Abstract
Background: Acute kidney injury (AKI) is a common complication after surgery. Because of unpredictable and variable age-dependent physical decline, the incidence, risk factor of postsurgical AKI and the predictive power of estimated glomerular filtration rate prior to surgery (eGFRpreSurg) has not been fully elucidated in very elderly patients.
Methods: All discharged patients aged ≥80 years without chronic kidney disease who underwent surgery prior to intensive care unit (ICU) admission from 2017 through 2018 were included. Clinical, biological and surgical data were recorded. Mean of outpatient creatinine values from the year prior to ICU admission was used as the baseline value to determine the occurrence of AKI. Postoperative AKI was diagnosed according to Kidney Disease Improving Global Outcomes criteria.
Results: Among 243 very elderly postoperative patients admitted during the study period, 48 had AKI during their ICU stay. The occurrence of postsurgical AKI was associated with longer ventilation times (p < .001) and higher mortality (p < .001). The eGFRpreSurg, which is calculated based on the Modification of Diet in Renal Disease study equation, was a risk factor for postoperative AKI (OR = 2.662, p = .010). The incidence of postoperative AKI was significantly higher among patients with lower eGFRpreSurg than among those with an eGFRpreSurg ≥ 70 mL/min/1.73 m2 (p = .003).
Conclusion: Postsurgical AKI in very elderly patients has a high incidence and is a risk factor for mortality. Our study confirmed that eGFRpreSurg could be used as an index for AKI risk stratification.
Introduction
Postoperative acute kidney injury (AKI) is a common and serious complication that is related to several adverse outcomes in critically ill patients [Citation1]. The reported incidence of AKI after surgery is as high as 20%, depending on the patient population [Citation1,Citation2]. Patients who develop AKI after surgery often need continuous renal replacement therapy, which prolongs intensive care unit (ICU) stay and can exacerbate long-term morbidity [Citation3]. Despite recent improvements in diagnosis and therapy, the mortality associated with AKI remains high, and those who survive have the additional burden of chronic kidney disease (CKD) [Citation4].
One of the big challenges in preventing postsurgical AKI is to identify patients with high risk. Although the very elderly population is widely accepted to be more prone to AKI than younger populations either due to kidney senility or because of the high prevalence of comorbidities present, the exact incidence of postsurgical AKI among those patients is unclear [Citation5–7]. Meanwhile, determining which patients will develop AKI after surgery remains very challenging [Citation8]. New biomarkers are widely used in the clinical setting to monitor the occurrence and progress of postsurgical AKI. For example, neutrophil gelatinase-associated lipocalin, which is expressed by renal tubular cells as the earliest induced proteins after ischemic AKI, has been shown to be an early predictor of AKI [Citation9]. However, limited data have been obtained on the use of these biomarkers in elderly populations, which have unique characteristics and poor representation in clinical trials [Citation10]. In addition, these new biomarkers are not always available in developing countries.
The estimated glomerular filtration rate (eGFR) is widely used to detect CKD because of the difficulty of measuring the actual glomerular filtration rate. In surgical populations, the eGFR is thought to reflect preoperative renal function and is used to guide postoperative fluid management to prevent postoperative AKI [Citation11]. But, the rate of decline of eGFR with age varies widely among individuals, which may restrict the use of this parameter. As the elderly population continues to grow at an unprecedented rate worldwide, appropriate care for the elderly has attracted increasing attention. The usefulness of eGFR in predicting postsurgical AKI has not been fully elucidated; the aim of this study was to investigate the incidence of postsurgical AKI and the clinical utility of eGFR prior to surgery (eGFRpreSurg) for AKI prediction in very elderly patients.
Materials and methods
Setting
This single-center retrospective study was conducted in the Department of Critical Care Medicine, West China Hospital, Sichuan, China. The clinical research ethics boards of the West China Hospital approved the study and waived the need for participants’ informed consent because of the study’s retrospective, anonymous, and non-interventional nature (ethic committee's approval number: 2018-S-87). All methods were performed in accordance with the relevant guidelines and regulations.
