Abstract
Background: Biomarkers are commonly used to estimate the presence of subclinical cardiovascular disease (CVD) in patients with essential arterial hypertension (HT). In addition to known association between cystatin C and glomerular filtration rate (GFR), elucidating the association between cystatin C and vascular biomarkers (intima-media thickness of common carotid arteries (CCIMT), carotid plaque and renal artery resistance index (RRI)) in patients with unresponsive hypertensive phenotype could be of significant clinical interest.
Methods: Participants (n = 200, median age 58 (52–64) years, 49% female) under treatment with antihypertensive drugs were stratified into two subgroups based on their blood pressure level as having responsive hypertension (RHT – compliant and responsive to treatment, n = 100), or nonresponsive (URHT – compliant but nonresponsive to treatment, n = 100). GFR was measured by isotopic (slope-intercept) method (99m Tc diethylene triamine penta-acetic acid – mGFR).
Results: The URHT group had significantly higher median cystatin C serum concentration (p = 0.02) and CCIMT (p = 0.00) compared to the RHT group, with no significant difference in RRI (p = 0.51) and mGFR among subgroups [69.9 ± 28.2 vs 76.74 ± 23.61 ml/min/1.73m2, p = 0.27]. In the URHT group, cystatin C was found to be associated with CCIMT (p = 0.02), hsCRP (p = 0.01) and duration of HT (p = 0.02), independently of mGFR and age. Independent predictors of URHT phenotype were CCIMT (p= 0.02) and hsCRP (p= 0.04).
Conclusion: In addition to GFR, cystatin C serum concentration is positively and independently associated with CCIMT in patient with URHT phenotype and subclinical CVD. Prospective larger studies should further investigate the clinical importance of this relationship.
Introduction
In the cardiovascular disease (CVD) continuum, essential hypertension (HT) is the most important modifiable risk factor for CVD, with an incidence of 30–50% in the adult European population.Citation1 Even though blood pressure (BP) treatment and control has been improving over past decades around Europe,Citation2 significant proportion of hypertensive patients are still not achieving their BP goals.Citation3 Patients with poor BP control, who are compliant but nonresponsive to treatment (URHT), are representing a particular phenotype of HT, and are characterized with higher prevalence of the target organ damage (TOD) and unfavorable prognosis.Citation4
Biomarkers play a significant role in the evaluation of the subclinical cardiovascular disease (CVD), by establishing the structural and/or functional hypertensive TOD, that usually indicates a high total cardiovascular risk. Additionally, they may have an substantial role in treatment evaluation, indirectly indicating adequate BP control.Citation4,Citation5 Cystatin C is a useful biochemical biomarker in assessing the subclinical hypertensive renal TOD, since it could accurately reflect glomerular filtration rate (GFR).Citation6 Furthermore, epidemiologic studies showed that cystatin C is an independent predictor of CVD in older adults, more sensitive than creatinine and GFR.Citation7 Recent studies suggested that, in hypertensive patients, higher serum cystatin C concentration is associated with stiffness of large arteries in older adults, and also with cardiac structural and functional alterations.Citation8,Citation9 However, the clinical use of cystatin C is relatively limited, partly due to factors which affect cystatin C levels independently from GFR, high cost of analysis, and the inconsistency of reference values.Citation10
Vascular imaging biomarkers can be used for the assessment of the hypertensive vascular injury that may be a more commonly observed in patients with URHT phenotype.Citation11 Additionally to common carotid artery intima-media thickness (CCIMT) and carotid plaque, renal artery resistance index (RRI) could be useful for evaluating the vascular and renal hypertensive consequences.Citation12 It has been established that decreased GFR is one of the main causes of increase in RRI. However, the role of RRI in the assessment of subclinical TOD has not yet been precisely defined since most studies lacked the clinical measurement of GFR by methods of valued reference (99mTc DTPA, 51Cr EDTA).Citation13,Citation14 Moreover, studies focusing on the association between cystatin C and RRI are limited. Thus, the present study was designed to evaluate the association between cystatin C and vascular biomarkers (CCIMT, carotid plaque and RRI) in patients with URHT phenotype and subclinical CVD.
