle morphology and capillarization of renal failure patients receiving different dialysis therapies. Clin Sci. 2004;107:617–624.
- Vega A, Ruiz C, Abad S, et al. Body composition affects the response to erythropoiesis-stimulating agents in patients with chronic kidney disease in dialysis. Ren Fail. 2014;36:1073–1077.
- Yavuz A, Akbas SH, Tuncer M, et al. Influence of inflammation on the relation between markers of iron deficiency in renal replacement therapy. Transplant Proc. 2004;36:41–43.
- Regidor DL, Kopple JD, Kovesdy CP, et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191.
- Szczech LA, Barnhart HX, Sapp S, et al. A secondary analysis of the CHOIR trial shows that comorbid conditions differentially affect outcomes during anemia treatment. Kidney Int. 2010;77:239–246.
- Meytes D, Bogin E, Ma A, Dukes PP, Massry S. Effect of parathyroid hormone on erythropoiesis. J Clin Invest. 1981;67:1263
- Macdougall IC. Role of uremic toxins in exacerbating anemia in renal failure. Kidney Int Suppl. 2001;78:S67–S72.
- Dhondt A, Vanholder R, Van Biesen W, Lameire N. The removal of uremic toxins. Kidney Int. 2000;58:S47–S59.
- Vos FE, Schollum JB, Coulter CV, Doyle TC, Duffull SB, Walker RJ. Red blood cell survival in long-term dialysis patients. Am J Kidney Dis. 2011;58:591–598.