Study population
All records of patients aged ≥80 years at admission who have undergone a surgery of any type and discharged from the ICU during the observation period (from 1 January 2017 to 31 December 2018) were considered for study inclusion. The following exclusion criteria were used: (a) non-postsurgical ICU admission, (b) ICU stay <24 h, (c) insufficient data for analysis, (d) occurrence of AKI prior to surgery and e) established of CKD before ICU admission. CKD was defined as a clear diagnosis prior to ICU admission, from both previous inpatient and outpatient records. Records of patients readmitted to the ICU during the observation period were discarded and only the records of the first ICU visit were kept.
The the primary aim of the study is to assess the association between eGFRpreSurg and post-op AKI. A twostep strategy is adapted in our study. We first calculate eGFR with four equations: the Modification of Diet in Renal Disease (MDRD) study equation, the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation, the CKD-EPI Cys-C equation and the CKD-EPI creatinine and Cys-C equation [Citation12,Citation13]. The eGFR from equation with the highest AUC in predicting the occurrence of AKI was used to reflect eGFRpreSurg to do further analysis. The secondary aim is to access the association between AKI events and mortality.
According to occurrence of AKI or not, we divided our cohort into AKI group and non-AKI group. AKI diagnosis and severity stage were based on serum creatinine, in accordance with the Kidney Disease Improving Global Outcomes (KDIGO) practice guidelines [Citation14]. In simple terms, mean of outpatient values from the year prior to admission were used as baseline values; AKI was defined as an increase to at least 1.5 times baseline within 7 days after surgery or an increase of ≥0.3 mg/dL in serum creatinine from baseline within 48 h after surgery. The staging system for evaluating the severity of AKI was also in accordance with KDIGO practice guidelines: stage I, serum creatinine increase to 1.5–1.9 times baseline within 7 days after surgery or ≥0.3 mg/dL increase from baseline within 48 h after surgery; stage II, serum creatinine increase to 2.0–2.9 times baseline within 7 days after surgery; and stage III, serum creatinine increase to at least 3.0 times baseline within 7 days after surgery, ≥4 mg/dL increase from baseline within 48 h after surgery or initiation of renal replacement therapy during the postsurgical ICU stay. The eGFRpreSurg was calculated based the last time laboratory test before surgery.
Data collection
The following information was obtained from the hospital information system: underlying medical conditions, type of surgery and results of all biochemical testing. Biochemical testing included red blood cell count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red blood cell distribution width, platelet count, white blood cell count, international normalized ratio, prothrombin time, activated partial thromboplastin time, fibrin and fibrinogen degradation products, fibrinogen, thrombin time, arterial oxygen partial pressure, arterial carbon dioxide partial pressure, base excess, anion gap, lactate, procalcitonin, total bilirubin, direct bilirubin, indirect bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total protein, albumin, creatinine, glucose, creatine kinase, lactate dehydrogenase, Cys-C, gamma-glutamyl transferase, triglycerides, cholesterol, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol. Coagulated and non-coagulated blood samples were collected after ICU admission. Underlying medical conditions were confirmed according to the codes of the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) from the inpatient and outpatient hospital information system. Additional information obtained from the critical care information system included: sex, age, date of ICU admission, date of ICU discharge, patient admission type, and the Acute Physiology and Chronic Health Evaluation (APACHE) II score on the day of ICU admission. Of notes, intraoperative hypotension was defined as usage of dopamine or noradrenaline or both during operation. Data were collected and integrated with Excel (Microsoft Corporation, Redmond, WA, USA), and were screened and monitored by two dedicated intensive care specialists for any missing information and to ensure data accuracy.