Material and methods
Study group
This cross-sectional study was done at the Clinical Center of Vojvodina (CCV), from February 2013 to October 2015. Consecutive participants (n = 200, aged <65 years) with HT and stable antihypertensive treatment regimen for at least a 6 months have been enrolled into the study. They attended in the CCV laboratory unit to evaluate the presence of subclinical CVD – presence of renal and vascular hypertensive TOD. Inadequate BP control was defined as a mean 24-h ambulatory BP ≥130/80 mmHg.Citation15 The antihypertensive therapy compliance was assessed by using Medication Adherence Assessment questionnaire.Citation16 Patient were stratified into two subgroups by their blood pressure (BP) level as having responsive hypertension (RHT – compliant and responsive to treatment, n = 100), or nonresponsive (URHT – compliant but nonresponsive to treatment – three different antihypertensive classes of agents with inadequate BP control, n = 100).
The study excluded patients with clinically confirmed CVD (AMI, percutaneous coronary angioplasty or revascularization, stroke and peripheral arterial disease); GFR <30 mL/min/1.73 m2, endocrinological diseases (diabetes mellitus, diseases of the thyroid gland, Cushing’s and Conn’s syndrome, pheochromocytoma) renal (glomerulonephritis and tubulointerstitial disease) and renovascular diseases; sleeping disorders; inflammatory and infectious diseases; malignancies; smokers, as well as those who use corticosteroids and hypolipemic drugs.
The study was conducted according to the principles of the Helsinki Declaration and approved by the Ethics Committee of CCV. Informed consent for participating in the study was obtained from all participants prior to the inclusion in the study.
Study protocol
Simultaneously, all participants underwent the following procedures in the same day: the anthropometric measures (height, weight and waist circumference), clinical BP measurement, blood sampling, handing out their 24 h urine samples and measurement of GFR. Ambulatory blood pressure monitoring (24 h AMBP), renal and carotid ultrasonography were performed within one month. Evaluation of the subclinical renal and vascular TOD was performed according to the recommendations of the European Society of Hypertension and the European Society of Cardiology. Subclinical renal TOD was defined as a GFR from 30 to 60 ml/min/1.73 m2 or urinary albumin excretion 30–300 mg/day, and subclinical vascular TOD was defined as CCIMT >0.9 mm.Citation4
Clinical BP measurement and 24-h ambulatory BP monitoring
Clinic blood pressure was measured three times after at least 10 min rest in a seated position by the Riva Rocci method using mercury sphygmomanometer. The average of the last two readings was used for analyses. Noninvasive 24-h ABPM was performed using a Meditech Cardiotens (Meditech Ltd., Budapest, Hungary) according to the standard procedure.Citation15
Biochemical analysis
Serum levels of creatinine (measured by the Jaffé method – alkaline picrate reaction), urea and uric acid were determined by standard biochemical methods (commercial kits-Beckman Coulter, Maryfort, Ireland on Olympus AU 400); cystatin C by the immunoturbidimetric method (Dyazime, Poway, CA, on Olympus AU 400, reference range: 0.5–1.03 mg/l); high-sensitive C-reactive protein (hsCRP) by the immunoturbidimetric method (Beckman Coulter, Maryfort, Ireland), lipid profile was measured by standard biochemical methods (commercial Siemens kits, Camberley, UK, on Olympus AU 400). Albuminuria (24 h urinary sample in the absence of laboratory evidence of urinary tract infection) was determined by the sandwich-immunometric method (NycoCard tests, Olso, Norvage). Estimated GFR (eGFR) was calculated using the CKD-EPI.Citation17,Citation18
GFR measurement
GFR was measured by the isotopic clearance method with diethylenetriamine penta-acetic acid marked with technetium-99mTc (99mTc-DTPA), using a single injection at a dose of 37 MBqCitation19 and by taking two samples of blood after 180 and 240 min (slope–intercept method). For quality control of the radiochemical purity of 99mTc-DTPA complex (>95%), paper chromatography was performed. For measuring the radioactivity of samples gamma counter (Captus 3000, Capintec Inc., Florham Park, NJ) with hollow crystal NaI (Tl) was used, with energy range of 99mTc and “window” width of 20%.
