Abstract
To evaluate the relationship between the aryl hydrocarbon receptor (AHR) rs2066853 gene polymorphism and the risk of male infertility. PubMed, Embase, Web of Science, and Chinese National Knowledge Infrastructure (CNKI) were searched for relevant case–control studies up to 31 July 2019. Odds ratio (OR) and 95% confidence interval (95% CI) were used to assess the strength of associations. Finally, seven case–control studies involving 1247 cases and 1762 controls were included in this meta-analysis. The pooled results showed that there was no significant association between AHR rs2066853 gene polymorphism and male infertility risk (A vs. G: OR = 1.08, 95% CI = 0.83–1.39; AA vs. GG: OR = 1.16, 95% CI = 0.65–2.04; AA vs. GA + GG: OR = 1.17, 95% CI = 0.66–2.07; AA + GA vs. GG: OR = 0.99, 95% CI = 0.85–1.15). Subgroup analysis by ethnicity showed the same result. However, significant association was found between AHR rs2066853 gene polymorphism and male infertility risk in oligoasthenotspermia (A vs. G: OR = 2.52, 95% CI = 1.72–3.70). In conclusion, our meta-analysis indicated that AHR rs2066853 gene polymorphism might be associated with an increased susceptibility to oligoasthenotspermia.
Introduction
Infertility is a worldwide health problem, which affects an estimated 15% of all couples trying to conceive a child [Citation1,Citation2]. Approximately half of all infertility cases caused by factors related to the male partner [Citation3]. The etiology of male infertility is complicated and not yet fully established, environment, lifestyle risk factors and genetic causes may be possible risk factors [Citation4,Citation5]. Genetic causes, such as chromosomal aberrations and single gene mutations, play important roles in developing male infertility among these risk factors [Citation4–6]. Researches have indicated that genetic abnormalities may contribute to approximately 15% of male infertility [Citation6].
The AHR is a ligand-activated transcription factor (TF) that belong to the basic helix–loop–helix.
Per-Arnt-Sim (bHLH/PAS) family which regulates a wide range of biological and toxicological effects [Citation7,Citation8]. AHR, in association with Hsp90, usually existing in cytoplasm, where dioxins plays as ligands and bind to AHR. After translocating to the nucleus, Hsp90 switches to AHR nuclear translocator (Arnt) and the ligand-AHR-Arnt complex binds to the cognate xenobiotic responsive elements (XREs) in the promoter/enhancer region, which are evolved in the upstream of the target genes for CYP1A1, GST, and others to activate their expressions [Citation9–11]. Seventy-five dioxin congeners and 135 furan congeners, widely existing in environmental pollutants, are capable of binding to and activating the aryl hydrocarbon receptor (AHR) signaling pathway [Citation12]. It has been well revealed in animal studies that AHR plays an important role in reproductive function in both sexes [Citation13,Citation14]. Adult female animals exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) were found a general decrease in reproductive health, including decreased fertility and litter size, alterations in estrous/menstrual cycles, and an increased abortion rate [Citation15,Citation16]. AHR pathway protein distributes widely in the testicular tissues. Testicular functions, especially spermatogenesis and sperm motility, are sufficiently reduced by exposure to PAHs through activation of AHR [Citation17]. AHR plays an important role in mediating the toxic actions of POPs, which inspires researchers to study the association between polymorphisms of genes involved in AHR pathway and sensitivity to POPs exposure.
The c.1661G>A transition (rs2066853) is the most widely studied SNP in the AHR gene which leads to an arginine to lysine change at codon 554 (p.Arg554Lys) in the transcriptional activation domain (TAD) of the receptor. Several researches have explored the associations between AHR rs2066853 gene polymorphism and male infertility risk. However, most of those researches had small patient sample sizes, and the results were inconclusive rather than consistent. Therefore, we conducted a meta-analysis to evaluate the association between the AHR rs2066853 gene polymorphism and risk of male infertility.
Materials and methods
Searching strategy
Two authors conducted a systematic search on PubMed, Embase, Web of Science, and CNKI independently up to 31 July 2019. The search terms were used as follows: ‘Aryl hydrocarbon receptor or AHR’ and ‘polymorphism or mutation or variant’ and ‘male infertility’. Moreover, the references of relevant articles were checked to identify additional studies on this topic. shows the strategy flowchart.
