Abstract
Background: Lotensin has been shown to have a protective function in the early stage of chronic renal failure. However, its role in the intermediate and late stages of chronic renal failure remains largely unknown. The present study aimed to investigate the role and underlying mechanism of lotensin in advanced chronic kidney disease.
Methods: Female Wistar rats were randomly divided into three groups (n = 10): sham group, 5/6 nephrectomy (5/6 Nx) group, and lotensin group (oral administration of lotensin for 9 weeks following 5/6 Nx). Rats were sacrificed and pathological parameters were measured. Western blot assay and immunohistochemical staining were performed to detect the expression of transforming growth factor-β1 (TGF-β1) and α-smooth muscle actin (α-SMA) in kidney tissues.
Results: Compared to the 5/6 Nx group, lotensin administration significantly decreased 5/6 Nx-induced elevation in blood urea nitrogen, serum creatinine and 24-h urinary protein excretion (UPE) rates, but markedly increased red blood cell count, plasma albumin and hemoglobin levels, along with improved renal morphology. Mechanistically, lotensin dramatically downregulated the renal expression of TGF-β1 and α-SMA induced by 5/6 Nx.
Conclusions: Lotensin protects against advanced chronic kidney disease in rats with 5/6 Nx through the downregulation of TGF-β1 and α-SMA.
Introduction
Chronic renal failure, which is currently more commonly classified as chronic renal disease, is one of the leading causes of morbidity and mortality worldwide. A variety of mechanisms participate in the progression of chronic renal failure, such as the accelerated proliferation of mesangial cells and excessive deposition of the extracellular matrix. Chronic renal failure may progress to end-stage renal disease, which requires costly renal replacement therapy, posing a heavy burden on the families involved and society [Citation1,Citation2]. Therefore, there is an urgent need to understand the pathophysiological mechanisms of chronic renal failure and identify effective therapeutic agents.
Transforming growth factor-β1 (TGF-β1) acts as a potent stimulator of glomerulus mesangial cell hyperplasia [Citation3,Citation4], and is one of the important factors responsible for the fibrotic process [Citation5]. The upregulation of TGF-β1 has been found to contribute to impaired renal function in chronic renal failure [Citation5].
Alpha-smooth muscle actin (α-SMA) is a marker protein of smooth muscle cells and myofibroblasts. It has been reported that high α-SMA expression in kidneys is a hallmark of tubular epithelial-myofibroblast transdifferentiation [Citation6]. In addition, mesangial cell activation is characterized by the induction of α-SMA expression, which further promotes the deposition of the extracellular matrix and glomerulosclerosis [Citation7].
A rat model of 5/6 nephrectomy (5/6 Nx) is commonly used for study of chronic renal failure and screening of therapeutic drugs. In the kidneys of 5/6 nephrectomized rats, the number of nephrons is greatly reduced with high glomerular capillary pressure and decreased glomerular filtration rate [Citation8]. These functional abnormalities lead to tubular atrophy, glomerular sclerosis and renal interstitial fibrosis, which is similar to the pathological features of advanced chronic renal failure [Citation9]. Previous studies have shown that rats are in the advanced stage of chronic renal failure at 9 weeks after 5/6 Nx [Citation10,Citation11].
Lotensin is an angiotensin-converting enzyme (ACE) inhibitor that confers substantial renal benefits in patients with advanced renal insufficiency, especially in patients who have increased urinary protein excretion (UPE) [Citation12,Citation13]. Our previous findings demonstrated that the administration of lotensin had a beneficial effect on early-stage chronic renal failure (5 weeks after 5/6 Nx) [Citation14]. However, it remains unclear whether lotensin has a protective function against advanced chronic renal failure.
In the present study, the potential renoprotective action of lotensin was investigated using a rat model of advanced chronic renal failure, and the possible molecular mechanisms underlying the renoprotection of lotensin were also suggested.
Materials and methods
Materials
TGF-β1, α-SMA and β-actin antibodies were purchased from Boster Biology Technology (Wuhan, China). Lotensin was obtained from Novartis AG (Basel, Switzerland).
Animals and experimental protocol
All animal experiments were conducted in accordance with The Guide for the Care and Use of Laboratory Animals issued by the Animal Centre of Jilin University. Female Wistar rats (210–250 g) were housed in a temperature-controlled room with free access to water and standard laboratory chow.