Doppler ultrasonography of the carotid arteries and kidneys
Measurement of the CCIMT was done according to the proposed standard procedure,Citation20 by high-resolution ultrasound in B mode, in real-time, using duplex ultrasonography and linear probe of 12 MHz (GE LOGIC 7). Measurements of the CCIMT have been taken on the back wall of both common carotid arteries, proximally 10 mm from the beginning of the carotid bulb. On a longitudinal cross-section of both right and left common carotid artery, which was about 20 mm long, in the posterior wall, 6 measurements have been taken, where there were no focal plaques, at the end of the diastole. From the obtained values, median values were calculated and used to define the thickness of the common carotid artery (CCIMC). Carotid plaque was defined as the presence of focal wall thickening that is at least 50% greater than that of the surrounding vessel wall or as a focal region with CIMT greater than 1.5 mm that protrudes into the lumen that is distinct from the adjacent boundary. Based on the plaque echogenicity, the plaque’s morphology was determined, lipid (hypo/anechogenic), fibrous (isoechogenic) and calcified (hyperechoic). Duplex scan of the renal arteries, as measurement of RRI was performed on the same device, in real-time was done using a multi-frequent convex probe of 3.75 MHz, according to the proposed standard procedure.Citation21
Reproducibility of the measurements, CC-IMC and RRI were observed in groups of 20 patients, whose measurements were taken within one hour by both radiologists, who were unaware of the clinical data of the subjects. The coefficient of variation (intraobserver and interobserver) for CCIMT measurements was 2.8% and 3.2%, respectively. The coefficient of variation (intraobserver and interobserver) for RI was 4.1% and 5.6%, respectively.
Statistical analysis
Normal distribution of continuous variable was assessed with the Kolmogorov–Smirnov test. Data are presented using descriptive statistical methods, continuous variables as mean ± standard deviation, median, interquartile range, while categorical data were summarized as percentages. Differences in characteristics between groups were tested using χ2 test for dichotomous variables, and for continuous variables t test, Man–Whitney test, or Kruskal–Wallis H nonparametric with post-test for multiple comparisons of mean ranks, as appropriate. The correlations among variables were assessed using the Spearman rank correlation coefficient. Stepwise multiple regression analysis was performed to assess the independent association between cystatin C and other markers. A two-tailed p < 0.05 was considered statistically significant. Statistical analysis was performed using the Statistika software 12.0 (StatSoft Inc., Tulsa, OK).
Results
Clinical and biochemical characteristics of the study population by groups are presented in . There was no significant difference found among groups in terms of age, sex, or BMI. Waist circumference was significantly higher in the URHT group compared to the RHT group (p = 0.01). Daytime SBP and DBP measurements showed significant differences between groups (p < 0.05, for all). As shown in , mGFR, eGFR, creatinine, urea, acid uric, as albuminuria were similar between groups. Patients in the URHT group had significantly higher cystatin C than in the RHT group (p = 0.02). Similarly, hsCRP levels were significantly higher in the URHT group than patients with RHT (p = 0.03). CCIMT was higher in the URHT group (1.1 (0.85–1.23) vs. 0.86 (0.76–1.1)) than the other group (p = 0.00). Presence of carotid plaque (fibrous and/or calcified) and RRI did not significantly differ between groups. No significant difference was observed in classes of antihypertensive drugs between groups ().
Table 1. Clinical and biochemical characteristics of the study population by groups.
Furthermore, in the URHT group serum concentration of cystatin C was analyzed based on the presence of subclinical cardiovascular damage (). Most of the patients in the group had subclinical cardiovascular damage [vascular TOD (n = 18), renal TOD (n = 24), and both (n = 43)]. Cystatin C serum concentration increased significantly in parallel with increasing the number of organs involved (no vascular or renal subclinical TOD – 0.90 (0.88–1.07) mg/l vs. vascular subclinical TOD – 1.13 (1.1–1.20) mg/l or renal TOD – 1.2 (1.07–1.31) mg/l vs. vascular and renal subclinical TOD – 1.51 (1.35–1.71) mg/l, to all p < 0.001). In post hoc analysis, cystatin C serum concentration was significantly higher in the presence of both vascular and renal TOD, compared to its concentration in patients with only renal TOD. No significant differences were found in median creatinine concentration, as median mGFR, between patients with renal compared to patients with both, renal and vascular TOD.
Table 2. Cystatin C and biomarkers of subclinical vascular and/or renal TOD in patents with URHT.
In all study patients, cystatin C serum concentration correlated with the mGFR (r= −0.86, p < 0.001), eGFR (r= −0.52, p < 0.001), albuminuria (r= 0.36, p < 0.001), hsCRP (r= −0.41, p < 0.001), CCIMT (r= 0.44, p < 0.001), RRI (r= 0.35, p < 0.001), waist circumference (r= 0.37, p < 0.001), duration of hypertension (r= 0.38, p < 0.001), URHT (r= 0.23, p = 0.04), and age (r= 0.28, p = 0.03). Cystatin C was not affected by sex, BMI, SBP, DBP, presence of atherosclerotic plaque, and the type of antihypertensive drugs (p > 0.05).