Statistical analyses
All statistical analyses were performed with SPSS Statistics 17 (SPSS Software, Chicago, IL, USA), GraphPad Prism (GraphPad Software, San Diego, CA, USA) and MedCalc statistical program package (MedCalc Software, Mariakerke, Belgium). The Kruskal–Wallis and/or Mann–Whitney U-test were used for comparisons of numerical variables between groups; the chi-square test was used for categorical variables. To identify the sensitivity and specificity of cutoff values in predicting AKI after surgery, receiver operator characteristic curve analysis was performed. The eGFRpreSurg was first used as a categorical variable to plot the sensitivity and specificity for identifying risk of AKI and the value as a categorical variable which has a maximize Youden’s index among those would be selected as the cutoff. The logistic analysis was used to analyze the correlation between eGFRpreSurg levels and the occurrence of AKI by forward conditional selection. Covariates were chosen for Cox proportional hazards regression models with 95% confidence intervals with regards to mortality. A two-sided p values <.05 was considered statistically significant.
Results
Baseline characteristics of study participants
From 1 January 2017 to 31 December 2018, 668 very elderly patients were admitted to the ICU without a specific history of CKD. After excluding rehospitalisation records, those with insufficient data and those with established AKI before ICU admission, our analysis included 243 postoperative patients with an ICU stay longer than 24 h (, Supplementary Material 1). The average age in the study cohort was 85.64 years and the average APACHE II score was 18.50 (). Most patients had undergone elective surgery, most commonly abdominal surgery. Hypertension was the most common underlying medical condition. The in-hospital mortality rate for the entire cohort was 7.41%. The mean eGFRpreSurg calculated with the four methods listed above were 91.23, 78.78, 85.36 and 72.07, respectively.
Figure 1. Flowchart of patients included in the study. The very elderly patients without chronic kidney disease discharged from ICU were scanned for occurrence of AKI according to the KDIGO definition. After excluding patients with re-admission, insufficient data, ICU stay <24 h and established AKI before surgery, 243 patients were enrolled for further analysis. ICU: intensive care unit; KDIGO: Kidney Disease Improving Global Outcomes.
Table 1. Characteristics of AKI patients and non-AKI patients.
Association between eGFRpreSurg and postsurgical AKI
According to our definition, 48 patients experienced AKI while hospitalized in the ICU and 195 patients did not (). Among non-cardiac surgery patients, the incidence of postoperative AKI was 16.89% (Supplementary Material 2). The eGFR-MDRD was used to reflect eGFRpreSurg in order to distinguish patients with potential risk of AKI because it had the highest AUC in predicting the occurrence of AKI (AUC: 0.703, supplementary material 3). We then conducted sensitivity analysis to determine whether eGFRpreSurg was associated with a sharp increase in AKI risk. Although we saw a significant association between decreasing eGFRpreSurg and the increasing rate of postsurgical AKI, using eGFRpreSurg as a categorical variable and plotting the sensitivity and specificity for identifying increased risk of AKI revealed no high specificity or sensitivity cutoff values for predicting the occurrence of AKI (Supplementary Material 4). We chose to classify patients according to a threshold of 70 mL/min/1.73 m2 which has a maximize Youden’s index among those categorical variables. The incidence of postoperative AKI was significantly higher among patients with lower eGFRpreSurg than among those with an eGFRpreSurg ≥70 mL/min/1.73 m2 (, p < .05). However, no difference was observed between the groups regarding AKI stage. In addition, eGFR calculated with other methods using the same cutoff may also predict the occurrence of AKI (). Logistic analysis showed that eGFRpreSurg was a risk factor for postoperative AKI (, OR = 2.662, p = .010).
Table 2. Characteristics of patients based on eGFRpreSurg stratification using cutoff 70 mL/min/1.73m2.
Table 3. Comparing AKI rate of patient cohort based on varied eGFR methods.
Table 4. Multivariable logistical analysis of eGFR and other covariates associated with AKI events.