The animals were randomly divided into three groups (n = 10, per group): sham group, 5/6 Nx group and lotensin group. In the 5/6 Nx group, rats were anesthetized by intraperitoneal injection of 2% Nembutal, and the upper 1/3 and lower 1/3 of the left kidney were removed (2/6 Nx). After 7 days, the right kidney was removed (5/6 Nx). In the sham group, rats underwent two flank incisions. In the lotensin group, rats subjected to 5/6 Nx were given lotensin (0.6 mg/100 g day−1) in 20 mL of drinking water on the day of the right nephrectomy until 9 weeks. Then, the animals were sacrificed, immediately followed by blood collection, and 24-h urine samples were collected before sacrifice. A portion of the remaining left kidney was stored in liquid nitrogen, and the remainder was fixed in 10% formalin for the following experiments.
Western blot assay
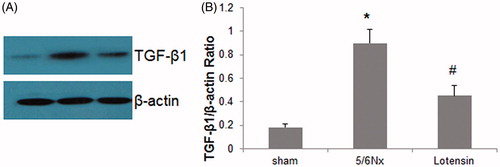
Renal tissue samples were grounded into powder in liquid nitrogen, and lysed in lysis buffer (2% SDS, 10 mmol L−1 of Tris-HCl, pH 6.8, 10% [v/v] glycerol). Lysates were centrifuged at 12,000 g for 15 min at 4 °C, and the supernatant was collected. Protein concentrations were measured using a Bio-Rad kit (Bio-Rad Laboratories; Hercules, CA). Fifty µg of protein were loaded per lane, separated on 10% SDS-PAGE, and transferred to nitrocellulose membranes. Then, the membranes were blocked in 5% BSA in TBST for 1 h, followed by overnight incubation with primary antibodies against TGF-β1 (1:1,000), β-actin (1:5,000) and α-SMA (1:1,000) at 4 °C. After washing with TBST for three times, the membranes were incubated with horseradish peroxidase-linked anti-rabbit secondary antibody (1:2,000) for 1 h at room temperature. Protein bands were detected using the Supersignal chemiluminescence reagent (Pierce; Thermo Fisher Scientific), and the band intensity was quantified using Quantitation One software (Bio-Rad). β-actin was used as an internal control.
Histological analysis and immunohistochemistry
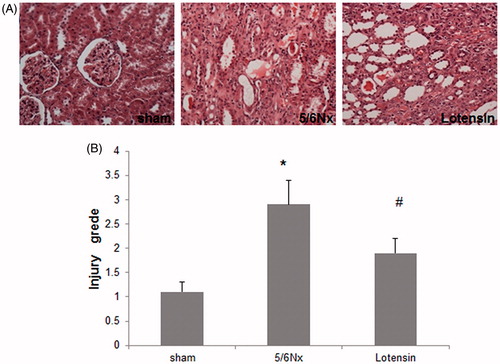
Renal tissues were fixed in 10% formalin, embedded in paraffin and prepared into 5-μm-thick sections. The sections were stained with hematoxylin-eosin (H&E) by standard methods for histological examination. A total of 10 randomly selected renal tubulointerstitial regions were observed under a light microscope (Japan, 100×), and the areas of renal tubular atrophy, interstitial inflammation and interstitial fibrosis were measured. The renal injury grades were defined as follows: Grade 1 = no injury; Grade 2 = injury area <25%; Grade 3 = injury area within 25–49%; Grade 4 = injury area >50%. All histological examinations were performed by two experienced renal pathologists in a double-blinded manner.
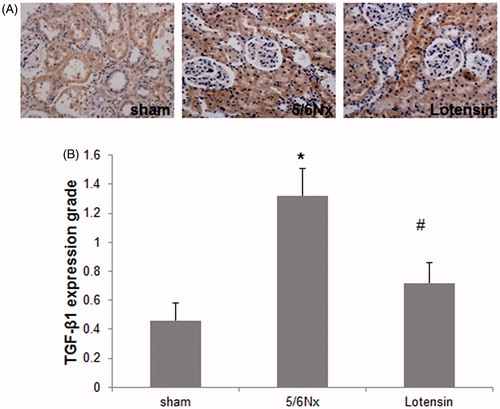
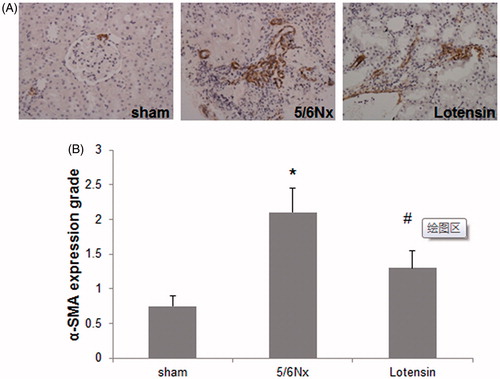
Following the standard protocol prior to H&E staining described above, the sections were subjected to antigen retrieval and blocking. The immunohistological staining of α-SMA and TGF-β1 was performed using a commercial kit (Dako; Carpinteria, CA), as previously described [Citation15]. The staining was observed under a Leica DMRE light microscope (200×). Staining intensity was quantified as follows: 0 = none or weak staining; 1 = stained areas <25% or weak-moderate staining; 2 = stained area within 25–49% or moderate staining; 3 = stained area within 50–75% or moderate-strong staining; 4 = stained areas >75% or strong staining. The sections were examined by two experienced renal pathologists in a double-blinded manner.