In the URHT group, as shown in , cystatin C serum concentration significantly correlated with mGFR and CCIMT, respectively (both p < 0.001). After controlling for mGFR and age, in regression analysis cystatin C significantly and independently was associated with hs CRP (β = 0.351, t = 3.861, p = 0.01), CCIMT (β = 0.331, t = 3.361, p = 0.017) and duration of hypertension (β = 0.351, t = 3.861, p = 0.02) in a model (R2 = 0.49, R adjusted = 0.45).
Figure 1. Correlations (a) between cystatin C serum concentration (log-transformed data) and the thickness of the common carotid artery (CCIMC) in the URHT group; (b) between cystatin C serum concentration (log-transformed data) and the thickness of the common carotid artery (CCIMC) in the RHT group; (c) between cystatin C serum concentration (log-transformed data) and the glomerular filtration rate measured with 99mTc-DTPA (mGFR) in the URHT group; (d) between cystatin C serum concentration (log-transformed data) and the glomerular filtration rate measured with 99mTc-DTPA (mGFR) in the RHT group.
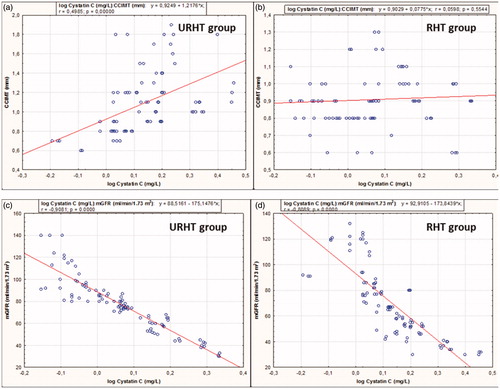
Correlation between cystatin C and CCIMT in the RHT group was not significant (p = 0.55). In RHT group, cystatin C serum concentration was found to be significantly and independently associated with mGFR (β = −0.795, t = −5.146, p = 0.00) and age (β = −0.246, t = −2.01, p = 0.04) in a model (R2 = 0.69, R adjusted = 0.62). Independent predictors of URHT phenotype were CCIMT (p = 0.017) and hsCRP (p = 0.04).
Discussion
The main finding that the present study suggests is that serum concentration of cystatin C is associated with subclinical TOD, namely GFR and CCIMT in patients with URHT phenotype. Moreover, cystatin C serum concentration increased significantly in parallel with the increasing number of subclinical TOD (renal vs vascular to renal and vascular) involved. In studied patients with URHT, regardless of traditional cardiovascular risk factors, association of cystatin C with CCIMT was independent of mGFR.
Cystatin C has been shown to be associated with subclinical CVD in patients with HT.Citation8 Also, the cross-sectional relationship between cystatin C and CCIMT has been examined previouslyCitation6 and their relationship was explained as a result of co-variation with traditional cardiovascular risk factors (age, male sex, duration of hypertension) and impaired renal function,Citation22 since they all contribute to the senile vascular changes and vascular intima media thickening.Citation23 Some authors showed no independent association between cystatin C level or eGFR with CCIMT in a population free of clinical CVD,Citation24 suggesting that accelerated atherosclerosis is unlikely to be the primary mechanism explaining the independent association of cystatin C level with CV risk. Further, others showed that microalbuminuria, but not cystatin C, was associated with carotid atherosclerosis beyond traditional cardiovascular risk factors among middle-aged adults.Citation25 In our, study cystatin C, but not albuminuria, was signifficantly associated with CCIMT and only in patients with URHT. Importantly, CCIMT and hs CRP were only independent predictors of URHT, suggesting the possible role of vascular inflammation in nonresponsiveness to treatment. Also, some studies suggested that increased level of cystatin C has been associated with arterial stiffness (aortic pulse wave velocity) in older adults.Citation21 Additionaly, as GFR is one of the most important determinants of cystatin C serum concentration and since most studies lacked measuring of the GFR, the relationship between cystatin C and carotid atherosclerosis, particularly in asymptomatic patients with subclinical renal TOD is still not entirely clear. Recent study that measured GFR (Iohexol Clearance) found associations between the values of GFR >60 ml/min/1.73m2 and CCIMT in non-diabetic individuals,Citation26 that resulted in speculation of possibilities that high values of GFR could be a CV risk factor.