Association between AKI events and mortality
In the entire patient cohort, the APACHE II score was significantly higher in the AKI group than in the non-AKI group (p < .001, ), along with other variables (Supplementary material 5). shows the trends of creatinine, Cys-C and urea levels over time after ICU admission. The occurrence of AKI in very elderly patients after surgery was associated with longer ventilation times and higher mortality, regardless of the surgery type (, Supplementary Material 2, p < .001). The characteristics of the study patients stratified according to KDIGO AKI stage are shown in Supplementary material 6 and we saw a significant association between increasing AKI stage and 28-day mortality (p < .05 for all models, ) after adjusting for age and other covariates.
Figure 2. Plasma Crea, Cys-c and Urea values stratified according to AKI occurrence and AKI stage. Patients who experienced AKI during ICU stay had a significantly higher level in Crea, Cys-C and Urea ad admission and during the hospitalization. Crea, creatinine; Cys-c, Cystatin C. * indicated p values <.05 between groups.
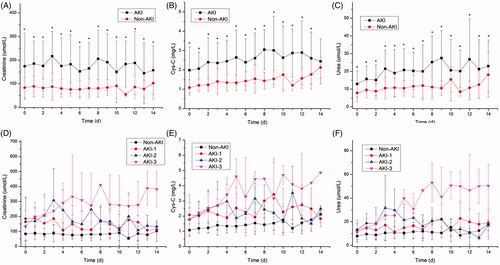
Table 5. Outcomes of patients stratifying based on KDIGO AKI stage using cox proportional hazards regression models.
Discussion
In this study, we observed that postoperative AKI is common after surgery in very elderly patients without CKD. The occurrence of AKI after surgery was associated with longer ventilation times and higher mortality. A lower eGFRpreSurg was related to increased risk of postoperative AKI. However, the level of eGFRpreSurg was not useful in predicting the severity of postsurgical AKI. Although the eGFR-MDRD and CKD-EPI equations have been shown to poorly estimate GFR in patients with muscle loss and resulting decreased creatinine levels [Citation15], our study confirmed that eGFRpreSurg could be an index for risk stratification in very elderly patients before surgery. To our knowledge, this is the first study to investigate the AKI incidence and validate the role of eGFRpreSurg in predicting postsurgical AKI among very elderly patients.
AKI is a common complication of surgery. Previous studies have demonstrated that almost 18% of patients undergoing cardiac surgery experience at least one episode of AKI; approximately 6% require haemodialysis [Citation16,Citation17]. In non-cardiac surgery, the incidence of postsurgical AKI is relatively lower and most cases are mild [Citation18]. In our study, 19.75% of patients experienced postoperative AKI, which is higher than the rates reported in other studies. In subgroup analysis, the incidence of AKI among non-cardiac surgical patients was as high as 16.89%. These data confirm that very elderly patients are prone to postsurgical AKI. There are several possible reasons for the relatively high incidence of AKI among very elderly patients. Although none of the patients in our study had CKD, they had many comorbidities over their lifetimes. Specific structural, functional and molecular changes in the kidneys over time reduce kidney reserves and increase susceptibility to serious injury in the face of insults. Apart from surgical procedures and perioperative management, the physical decline in kidney function increases the susceptibility of very elderly patients to AKI.
Postoperative AKI, along with infection and bleeding, is known to be associated with mortality and morbidity after surgery [Citation19]. In addition, postoperative ICU length of stay and total hospital stay are significantly longer in patients who develop AKI following cardiac surgery [Citation20]. Results from our study confirm that these associations also exist among very elderly postsurgical patients, regardless of the type of surgery. In addition, trend analysis indicated a significant association between increasing AKI stage and 28-day mortality. Considering the high incidence of postsurgical AKI, the relationship between postoperative AKI and mortality and the aging trend globally, more studies on this subject are urgently needed.