Statistical analysis
Data were presented as mean ± standard error of the mean (SEM). Comparisons among multiple groups were analyzed with Analysis of Variance (ANOVA) using SPSS version 17.0 software. p values <.05 was considered statistically significant.
Results
Pathological parameters
presents the pathological parameters measured at 9 weeks after lotensin administration. We found that there was a significant increase in the levels of blood urea nitrogen, serum creatinine and 24-h urinary protein excretion (UPE), and a marked decrease in red blood cell count, plasma albumin and hemoglobin levels in the 5/6 Nx group, when compared with the sham group. This suggests that 5/6 Nx greatly impairs renal function. However, the administration of lotensin can generally reverse the adverse effects of 5/6 Nx on renal function in rats, suggesting the protective effect of lotensin on renal function.
Table 1. Pathological parameters in the sham, 5/6 nx and lotensin groups.
Histological analysis
The H&E staining of renal tissues indicated that renal glomeruli, tubules and the interstitium were morphologically normal in the sham group, while morphological abnormalities such as focal lesions in the interstitial areas, excessive accumulation of fibrous elements, interstitial inflammation and tubular atrophy were present in the 5/6 Nx group (). In the lotensin group, renal morphology was greatly improved, as evidenced by the reduced number of focal lesions, attenuated interstitial inflammation, and modest tubular atrophy (). Consistently, tubulointerstitial injury grade was significantly lower in the lotensin group, compared to the 5/6 Nx group (). These data suggest that lotensin has a protective effect on renal morphology.
Immunohistochemical analysis of TGF-β1
To further investigate the underlying mechanism of the renoprotection of lotensin, we detected the local expression of TGF-β1, a potent pro-fibrotic factor, in renal tissues. The immunohistochemical staining revealed that TGF-β1 was majorly expressed in renal tubular epithelial cells in the sham group, and was dramatically unregulated in the 5/6 Nx group (), suggesting the possible involvement of TGF-β1 in renal injury. However, the 5/6 Nx-induced upregulation of TGF-β1 was remarkably inhibited by lotensin (), demonstrating that lotensin protects against 5/6 Nx-induced renal injury through the inhibition of TGF-β1 expression.
Western blot analysis of TGF-β1 expression
To confirm the involvement of TGF-β1 in the renoprotection of lotensin, western blot assay was performed to examine the protein expression of TGF-β1 in renal tissues. As shown in , TGF-β1 expression was greatly enhanced in the 5/6 Nx group, compared to the sham group. In addition, lotensin markedly diminished this enhancement, further demonstrating that the suppression of TGF-β1 expression is a mechanism underlying the renoprotection of lotensin.
Immunohistochemical analysis for α-SMA protein
We next sought to determine whether the renoprotection of lotensin is attributable to the regulation of α-SMA, an important fibrotic marker. Hence, the immunohistochemical staining of α-SMA was performed. As shown in , α-SMA was almost undetectable in renal tissues in the sham group, and 5/6 Nx induced the marked expression of α-SMA in renal interstitium. Furthermore, lotensin administration resulted in a significant decrease in the interstitial expression of α-SMA (), suggesting that the renoprotection of lotensin is due at least partially to the inhibition of the fibrotic process.
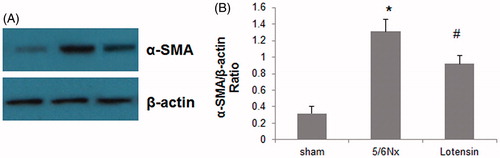
Western blot analysis of α-SMA expression
Western blot assay was also conducted to confirm the inhibition of 5/6 Nx-induced α-SMA expression by lotensin. As shown in -SMA was weakly expressed in renal tissues in the sham group, but was dramatically upregulated in the 5/6 Nx group. Furthermore, the upregulation of α-SMA was significantly suppressed in the lotensin group (). These data further indicate that the suppression of α-SMA expression is an alternative mechanism underlying the renoprotection of lotensin.