Inclusion criteria and exclusion criteria
Studies were considered eligible if they (1) were full text articles; (2) were case–control studies evaluating the association between AHR rs2066853 gene polymorphism and susceptibility to male infertility; (3) included available genotype distributions for both cases and controls; (4) contained no overlapping data. If the studies with the same or overlapping data by the same authors, we selected the ones with the most subjects, (5) were published in Chinese or English. Exclusion criteria including: (1) not for the association between AHR rs2066853 gene polymorphism and the male infertility risk; (2) studies with insufficient data; (3) animal studies, conference abstracts, editorial articles, review articles or meta-analyses.
Quality assessment
Two independent authors used the Newcastle-Ottawa scale (NOS) to assess the quality of the included studies [Citation18]. The NOS has eight items with three aspects including selection, comparability, and exposure for both cohort and case–control researches. The methodological quality was evaluated using a ‘star’ rating system. Researches that scored nine stars were considered high quality, that scored seven or eight stars were considered medium quality, and that scored less than seven stars were considered low quality. Inconsistent opinions were resolved by discussion and consensus with a third author.
Data extraction strategy
Two authors extracted all available data from each study independently using standard data collection form. The following information for each study were collected: first author’s name, publication year, country, genotyping method, sample size of the study case and control groups, the results of the Hardy–Weinberg equilibrium test.
Statistical analysis
Reviewer Manager 5.3 (Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and Stata 12.0 software (Stata Corporation, College Station, Texas, USA) were applied for statistical analyses. The strength of association between AHR rs2066853 gene polymorphisms and male infertility was evaluated by odds ratios (ORs) with 95% CIs. The pooled ORs were analyzed for four genetic models: allele comparison model, dominant model, recessive model and codominant model, respectively. We used chi-square-based Q-test and the I2 metric to assess heterogeneity between studies. An I2 >50% and the p values for heterogeneity <.10 was considered the high level of heterogeneity. If heterogeneity was observed among the individual studies (p values for heterogeneity was >.10 and I2 < 50%), a random-effect model was applied to evaluate the summary OR. Otherwise, a fixed-effect model was used. The significance of the summary OR was determined by the Z-test which was considered as statistically significant when p < .05.The potential publication bias were estimated by Begg’s test, Egger’s test and funnel plots. As for Sensitivity analysis, we excluded one study each time to evaluate the stability of the results.
Results
Study inclusion and characteristics
Eighty-three case–control studies were identified through first search in PubMed, Embase, Web of Science, and CNKI. Among these search results, 76 studies were excluded for full text review after screening titles and abstracts. Finally, 7 case–control studies involving 1247 cases and 1762 controls were included in this meta-analysis [Citation19–25]. The studies were published from 2004 to 2017. Three of these studies based in Caucasians, three in Asians, and one in African population. The AHR gene rs2066853 genotype frequencies of the controls from the seven studies were consistent with HWE. shows the main characteristics of all the included studies and shows the quality of the included studies based on the NOS score.
Table 1. Main characteristics of studies included in the meta-analysis.
Table 2. Quality assessment for all of the included studies.
Association between the AHR gene rs2066853 polymorphism and male infertility
Seven studies including 3254 individuals totally evaluated the influence of ARH rs2066853 gene polymorphism on the risk of male infertility. show the meta-analysis results for the allele model (A vs. G), additive model (AA vs. GG), dominant model (AA + GA vs. GG), and recessive model (AA vs. GA + GG), for which the I2 value, representing the among-study heterogeneity, was 77%, 74%, 29%, and 78%, respectively. Therefore, random-effects models were applied in the allele model (A vs. G), additive model (AA vs. GG) and recessive model (AA vs. GA + GG). All in all, the result revealed no significant association between the ARH rs2066853 gene polymorphism and male infertility risk (A vs. G: OR = 1.08, 95% CI = 0.83–1.39; AA vs. GG: OR = 1.16, 95% CI = 0.65–2.04; AA vs. GA + GG: OR = 1.17, 95% CI = 0.66–2.07; AA + GA vs. GG: OR = 0.99, 95% CI = 0.85–1.15).
Figure 2. Forest plot of the studies assessing the association between AHR rs2066853 gene polymorphisms and male infertility based on allelic model (allele model: A vs. G).