Mathematically estimated GFR, or eGFR, is derived from a patient’s serum creatinine or Cys-C level, age, sex and race. The MDRD equation and the CKD-EPI equation are the most widely used traceability equations for the estimation of GFR in patients 18 years of age and older [Citation21]. Although the eGFR-MDRD equation underestimates high eGFR levels and the CKD-EPI equation is less accurate for patients with extreme muscle mass, both equations are widely used in clinical practice. Traditionally, the eGFR equation is only suitable for evaluating CKD and not AKI [Citation22]. Besides, eGFR is not always sufficiently accurate for clinical decision making in very elderly patients because muscle mass and protein intake, which affect eGFR, are often abnormal in this population [Citation23,Citation24]. Indeed, both equations have been shown to provide risk stratification for AKI after surgery [Citation25]. Our results indicate that although serum creatinine is unstable and eGFR may significantly change within days in very elderly patients, current eGFR equations remain useful for AKI risk stratification.
Using eGFR as a categorical variable, we found significantly differences in eGFRpreSurg between patients with versus without postsurgical AKI, regardless of surgery type. In addition, in non-cardiac surgical subgroup analysis, eGFRpreSurg was lower among patients who experienced postsurgical AKI. This suggests a closer association between preoperative conditions and postsurgical AKI in non-cardiac surgery, whereas surgical procedure and perioperative management are more important in avoiding postsurgical AKI in cardiac surgery patients.
We used 70 mL/min/1.73 m2 as the cutoff to verify whether eGFRpreSurg was closely associated with the development of postsurgical AKI. In some previous literatures, 60 mL/min/1.73 m2 is chosen as a cutoff point but in our study we choose the cutoff value 70 mL/min/1.73 m2 instead the usual 60 mL/min/1.73 m2 of for the following three reasons: 1) it has the highest Youden’s index among all categorized eGFRpreSurg in our study, especially compared with 60 mL/min/1.73 m2; 2) using an irregular number which has the highest Youden’s index considering using 65.47 mL/min/1.73 m2 treating eGFRpreSurg as continues variables is not conducive for clinical use; 3) the cut off 70 mL/min/1.73 m2 although may overestimate risk compared with eGFRpreSurg as continues variables a little bit, it is acceptable since high mortality and extended ICU stay in patients who develop AKI [Citation26]. Our study demonstrated that patients whose eGFRpreSurg was below 70 mL/min/1.73 m2 were more likely than other patients to experience postsurgical AKI. Logistic analysis indicates that this association existed independent of underlying disease or surgery type. Results from our study indicate that eGFRpreSurg could be an index for assessing renal function in very elderly patients. Using a cutoff of 70 mL/min/1.73 m2 allows identification of patients with a high risk of postsurgical AKI. However, we also found that eGFRpreSurg was not an index for predicting the severity of postsurgical AKI, suggesting that additional factors are involved in the deterioration of AKI.
We evaluated the performance of the eGFR-MDRD equation and the CKD-EPI equation based on creatinine or Cys-C or both. The eGFR-MDRD had the highest AUC in predicting the occurrence of AKI. However, the CKD-EPI equation was also able to identify patients at high risk of AKI. Although, it has been suggested that serum Cys-C has better accuracy in predicting postsurgical AKI in adult cardiac surgery patients [Citation27], our study demonstrated that eGFR-MDRD was more suitable in very elderly patients, not only from the predictive value but also from the costs. The recent introduction of novel biomarkers of AKI is resulting in a gradual replacement of serum creatinine in diagnosing AKI before elevated creatinine level can be detected several days after kidney injury [Citation28]. Whether the new indicators are applicable in very elderly patients also needs to be verified in further studies, especially the costs regarding those new biomarkers.
Although body mass index, which reflects nutritional status, was not available for all the patients in our study, postoperative serum total protein and albumin were comparable in AKI and non-AKI patients, indicating that differences in eGFRpreSurg were not related to nutritional status. In addition, all patients enrolled in our study underwent surgery at a single center; most procedure-related factors as well as postoperative management parameters were likely controlled appropriately.