Discussion
Chronic renal failure poses a serious threat to human health. To better understand its pathogenesis and more effectively identify useful therapeutic approaches against it, the 5/6 Nx murine model has been commonly used to mimic the process of chronic renal failure [Citation15]. Following 5/6 Nx, renal morphology is pathologically altered, including renal interstitial fibrosis and tubular atrophy, eventually resulting in chronic renal failure [Citation9–11]. At present, the majority of studies have focused on the early stage of chronic renal failure (5 weeks after 5/6 Nx). The protective role of lotensin during early chronic renal failure has been well-investigated [Citation16]. However, it remains unknown whether lotensin also protects against late-stage chronic renal failure.
In the present study, a 5/6 Nx rat model of advanced chronic renal failure was established, and we found that lotensin had a renoprotective function in this model. Importantly, we demonstrated that renoprotection of lotensin was achieved through the downregulation of both TGF-β1 and α-SMA, although the detailed mechanism underlying the inhibition of the expression of these two factors by lotensin have not been addressed in the study.
TGF-β1 is a multifunctional cytokine involved in a variety of physiological and pathological processes such as smooth muscle differentiation, epithelial-mesenchymal transition and tissue fibrosis [Citation5]. It has been well-established that TGF-β1 is mainly responsible for the increased fibrosis in chronic kidney diseases [Citation17]. In vivo and in vitro studies have demonstrated that TGF-β1 also contributes to the pathology of glomerulosclerosis by activating glomerular mesangial cells, and subsequently promoting the accumulation of the extracellular matrix [Citation3,Citation4,Citation18,Citation19]. On the other hand, α-SMA, as a common marker for smooth muscle cells and myofibroblasts, is highly expressed in kidneys, and the overproduction of α-SMA partially results from tubular epithelial-myofibroblast transdifferentiation, which plays an important role in renal interstitial fibrosis [Citation6,Citation20,Citation21]. Consequently, the upregulation of TGF-β1 and α-SMA correlates with the risk of chronic renal failure.
In chronic renal diseases, angiotensin (Ang) II mediates renal injury through the regulation of blood pressure and cytokine production [Citation22,Citation23]. Lotensin, as an ACE inhibitor, can inhibit Ang II production [Citation24]. Furthermore, lotensin has been clinically used in reducing UPE and improving renal function in the early stage of chronic renal failure [Citation12,Citation13]. In the present study, the role and mechanism of lotensin in protecting against advanced chronic renal failure was investigated. Our results revealed that the 9-week administration of lotensin may greatly improve blood and urinary physicochemical parameters following 5/6 Nx. The histological examination of renal tissues also indicated that lotensin administration may improve renal morphology. These findings suggest that lotensin facilitates the restoration of renal structure and function in advanced chronic renal failure, which requires additional clinical validation.
Mechanistically, lotensin downregulated the 5/6 Nx-induced expression of TGF-β1 and α–SMA in renal epithelium and the interstitium, respectively, suggesting that Ang II may serve as an upstream regulator for both TGF-β1 and α–SMA. Further investigations are needed to determine how lotensin or Ang II regulates TGF-β1/α–SMA. Collectively, the present study may provide valuable clues for developing new applications of old agents or drugs.
Lotensin has been clinically used in reducing UPE and improving renal function in the early stage of chronic renal failure. However, it remains controversial whether ACE inhibitors can improve renal function and reducing UPE in the late stage of chronic renal failure. Some studies demonstrate that ACE inhibitors still provide renoprotection in this stage, whereas other ones show that they may accelerate the deterioration of renal function because of its hemodynamic function.
In this study, we examined the renoprotective role of Lotensin in rats 9 weeks after 5/6 Nx, which can mimic the late stage of chronic renal failure. We found that Lotensin indeed reduced UPE and improved renal function in this stage as evidenced in this manuscript. The renoprotection of Lotensin may be at least partially attributed to the decreased expression of transforming growth factor-β1 and α-smooth muscle actin. We believe that this study has provided strong evidence that Lotensin plays a renoprotective role in the late stage of chronic renal failure, deepening the understanding of functions of ACE inhibitors in chronic renal failure.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Grupp C, Troche I, Klass C, et al. A novel model to study renal myofibroblast formation in vitro. Kidney Int. 2001;59:543–553. Feb doi: 10.1046/j.1523-1755.2001.059002543.x.
- Ruiz-Ortega M, Ruperez M, Lorenzo O, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002;62:S12–S22. Dec doi: 10.1046/j.1523-1755.62.s82.4.x.
- Schnaper HW, Hayashida T, Hubchak SC, et al. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol. 2003;284:F243–F252. Feb doi: 10.1152/ajprenal.00300.2002.