Recent studies have revealed that an associated occurrence of more than one organ-specific biomarkers (e.g. decrease in GFR and albuminuria) increase CV morbidity when compared to their sole presence.Citation4,Citation6 Results of our study showed that cystatin C were independently associated with hsCRP and CCIMT in URHT group. We may assume that the link between these parameters is inflammation because of the small decline in GFR, which due to interaction of proatherogenic factors and hemodynamic disorders exponentially increases the risk for CVD and their complications. Possible mechanisms for the relationship between Cystatin C and hsCRP remain unclear. It was suggested that vascular endothelial damage and chronic inflammation in hypertension promote atherosclerosis.Citation8 Our results indicate that even though cystatin C serum concentration was primary reflecting GFR, his concentrations was significantly higher in the presence of both, vascular and renal TOD, compared to its concentration in patients with only renal TOD (with similar values of mGFR). That was not observed for creatinine concentration, indicating that cystatin C serum concentration could be more sensitive in evaluating subclinical CVD in hypertensive population.
The values of RRI were not significantly higher in patients with URHT group compared to RHT group. These findings could be due to the lack of significant differences between subgroups, firstly in mGFR. Using the RRI as a marker of subclinical vascular TOD and as a predictor of cardiovascular morbidity and mortality in patients with hypertension has become important in recent years.Citation25,Citation29 Some studies suggest correlation between RRI and other factors such as age, SBP and PP, GFR, albuminuria, left ventricular hypertrophy, Framingham score and calcium score in the coronary blood vessels.Citation13 Yet, one of the major shortcomings of wider application of RRI is insufficient specificity and the lack of its cutoff value that would separate the impact of intrarenal vascular damage compared to systemic vascular damage. In the majority of the studies that had been performed so far,Citation14 GFR less than 30 ml/min/1.73 m2 led to an increase in RRI due to the influence of local factors in the kidney. In this study, we analyze patients with uncontrolled hypertension and possibly nonresistant phenotype since they did not fulfill the criteria for resistant hypertension.Citation4 A recent study from Brasil demonstrated that patients with resistant hypertensive phenotype have more frequent arterial and renal consequences of high BP compared with the nonresistant phenotype.Citation29 Additionally, it is still undetermined whether for the same BP levels and duration of hypertension, the resistant compared with the nonresistant phenotype is characterized by a different CV prognosis.Citation30
Even though the advantages of our study include the measurement of GFR and simultaneous assessment of cystatin C and markers of subclinical renal and vascular TOD in asymptomatic hypertensive population, it also has some potential limitations. Firstly, small number of patients were included in the study. Further, since mGFR and biomarkers serum/plasma concentration were measured once, possible overestimation of true GFR and intraindividual variation in the concentration of these variables cannot be considered. Although the cross-sectional design of this study does not allow us to make conclusions regarding the pathogenetic mechanisms underlying associations between cystatin C, CCIMT, and hsCRP, these findings may further support the role of cystatin C as a marker of cardiovascular risk in hypertensive patients. Considering that cardiovascular morbidity and mortality in our region account for more than half of all death events, it would be necessary to carry out large and long-term prospective studies to confirm that serum cystatin C is a suitable marker for the detection of subclinical hypertensive TOD.
Conclusions
In addition to GFR, cystatin C serum concentration is positively and independently associated with CCIMT in patient with URHT phenotype and subclinical CVD. Prospective larger studies should further investigate the clinical importance of this relationship.
Disclosure statement
The authors report no conflicts of interest
Funding
This work was supported by [grant No. 114–451-2013/2016–01] approved by the Provincial Secretariat for Science and Technological Development, Autonomous Province of Vojvodina, Republic of Serbia.
References
- Grassi G, Cifkova R, Laurent S, et al. Blood pressure control and cardiovascular risk in hypertensive patients from central and eastern European countries: Results of the BP-CARE study. Eur Heart J. 2011;32:218–225.
- World Health Organization. World health statistics 2013. 2013. Geneva: WHO Press.
- Banegas JR, Lopez-Garcia E, Dallongeville J, et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: The EURIKA study. Eur Heart J. 2011;32:2143–2152.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357.
