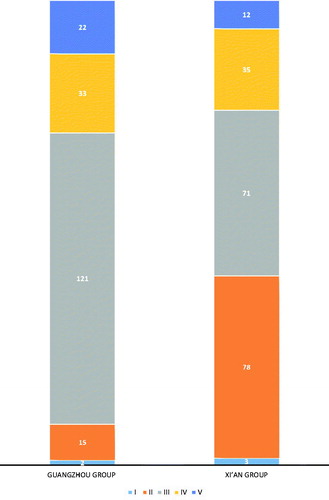Abstract
Objectives: The effects of regional characteristics of IgAN patients in different areas of China were investigated.
Methods: Patients who were identified to have primary IgAN by renal biopsy diagnosis were recruited both from Shaanxi province hospital of traditional Chinese medicine and Guangdong province hospital of traditional Chinese medicine. Besides renal histopathology data, a number of clinical and laboratory data were collected.
Results: It was shown that the frequency of the patients with no mucosal infection in the urinary tract was higher in the Guangzhou group, while the frequencies of upper respiratory tract and biliary infections were lower when compared with those in the Xi’an group. Serum uric acid, alexin C3, creatinine and serum cholesterol concentrations were increased in the Guangzhou group, while triglyceride, glomerular filtration rate, and urine red blood cell count level decreased. IgA + IgM + C3 and IgA + IgG + IgM + C3 were found in most patients of the Xi’an group, whereas IgA + C3, IgA + IgM + C3 and IgA were more frequent in the Guangzhou group.
Conclusion: It was found that differential environment, life habits and patterns in the two investigated areas obviously may influence the variable characteristics of IgAN patients.
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common glomerulonephritis and a principal cause of end-stage renal disease. The incidence of IgAN is known to have geographic and ethnic variations. For example, frequencies in some parts of Asia–Pacific and southern Europe are known to be higher, while the incidence is significantly lower among individuals of African descendants [Citation1]. Japan has the highest frequency of the disease in the world, where >30% of adults and >20% of children with chronic glomerulonephritis has the disease [Citation2]. However, the true incidence of this disease remains unknown because renal biopsies, which are a prerequisite for diagnosis, are not always collected [Citation3]. Multiple genes and environmental factors are known to influence the onset and progression of the disease [Citation4]. A number of studies have identified specific demographic populations, which are associated with an increased prevalence of IgAN [Citation5].
IgAN constitutes about 45.3–54.3% of all the primary focal segmental glomerulosclerosis (FSGS) cases diagnosed in China, and of these, 61.7–68.6% have been identified based on renal biopsies. The frequency of FSGS has been shown to vary across China [Citation6]. Indeed, China is a large country and significant regional variations would be expected. However, to date, there has been no study published, which specifically focuses on identifying correlations between clinical manifestations and physicochemical index with regional variation. Consequently, we have chosen to carry out a retrospective cohort study between two large Chinese cities, Xi’an located in the northern China, and Guangzhou located in the southern China. These two cities differ significantly in respect to their demographics, life style and environment.
Methods
Study subjects
The dataset, which includes clinical records from patients with biopsy-proven IgAN diagnosis from two regions of China, Guangzhou and Xi’an, was established. The study period covered time from January 2010 to December 2014. A total of 392 adult patients, who were diagnosed to have primary IgAN at Shaanxi province hospital of traditional Chinese medicine and Guangdong province hospital of traditional Chinese medicine during this period, were recruited. The subjects were enrolled in the IgAN study based on the following criteria: the results of their renal biopsies, changes in mesangial proliferation as judged by light microscopy (LM) examination, detection of electron-dense deposits in glomerular mesangial tissue using electron microscopy (EM) and detection of mesangial IgA and C3 deposits using immunofluorescence (IF) microscopy.
The exclusion criteria were as follows: the patients who suffered from lupus nephritis, allergic purpura, chronic advanced liver disease or other secondary IgANs were excluded. Patients who were under 18 years old were also excluded, as well as the patients with incomplete records and those who had undergone repeated biopsies.