- Dai C, Liu Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J Am Soc Nephrol. 2004;15:1402–1412. JunPubMed PMID: 15153551; eng.
- Tsuchida K, Zhu Y, Siva S, et al. Role of Smad4 on TGF-beta-induced extracellular matrix stimulation in mesangial cells. Kidney Int. 2003;63:2000–2009. Jun doi: 10.1046/j.1523-1755.2003.00009.x.
- Jiang T, Zhou QS, Pi L, et al. Role of angiotensin II and JAK2 signal pathway in transdifferentation of renal tubular cells in mice after acute ischemic followed by reperfusion. Zhonghua Bing Li Xue Za Zhi 2009;38:466–471. Jul
- Dai C, Yang J, Bastacky S, et al. Intravenous administration of hepatocyte growth factor gene ameliorates diabetic nephropathy in mice. J Am Soc Nephrol. 2004;15:2637–2647. Oct doi: 10.1097/01.asn.0000139479.09658.ee.
- Brenner BM. Nephron adaptation to renal injury or ablation. Am J Physiol. 1985;249:F324–F337. Sep doi: 10.1152/ajprenal.1985.249.3.F324
- Wang HY, Yang LZ, Cui MJ, et al. Hepatocyte growth factor-induced amelioration in chronic. Renal failure is associated with reduced expression of alpha-smooth muscle actin. Renal Fail. 2012;34:862–870. doi: 10.3109/0886022x.2012.687344.
- Shen Y-C, Yang X, Chen Q-X. Renal pathological changes in rat with 5/6 nephrectomy [J]. Shanghai Lab Anim Sci. 2005;1:006.
- Zhao G, Zhao H, Tu L, et al. Effects and mechanism of irbesartan on tubulointerstitial fibrosis in 5/6 nephrectomized rats. J Huazhong Univ Sci Technol [Med Sci]. 2010;30:48–54. Feb doi: 10.1007/s11596-010-0109-1.
- Ihle BU, Whitworth JA, Shahinfar S, et al. Angiotensin-converting enzyme inhibition in nondiabetic progressive renal insufficiency: a controlled double-blind trial. Am J Kidney Dis. 1996;27:489–495.
- Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–140. Jan 12 doi: 10.1056/NEJMoa053107.
- Wang HY, Wang YJ, Cui MJ, et al. Hepatocyte growth factor-induced amelioration in renal interstitial fibrosis is associated with reduced expression of alpha-smooth muscle actin and transforming growth factor-beta1. Indian J Biochem Biophys. 2011; 48:308–315.
- Barata K, Yoshida M, Hokao R, et al. Sequential alterations in clinical biochemical indicators of renal function in 5/6 nephrectomized rats–basic study for renal toxicity using 5/6 nephrectomized rats. J Toxicol Sci. 1998;23:433–442.
- Wang HY, Yang LZ, Gu CM, et al. Pathological changes, TGF-beta1 expression, and the effects of hepatocyte growth factor in 5/6 nephrectomized rats. Renal Fail. 2014;36:393–399. doi: 10.3109/0886022x.2013.867797.
- Reeves WB, Andreoli TE. Transforming growth factor beta contributes to progressive diabetic nephropathy. Proc Natl Acad Sci USA 2000;97:7667–7669.
- Han DC, Isono M, Hoffman BB, et al. High glucose stimulates proliferation and collagen type I synthesis in renal cortical fibroblasts: mediation by autocrine activation of TGF-beta. J Am Soc Nephrol 1999; 10:1891–1899.
- Sato F, Narita I, Goto S, et al. Transforming growth factor-beta1 gene polymorphism modifies the histological and clinical manifestations in Japanese patients with IgA nephropathy. Tissue Antigens. 2004;64:35–42. Jul doi: 10.1111/j.1399-0039.2004.00256.x.
- Rastaldi MP. Epithelial-mesenchymal transition and its implications for the development of renal tubulointerstitial fibrosis. J Nephrol. 2006; 19:407–412.
- Masszi A, Fan L, Rosivall L, et al. Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am J Pathol. 2004;165:1955–1967.
- Rekola S, Bergstrand A, Bucht H. Deterioration rate in hypertensive IgA nephropathy: comparison of a converting enzyme inhibitor and beta-blocking agents. Nephron 1991;59:57–60. doi: 10.1159/000186518.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int. 2000;57:1803–1817. doi: 10.1046/j.1523-1755.2000.00031.x.
- Coppo R, Amore A, Gianoglio B, et al. Angiotensin II local hyperreactivity in the progression of IgA nephropathy. Am J Kidney Dis. 1993;21:593–602.