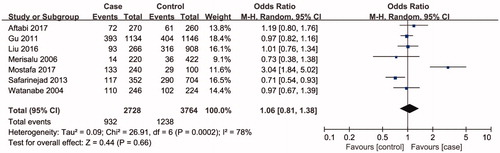
Figure 3. Forest plot of the studies assessing the association between AHR rs2066853 gene polymorphisms and male infertility based on additive model (additive model: AA vs. GG).
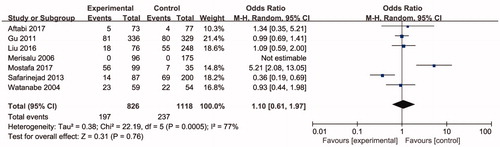
Figure 4. Forest plot of the studies assessing the association between AHR rs2066853 gene polymorphisms and male infertility based on recessive model (recessive model: AA vs. GG + GA).
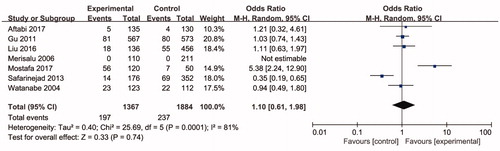
Figure 5. Forest plot of the studies assessing the association between AHR rs2066853 gene polymorphisms and male infertility based on dominant model (dominant model: AA + GA vs. GG).
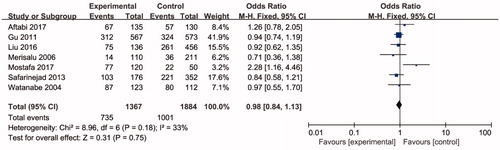
presents subgroup analyses results of male infertility risk by ethnicity, there was no significant association between ARH rs2066853 gene polymorphism and male infertility risks in Asian, European, and African. However, significant association was found between ARH rs2066853 gene polymorphism and oligoasthenozoospermia (A vs. G: OR = 2.52, 95% CI =1.72–3.70; AA vs. GG: OR = 4.66, 95% CI = 2.22–9.80; AA vs. GA + GG: OR = 3.99, 95% CI = 2.09–7.61; AA + GA vs. GG: OR = 2.36, 95% CI = 1.35–4.13) ().
Table 3. Meta-analysis of the association of AHR rs2066853 gene polymorphisms with male infertility.
Sensitivity analyses and publication bias
Begg’s test, Egger’s test, and funnel plots were applied to assess the publication bias on ARH rs2066853 gene polymorphism. No publication bias was observed based on visual inspection of funnel plots or according to the results of Begg’s and Egger’s test (; ). The sensitivity analyses were performed to calculate the pooled ORs by sequentially excluding individual studies, the results revealed that no individual study influenced the overall pooled ORs, suggesting the results of this meta-analysis are stable ().
Figure 6. Funnel plot of the studies assessing the association between AHR rs2066853 gene polymorphisms and male infertility (allele model: A vs. G; additive model: AA vs. GG; recessive model: AA vs. GG + GA; dominant model: AA + GA vs. GG).
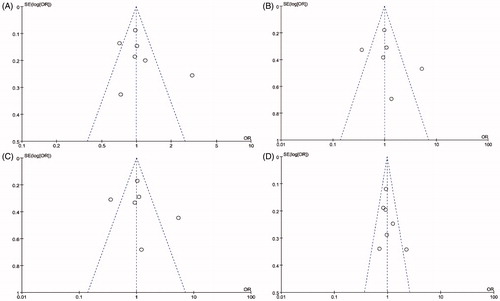
Figure 7. Sensitivity analysis diagram for each study used to assess the relative risk estimates for the AHR rs2066853 gene polymorphisms and male infertility in all the included studies (allele model: A vs. G; additive model: AA vs. GG; recessive model: AA vs. GG + GA; dominant model: AA + GA vs. GG).
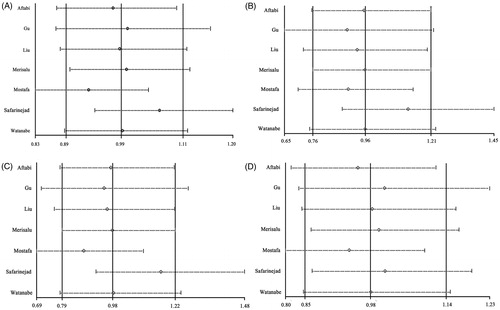
Table 4. Publication bias test for the AHR rs2066853 gene polymorphism.