This study was approved by the Ethics Committee of both Shaanxi province hospital of traditional Chinese medicine and Guangdong province hospital of traditional Chinese medicine. All of the participants provided written informed consent agreements. Baseline characteristics of all the patients were retrieved from the medical records at the time their renal biopsies were taken. The clinical data and renal biopsy pathology information were stored by using PASM 18.0 Statistics software. After input, the data were cross-checked carefully to ensure the consistence with the medical records. As the influence of age, gender, duration of disease and onset seasons might be confounding factors, the patients' age, gender, duration of disease and onset seasons in two groups were given the balance test to ensure the validity of the results.
The assessment of renal histopathology
Each renal biopsy specimen was routinely divided into three tissue sections. The first section was used for LM analysis, the second for IF analysis and the third for EM analysis. At least three experienced renal pathologists performed the histological evaluation of renal biopsy tissues. The histological classification of the renal biopsies was divided into five levels of severity, as previously described [Citation7].
The criteria upon which biopsies were classified was based on the analysis of IgA-stained mesangial immune deposits revealed by IF microscopy, LM analysis showing mesangial proliferation, and finally EM to confirm the presence of mesangial electron-dense deposits. The pathologists also evaluated renal histological features, such as the global glomerulosclerosis rate, the crescent formation rate, and the intensity of tubulointerstitial cell infiltration.
Pathology datasets
Patient baseline clinical and laboratory data were collected, including blood pressure, duration of disease (duration of symptoms before biopsy), hematuria, proteinuria, blood urea nitrogen, serum uric acid (UA), serum creatinine, triglyceride and alexin C3, glomerular filtration rate (GFR), serum cholesterol, urine erythrocyte, serum IgA level and 24-h urinary protein quantity. The patients with infection(s) during the week prior to onset of IgAN were tracked to rule out the possibility that other diseases triggered IgAN. The initial clinical presentation, such as gross hematuria, edema, hypertension, lumbago, debilitation, urinary irritation, change in urinary volume and abnormal urinalysis were also reviewed. EM was performed using the biopsy material to detect deposition of IgG, IgA, IgM and C3. We defined mesangial IgA with/without C3 deposition as ‘IgA nephropathy (IgAN)’. The clinical and laboratory findings at the time of biopsy were compared.
Statistical analysis
The measurement data are expressed as mean ± standard deviation (SD) or median. The count data are expressed as constituent ratios. The difference of measurement data between the two groups was compared with t-test or rank sum test. If the data differed from normal distribution, then the rank sum test was used. Chi-square test (Fisher’s exact test) was used to compare the count data. The SPSS 18.0 statistical software (version 18.0; Chicago, IL) was utilized to compare the two groups. p < .05 was considered to be statistically significant in all of the analyses.
Results
Effect of patient demographics
All of the patients in this study were Han Chinese. The ratio of male to female of IgAN patients in Guangzhou group was 1:1.35, while the ratio for Xi’an group was 1.07:1. When comparing the gender in two groups, the values of χ2= 1.235, p = .266 was obtained by using the chi-square test. The age of the patients ranged from 18 to 74 years old at the time when biopsies were taken. The mean age was 34.44 ± 10.82 in the Guangzhou group, whereas 35.05 ± 11.74 years in the Xi’an group. Therefore, there were no significant differences in the gender and the age between the two groups.
One-way analysis of variance indicated also that there were no significant differences in the duration of disease (p = .758) or the onset seasons (p = .258) between the two groups.
Comparison of the clinical and histological findings
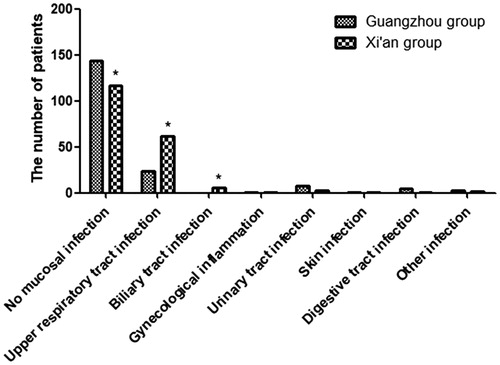
The frequency of the patients with no mucosal infection was higher in Guangzhou group, p < .001. Although many patients lacked mucosal infection, there were still 131 patients, who suffered from mucous membrane infection, such as upper respiratory infection (). On the other hand, the frequencies of upper respiratory tract and biliary infections were lower in Guangzhou group ( p < .001 and p = .030, respectively). There were no significant differences in the frequencies of urinary tract and digestive tract infections between the two groups.