- Ruilope LM, Bakris GL. Renal function and target organ damage in hypertension. Eur Heart J. 2011;32:1599–1604.
- Čabarkapa V. Cystatin C: More than the marker of the glomerular filtration rate. Med Pregl. 2016;68:173–179.
- Helmersson-Karlqvist J, Ärnlöv J, Larsson A. Cystatin C-based glomerular filtration rate associates more closely with mortality than creatinine-based or combined glomerular filtration rate equations in unselected patients. Eur J Prev Cardiol. 2016;23:1649–1657.
- Salgado V, Francival L, Bernardete J. How to understand the association between cystatin C levels and cardiovascular disease: Imbalance, counterbalance, or consequence? J Cardio. 2013;62:331–335.
- Agarwal S, Thohan V, Shlipak MG, et al. Association between cystatin C and MRI measures of left ventricular structure and function: Multi-ethnic study of atherosclerosis. Int J Nephrol. 2011;2011:153868. doi: 10.4061/2011/153868.
- Delanaye P, Cavalier E, Cristol JP, Delanghe JR. Calibration and precision of serum creatinine and plasma cystatin C measurement: Impact on the estimation of glomerular filtration rate. J Nephrol. 2014;27:467–475.
- Roman MJ, Kizer JR, Best LG, et al. Vascular biomarkers in the prediction of clinical cardiovascular disease: The Strong Heart Study . Hypertension. 2012;59:29–35.
- Prejbisz A, Warchoł-Celińska E, Florczak E, et al. Renal resistive index in patients with true resistant hypertension: Results from the RESIST-POL study. Kardiol Pol. 2016;74:142–150.
- Heine GH, Rogacev KS, Fliser D, Krumme B. Renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension. 2013;61:e22. doi: 10.1161/HYPERTENSIONAHA.111.00655.
- Boddi M, Natucci F, Ciani E. The internist and the renal resistive index: Truths and doubts. Intern Emerg Med. 2015;10:893–905.
- Parati G, Stergiou G, O'Brien E, European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–1366.
- Morisky DE, Ang A, Krousel‐Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10:348–354.
- National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update (2012). Am J Kidney Dis. 2012:60:850–886.
- Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627.
- Fleming JS, Zivanovic MA, Blake GM, Burniston M, Cosgriff PS. British Nuclear Medicine Society. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun. 2004;8:759–769.
- Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296.
- Darmon M, Schnell D, Zeni F. Doppler-based renal resistive index: A comprehensive review. In: Vincent JL, ed. Yearbook of Intensive Care and Emergency Medicine. Heidelberg: Springer; 2010:331–338.
- Monteiro Junior F, Ferreira PA, Nunes JA, et al. Correlation between serum cystatin C and markers of subclinical atherosclerosis in hypertensive patients. Arq Bras Cardiol. 2012;99:899–906.
- Kastarinen H, Ukkola O, Kesaniemi YA. Glomerular filtration rate is related to carotid intima-media thickness in middle-aged adults. Nephrol Dial Transplant. 2009;24:2767–2772.
- Bui AL, Katz R, Kestenbaum B, et al. Cystatin C and carotid intima-media thickness in asymptomatic adults: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2009;53:389–398.
- Rodondi N, Yerly P, Gabriel A, et al. Microalbuminuria, but not cystatin C, is associated with carotid atherosclerosis in middle-aged adults. Nephrol Dial Transplant. 2007;22:1107–1114.
- Eriksen BO, Løchen ML, Arntzen KA, et al. Subclinical cardiovascular disease is associated with a high glomerular filtration rate in the nondiabetic general population. Kidney Int. 2014;86:146–153.
- Shankar A, Teppala S. Relationship between serum cystatin C and hypertension among US adults without clinically recognized chronic kidney disease. J Am Soc Hypertens. 2011;5:378–384.
- Lotufo PA, Pereira AC, Vasconcellos PS, Santos IS, Mill JG, Bensenor IM. Resistant hypertension: Risk factors, subclinical atherosclerosis, and comorbidities among adults-the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J Clin Hypertens (Greenwich). 2015;17:74–80.
- Lubas A, Kade G, Niemczyk S. Renal resistive index as a marker of vascular damage in cardiovascular diseases. Int Urol Nephrol. 2014;46:395–402.
- Thomopoulos C, Skalis G, Makris T. Resistant hypertension: A real entity or a phantom diagnosis? J Clin Hypertens (Greenwich). 2015;17:578–579.