Discussion
Testis is the most sensitive organ to TCDD toxicity. It was demonstrated that testicular functions, especially spermatogenesis and sperm motility, were specifically reduced by exposure to PAHs due to activation of AHR [Citation26–28]. More and more evidence from evidence-based studies certificates the critical role of genetic factors in the development of male infertility. The c.1661G>A transition (rs2066853) is the most widely studied SNP in the AHR gene. Hence, we performed a meta-analysis to provide a clear understanding between the ARH rs2066853 gene polymorphisms and risk of male infertility.
In the present studies, no overall association was observed between the ARH rs2066853 gene polymorphisms and male infertility risk, similar results were obtained in subgroup analysis by ethnicity. However, association between AHR rs2066853 gene polymorphisms and oligoasthenotspermia risk was observed significant. The present meta-analysis included only five studies that reported the relationship between the AHR rs2066853 gene polymorphisms and male infertility risk in Asian, one study in European and one study in African and two studies reported the relationship between the AHR rs2066853 gene polymorphisms and oligoasthenotspermia risk, the sample size was small; Thus, studies with larger sample sizes are demanded for further investigation on the potential relationships between AHR rs2066853 gene polymorphisms and male infertility risk.
Safarinejad et al. found strong evidence that AHR rs2066853 gene polymorphism significantly contributed to susceptibility of male factor infertility with impaired semen quality in the Iranian population. Similarly, Liu et al. and Mostafa et al. clarified that the AHR rs2066853 gene polymorphisms may correlated with sperm quality, which were in contrast to the conclusions of other studies include in the meta-analysis. The inconsistency between the studies could arise from study patients, race, geography or genetic differences of the study population. However, we enrolled only seven studies in the present studies. Well designed, unbiased, and large case–control studies should be performed to acquire a more precise association between the AHR rs2066853 gene polymorphism and male infertility risk.
In the current meta-analysis, I2 statistics and Q-test were performed to evaluate the significance of heterogeneity. Obvious heterogeneity among the included studies was found in Allele model, Recessive model and Additive model. After sub-grouped by ethnicity, the heterogeneity among the studies was removed in Asian population, whereas certain degree of heterogeneity was observed in the Caucasian population. We considered the heterogeneity may result from difference in genotyping method, the intricate substructure in Caucasian population, and some other unknown factors. Sensitivity meta-analyses were performed in this meta-analysis. We removed each study in turn in every comparison, finding that none of the individual studies significantly affected the pooled ORs, and the association between the AHR rs2066853 gene polymorphism and male infertility remained unchanged in each genetic models, proving the high stability of the meta-analysis. As well, there were no publication biases in our meta-analysis.
However, some limitations of the present study should be noted. First, heterogeneity is a major issue that needs to be mentioned when interpreting the meta-analysis results, and after performing subgroup analyses by ethnicity, the heterogeneity was effectively reduced or removed in most of genetic models. Second, only seven studies were included in the meta-analysis, the sample sizes were small; therefore, more case–control studies that evaluate the association between AHR rs2066853 gene polymorphism and male infertility are needed. Third, the potential gene–gene and gene–environment interactions were not estimated due to the limited information in the original studies. Fourth, sources of control, genotyping procedure, and semen quality were not applied in the pooled results assessment, due to lack of information. Finally, there were no enough studies investigating the association between the AHR rs2066853 gene polymorphism and different type of male infertility in this meta-analysis. Thus, the association between the AHR rs2066853 gene polymorphism and different type of male infertility needed further confirmation.
Conclusion
Results of the present meta-analysis indicate that the AHR rs2066853 gene polymorphism may contribute to genetic susceptibility to the risk of oligoasthenotspermia. Nevertheless, future studies are needed to further investigate the association between the AHR gene polymorphisms and different type of male infertility.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gurkan H, Tozkir H, Goncu E, et al. The relationship between methylenetetrahydrofolate reductase c.677TT genotype and oligozoospermia in infertile male patients living in the Trakya region of Turkey. Andrologia. 2015;47(9):1068–1074.
- Sharma RK, Said T, Agarwal A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl. 2004;6(2):139–148.