This study has limitations. First, although all patients who underwent surgery during the study period were included in the analysis to minimize selection bias, patient selection was inevitable because of the study’s retrospective design. The lack of follow-up data prevents our analysis of out-of-hospital outcomes. Our study did not use urine output to determine AKI because urine volume data were not available in our hospital information system for most patients. This may have resulted in underestimation of the occurrence of AKI and caused a lack of increasing trends in AKI severity grades. We enrolled patients without CKD to avoid the bias from the inclusion of end-stage renal failure patients, according to both previous inpatient and outpatient records, some undiagnosed CKD patients have potential to be enrolled since CKD is not defined by previous eGFR or albuminuria data which is not available in our dataset. Although the eGFR calculated among very elderly patients without CKD in our study is comparable to other study [Citation29], it still may have introduced selection bias, making our conclusions not universally applicable. We did not evaluate interactions between preoperative kidney function and intraoperative management. We did not calculate creatinine clearance with the Cockcroft–Gault equation because we did not have body weight for most patients. We compared the rate of renal replacement therapy between AKI and non-AKI patients, but we did not consider the reason for renal replacement therapy.
In conclusion, we found an increased risk of postoperative AKI in very elderly postsurgical patients and an association between AKI and mortality. Although eGFR is not an accurate reflection of renal function among very elderly patients, it can help in assessing the risk of postoperative AKI in very elderly patients. Regarding all the potential Equations which have been used for evaluation, the eGFR-MDRD has the best predictive value and may be considered to use in daily practice.
Author contributions
QW and YK designed the whole study. HY and HB supervised the whole project and performed data analysis. HY, MF, XZ, GL and XJ supervised patient diagnosis and recruitment. QW, YX and ZH conducted data analyses and drafted the manuscript. HY and ZZ participated in the manuscript writing and contributed to protocol version development, on which this paper is based. All authors critically reviewed the article and approved the final manuscript.
Ethics approval and consent to participate
The clinical research ethics boards of the West China Hospital approved the study and waived the need for participants’ informed consent because of the study’s retrospective, anonymous, and non-interventional nature (ethic committee's approval number: 2018-S-87). All methods were performed in accordance with the relevant guidelines and regulations.
| Abbreviations | ||
| AKI | = | Acute kidney injury |
| eGFRpreSurg | = | estimated glomerular filtration rate prior to surgery |
| CKD | = | chronic kidney disease |
| eGFR | = | estimated glomerular filtration rate |
| Cys-C | = | cystatin C |
| KDIGO | = | Kidney Disease Improving Global Outcomes |
| ICD-10-CM | = | international Classification of Diseases, 10th Revision, Clinical Modification |
| APACHE | = | the Acute Physiology and Chronic Health Evaluation |
| MDRD | = | the Modification of Diet in Renal Disease |
| CKD-EPI | = | the CKD Epidemiology Collaboration |
Supplemental Material
Download PDF (337 KB)Acknowledgements
We thank Shuya Zhang and Yuan Li for their help in coordination of team work. We thank Rebecca Tollefson, DVM, from Liwen Bianji, Edanz Editing China, for editing the English text of a draft of this manuscript.
Disclosure statement
The authors declare that they have no competing interests.
Availability of data and material
The datasets generated and analyzed in this article are not publicly available due to health privacy concerns. However, they are available from the corresponding author and will be obtainable by the public when the database construction is complete.
Additional information
Funding
References
- Park JT. Postoperative acute kidney injury. Korean J Anesthesiol. 2017;70(3):258–266.
- Malhotra R, Kashani KB, Macedo E, et al. A risk prediction score for acute kidney injury in the intensive care unit. Nephrol Dial Transplant. 2017;32(5):814–822.
- Wen J, Cheng Q, Zhao J, et al. Hospital-acquired acute kidney injury in Chinese very elderly persons. J Nephrol. 2013;26(3):572–579.
- Venkatachalam MA, Weinberg JM, Kriz W, et al. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26(8):1765–1776.
- Lagny MG, Jouret F, Koch JN, et al. Incidence and outcomes of acute kidney injury after cardiac surgery using either criteria of the RIFLE classification. BMC Nephrol. 2015;16(1):76.