Figure. 1. The ratio of mucosal infections for IgAN patients in two groups. No mucosal infection in the Xi’an group was lower than that in the Guangzhou group. Upper respiratory infection and biliary tract infection in the Xi’an group was higher than that in the Guangzhou group. *p < .05 versus control.
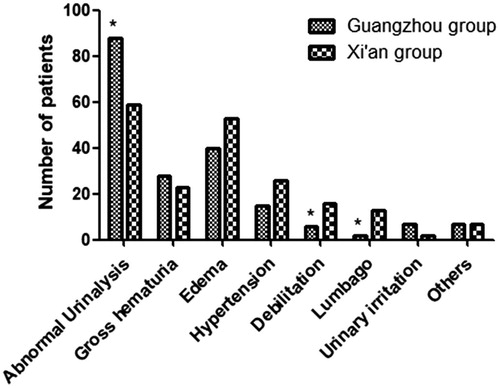
Abnormal urinalysis, accounting for 45.60%, was the most common initial presentation of IgAN in the patients of the Guangzhou group and significantly more common than in the Xi’an group. The frequency of abnormal urinalysis also had the highest incidence percentage in the IgAN patients of the Xi’an group. The frequencies of debilitation and lumbago were more frequent in the Xi’an group ().
Figure. 2. The clinical presentations of patients with IgAN in two groups. Abnormal urinalysis in the Xi’an group was lower than that in the Guangzhou group. Debilitation and lumbago in the Xi’an group was higher than that in the Guangzhou group. *p < .05 versus control.
As shown in , the serum UA, triglyceride, alexin C3, creatinine and cholesterol concentrations, GFR, and urine red blood cell count level between the two groups were statistically significant ( p < .05). The serum UA, alexin C3, creatinine concentrations and cholesterol levels were increased in the Guangzhou group, while triglyceride, GFR, and urine red blood cell count level decreased.
Table 1. The comparison of clinical characteristics between the two groups.
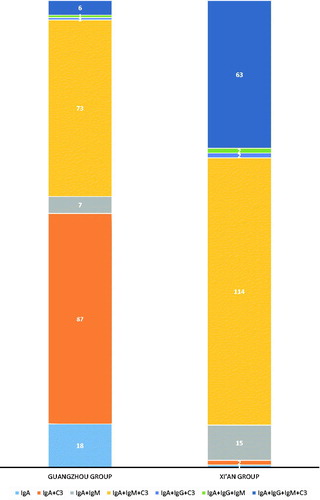
We also examined the IgA deposits between the two groups. At least three pathologists independently examined the glomerular tissue for mesangial IgG deposits in biopsies stained with IF techniques. The presence of immune complex depositions in the mesangial area and capillary wall was as follows: IgA; IgA + C3; IgA + IgM; IgA + IgM + C3; IgA + IgG + C3; IgA + IgG + IgM; and IgA + IgG + IgM + C3. In the Xi’an group, codepositions of IgA + IgM + C3 and IgA + IgG + IgM + C3 were found to be 57.3% and 31.7%, respectively. In the Guangzhou group, the patterns of immune complexes for IgA + C3, IgA + IgM + C3 and IgA were 45.5%, 38.2% and 8.9%, respectively ().
Figure. 3. The ratio of immune fluorescence components in two groups. The percentage of immune complex depositions in the mesangial area and capillary wall is shown. The number of the patients with the immune complex deposition was also shown.
All biopsies were assigned into a histological subclass (I–V) according to Lee’s classification [Citation7]. All specimens were examined and graded by three pathologists independently. Distribution into the 5 different histological grades at the time of renal biopsy was the following: grade I, 5 patients (1.28%); grade II, 93 patients (23.72%); grade III, 192 patients (48.98%); grade IV, 68 patients (17.35%); and grade V, 34 patients (8.67%). There was a significant difference between the two groups for renal lesions at renal biopsy (p < .001) (). Grade II was more common in the Xi’an group, while grade III was more common in the Guangzhou group.
Discussion
The etiology and pathogenesis of IgAN are not entirely clear, both genetic and environmental factors are thought to play an important role in IgAN pathogenesis.