- Miyamoto T, Tsujimura A, Miyagawa Y, et al. Male infertility and its causes in human. Adv Urol. 2012;2012:384520.
- Wong EWP, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci. 2011;32(5):290–299.
- Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab. 2011;25(2):271–285.
- Krausz C, Chianese C. Genetic testing and counselling for male infertility. Curr Opin Endocrinol Diabetes Obes. 2014;21(3):244–250.
- Nuti R, Gargaro M, Matino D, et al. Ligand binding and functional selectivity of L-tryptophan metabolites at the mouse aryl hydrocarbon receptor (mAhR). J Chem Inf Model. 2014;54(12):3373–3383.
- Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40(1):519–561.
- Fujisawa-Sehara A, Sogawa K, Yamane M, et al. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucl Acids Res. 1987;15(10):4179–4191.
- Telakowski-Hopkins CA, King RG, Pickett CB. Glutathione S-transferase Ya subunit gene: identification of regulatory elements required for basal level and inducible expression. Proc Natl Acad Sci U S A. 1988;85(4):1000–1004.
- Mimura J, Ema M, Sogawa K, et al. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13(1):20–25.
- Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223–241.
- Hernandez-Ochoa I, Karman BN, Flaws JA. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol. 2009;77(4):547–559.
- Izawa H, Kohara M, Watanabe G, et al. Diesel exhaust particle toxicity on spermatogenesis in the mouse is aryl hydrocarbon receptor dependent. J Reprod Dev. 2007;53(5):1069–1078.
- Li XL, Johnson DC, Rozman KK. Reproductive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (Tcdd) in female rats - ovulation, hormonal-regulation, and possible mechanism(S). Toxicol Appl Pharmacol. 1995;133(2):321–327.
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol. 1993;23(3):283–335.
- Esakky P, Hansen DA, Drury AM, et al. Cigarette smoke condensate induces aryl hydrocarbon receptor-dependent changes in gene expression in spermatocytes. Reprod Toxicol. 2012;34(4):665–676.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- Aftabi Y, Hosseinzadeh Colagar A, Mehrnejad F, et al. Aryl hydrocarbon receptor gene transitions (c.-742C>T; c.1661G>A) and idiopathic male infertility: a case-control study with in silico and meta-analysis. Environ Sci Pollut Res. 2017;24(25):20599.
- Gu A, Ji G, Long Y, et al. Assessment of an association between an aryl hydrocarbon receptor gene (AHR) polymorphism and risk of male infertility. Toxicol Sci. 2011;122(2):415–421.
- Shu-Yuan L, Chang-Jun Z, Hai-Ying P, et al. AHR Gene variants correlated with sperm count. Chin J Birth Health Hered. 2016; 24(5):25–29.
- Merisalu A, Punab M, Altmae S, et al. The contribution of genetic variations of aryl hydrocarbon receptor pathway genes to male factor infertility. Fertil Steril. 2007;88(4):854–859.
- Mostafa T, Fouad H, Nabil N, et al. Aryl hydrocarbon receptor (AhR) rs2066853 gene polymorphism association with infertile oligoasthenoteratozoospermic men and seminal oxidative stress. Environ Sci Pollut Res. 2017;24(9):8297–8301.
- Safarinejad MR, Shafiei N, Safarinejad S. Polymorphisms in aryl hydrocarbon receptor gene are associated with idiopathic male factor infertility. Reprod Sci. 2013;20(12):1423–1432.
- Watanabe M, Sueoka K, Sasagawa I, et al. Association of male infertility with Pro185Ala polymorphism in the aryl hydrocarbon receptor repressor gene: implication for the susceptibility to dioxins. Fertil Steril. 2004;82(Suppl 3):1067–1071.
- Jenardhanan P, Panneerselvam M, Mathur PP. Effect of environmental contaminants on spermatogenesis. Semin Cell Dev Biol. 2016;59:126–140.
- Mably TA, Moore RW, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 1. Effects on androgenic status. Toxicol Appl Pharmacol. 1992;114(1):97–107.
- Iseki M, Ikuta T, Kobayashi T, et al. Growth suppression of Leydig TM3 cells mediated by aryl hydrocarbon receptor. Biochem Biophys Res Commun. 2005;331(4):902–908.