- da Rocha EP, Yokota LG, Sampaio BM, et al. Urinary neutrophil gelatinase-associated lipocalin is excellent predictor of acute kidney injury in septic elderly patients. Aging Dis. 2018;9(2):182–191.
- Yokota LG, Sampaio BM, Rocha EP, et al. Acute kidney injury in elderly patients: narrative review on incidence, risk factors, and mortality. Int J Nephrol Renovasc Dis. 2018;11:217–224.
- Gong Y, Zhang F, Ding F, et al. Elderly patients with acute kidney injury (AKI): clinical features and risk factors for mortality. Arch Gerontol Geriatr. 2012;54(2):e47–51.
- Gaipov A, Solak Y, Turkmen K, et al. Serum uric acid may predict development of progressive acute kidney injury after open heart surgery. Ren Fail. 2015;37(1):96–102.
- Shenoy P, Harugeri A. Elderly patients' participation in clinical trials. Perspect Clin Res. 2015;6(4):184–189.
- Shimomura A, Obi Y, Fazl Alizadeh R, et al. Association of pre-operative estimated GFR on post-operative pulmonary complications in laparoscopic surgeries. Sci Rep. 2017;7(1):6504.
- Fan L, Levey AS, Gudnason V, et al. Comparing GFR estimating equations using cystatin C and creatinine in elderly individuals. J Am Soc Nephrol. 2015;26(8):1982–1989.
- Matsushita K, Selvin E, Bash LD, et al. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55(4):648–659.
- Fujii T, Uchino S, Takinami M, et al. Validation of the kidney disease improving global outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin J Am Soc Nephrol. 2014;9(5):848–854.
- Bragadottir G, Redfors B, Ricksten SE. Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury–true GFR versus urinary creatinine clearance and estimating equations. Crit Care. 2013;17(3):R108.
- Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10(3):500–514.
- Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13(11):697–711.
- O'Connor ME, Hewson RW, Kirwan CJ, et al. Acute kidney injury and mortality 1 year after major non-cardiac surgery. Br J Surg. 2017;104(7):868–876.
- Deng Y, Yuan J, Chi R, et al. The incidence, risk factors and outcomes of postoperative acute kidney injury in neurosurgical critically ill patients. Sci Rep. 2017;7(1):4245.
- Jayaraman R, Sunder S, Sathi S, et al. Post cardiac surgery acute kidney injury: a woebegone status rejuvenated by the novel biomarkers. Nephrourol Mon. 2014;6(4):e19598.
- Inal BB, Oguz O, Emre T, et al. Evaluation of MDRD, Cockcroft-Gault, and CKD-EPI formulas in the estimated glomerular filtration rate. Clin Lab. 2014;60(10):1685–1694.
- Masson I, Flamant M, Maillard N, et al. MDRD versus CKD-EPI equation to estimate glomerular filtration rate in kidney transplant recipients. Transplantation. 2013;95(10):1211–1217.
- Martensson J, Bellomo R. Perioperative renal failure in elderly patients. Curr Opin Anaesthesiol. 2015;28(2):123–130.
- Maltais F, Decramer M, Casaburi R, et al. An Official American Thoracic Society/European Respiratory Society Statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–e62.
- Tarantini L, Barbati G, Cioffi G, et al. Clinical implications of the CKD epidemiology collaboration (CKD-EPI) equation compared with the modification of diet in renal disease (MDRD) study equation for the estimation of renal dysfunction in patients with cardiovascular disease. Intern Emerg Med. 2015;10(8):955–963.
- Bell S, Dekker FW, Vadiveloo T, et al. Risk of postoperative acute kidney injury in patients undergoing orthopaedic surgery–development and validation of a risk score and effect of acute kidney injury on survival: observational cohort study. BMJ. 2015;351(19):h5639.
- Shlipak MG, Coca SG, Wang Z, et al. Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis. 2011;58(3):366–373.
- Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10(1):147–155.
- Wetzels JF, Kiemeney LA, Swinkels DW, et al. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007;72(5):632–637.