The URI was a common finding in IgAN patients. The annual average temperature of Guangzhou is 20.0–22.0 °C, the annual precipitation approximately 1720 mm, and average relative humidity 77%, whereas in Xi’an they are 13.0–13.7 °C, 561 mm and 69.6%, respectively (data from China Meteorological Administration). The weather in Guangzhou is warm, humid and rainy, so the ratios of urinary tract and gastrointestinal infections are higher in the southern area [Citation8]. This study showed that the ratio of URI was much higher in the Guangzhou group than in the Xi’an one.
IgAN is often accompanied with mucous membrane infection, of which the majority is URI. Some studies have shown that the total polycyclic aromatic hydrocarbons (PAHs) ranged from 3.35 to 80.45 ng/m3 air, being highest in Beijing, followed by Xi’an, Xiamen, and Guangzhou. In a cell culture study, transcript levels of pro-inflammatory cytokine interleukin-6 (IL-6) were found to be induced in the treatment. This can be hypothesized to explain why the rates of URI and biliary infection in the Guangzhou group were lower than those in the Xi’an group, and the percentage of infection-free mucosa was higher than that in the Xi’an group [Citation9]. Xi’an is relatively dry and cold, then the ratio of the URI was higher than that in Guangzhou. Besides, diet and living habits of people from different areas are different. People living in the northern cold area might like eating spicy food, hot drinks (coffee) and roasted food, so the ratio of biliary infection in Xi’an area is higher [Citation10].
One study has found that sleeping disorders were more common in the patients, and 65.0% (39/60) of them were from Xi'an. It was higher than in the Guangzhou group (29.0%, 9/31, p = .001) [Citation11]. So we infer that is why the ratio of debilitation and lumbago in the Xi’an group was significantly higher. Many IgAN patients, especially those with gross hematuria and/or proteinuria, are detected on routine urine screening [Citation12]. Therefore, early health physical examination is very important for the diagnosis and treatment of the disease. Most clinical symptoms in patients from the Xi'an area were more serious, so blood pressure monitoring and the reason of edema should be paid good attention in Xi'an area.
Many studies have shown that serum UA, proteinuria, elevated serum creatinine, impairment of renal function, and a severe histological grade are associated with increased risk of progression in IgAN patients [Citation13–17]. In this study, serum UA, serum alexin C3, serum creatinine, serum cholesterol and decreased GFR were more common in patients of the Guangzhou group. These findings indicate that IgAN patients in the Guangzhou group were more likely to have a more serious course compared with patients in the Xi’an group. This conclusion was also supported by histological assessment. Although a similar proportion of grade I and grade IV–V histological lesions occurred in both groups, the frequency of grade II was significantly higher in patients of the Xi’an group, whereas the frequency of grade III was significantly higher in patients of the Guangzhou group. The hyperuricemia and complement activation are independent risk factors of IgAN progression, and it can be speculated that life style predisposing to hyperuricemia may modify the pathogenesis and progression of IgAN [Citation18].
Contrary to the above parameters, the degree of urine erythrocyte was more severe in patients of the Xi’an group. Hematuria seemed to correlate with a benign clinical course. Similar findings have been shown in several studies [Citation19,Citation20], but the mechanism is unknown.
IgA deposition alone or accompanied only by C3 was common in patients of the Guangzhou group, while the co-deposition of IgA, IgG and IgM two or more of C3 was common in patients of the Xi'an group. It has been reported that serum creatinine was significantly elevated in the IgA deposition group [Citation21]. IgA deposition has also been shown to have a significant positive association with endocapillary proliferation and segmental glomerulosclerosis variables of Oxford classification [Citation22].
As shown in another study, IgG deposition in the normal renal function group was higher than that of renal insufficiency group (p < .01). The high serum IgA level and serious kidney pathology change were related to poor renal survival [Citation23]. Therefore, codeposition of several immunoglobulin complexes might be beneficial to the patients from Xi’an, although this conclusion warrants further research.
Conclusions
The urinary tract infection should be paid more attention among the patients from Guangzhou, while the patients in the Xi’an group need to pay more attention to upper respiratory tract and biliary tract infection. The patients in the Xi’an group also need to take regular health physical examination. Our findings suggest that urine analysis is important for the timely discovery of IgAN. The climate characteristics, living habits, diet and lifestyle factors may have influence on IgAN.
Acknowledgments
Editorial help of Prof Mikko Lammi at the University of Umeå is acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wada Y, Ogata H, Takeshige Y, et al. Clinical significance of IgG deposition in the glomerular mesangial area in patients with IgA nephropathy. Clin Exp Nephrol. 2013;17:73–82.
- Saka S, Hirawa N, Oka A, et al. Genome-wide association study of IgA nephropathy using 23 465 microsatellite markers in a Japanese population. J Hum Genet. 2015;60:573–580.
- Hsu S, Ramirez SB, Winn MP, et al. Evidence for genetic factors in the development and progression of IgA nephropathy. Kidney Int. 2000;57:1818–1835.
- Kiryluk K, Li Y, Scolari F, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196.
- Wyatt RJ, Julian BA, Baehler RW, et al. Epidemiology of IgA nephropathy in central and eastern Kentucky for the period 1975 through 1994. Central Kentucky Region of the Southeastern United States IgA Nephropathy DATABANK Project. J Am Soc Nephrol. 1998;9:853–858.
- Xie J, Chen N. Primary glomerulonephritis in mainland china: an overview. Contrib Nephrol. 2013;181:1–11.
- Manno C, Strippoli GFM, D Altri C, et al. A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. Am J Kidney Dis. 2007;49:763–775.
- Liu L, Liu X, Wang T, et al. The study of the constitution, mucosal inflammation, chinese medicine syndrome types and clinical pathology in IgA nephropathy. Int J Clin Exp Patho. 2016;9:9641–9645.
- Leung PY, Wan HT, Billah MB, et al. Chemical and biological characterization of air particulate matter 2.5, collected from five cities in China (vol 194, pg 188, 2014). Environ Pollut. 2014;194:188.
- Hu H, Li P, Lin M. The Chinese medicine constitution and life style of physical examinees in Guangzhou and Beijing. Chinese Gen Pract. 2015;18:3852–3855.
- Zhu L, Fang X, Liu S, et al. Multi-centered stratified clinical studies for psychological and sleeping status in patients with chronic constipation in China. Zhonghua Yi Xue Za Zhi 2012;92:2243–2246.
- Shen P, He L, Huang D. Clinical course and prognostic factors of clinical early IgA nephropathy). Neth J Med. 2008;66:242.
- Nagasawa Y, Yamamoto R, Shoji T, et al. Serum uric acid level predicts progression of IgA nephropathy in females but not in males. PLoS One. 2016;11(8):e0160828.
- Rafalska A, Franczuk J, Franczuk P, et al. Stratifying risk for progression in IgA nephropathy: How to predict the future? Pol Arch Med Wewn. 2014;124:365–372.
- Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5:425–430.
- Xu L, Yang H, Hao C, et al. Podocyte number predicts progression of proteinuria in IgA nephropathy. Mod Pathol. 2010;23:1241–1250.
- Song YS, Kim JE, Park JE, et al., Is it possible to predict the progression rate in Korean IgA nephropathy patient? Kidney Res Clin Pract. 2006;25:35–44.
- Caliskan Y, Ozluk Y, Celik D, et al. The clinical significance of uric acid and complement activation in the progression of iga nephropathy. Kidney Blood Press Res. 2016;41:148–157.
- Kusano T, Takano H, Kang D, et al. Endothelial cell injury in acute and chronic glomerular lesions in patients with IgA nephropathy. Hum Pathol. 2016;49:135–144.
- Deng W, Zhou Q, Liu W, et al. Impact of gender on the clinicopathological features of patients with primary IgA nephropathy. Chinese J Nephrol. 2015;31:561–566.
- Moriyama T, Shimizu A, Takei T, et al. Characteristics of immunoglobulin a nephropathy with mesangial immunoglobulin G and immunoglobulin M deposition. Nephrology. 2010;15:47–754.
- Nasri H, Sajjadieh S, Mardani S, et al. Correlation of immunostaining findings with demographic data and variables of Oxford classification in IgA nephropathy. J Nephropathol. 2013;2:190–195.
- Xue C, Liao Y. A study on risk factors of chronic renal failure in IgA nephropathy. Chinese J Pract Int Med. 2007;27:438–440.