Abstract
Purpose
To compare the efficacy and safety of two distinct doses of ulinastatin on late-onset acute renal failure (LARF) following orthotopic liver transplantation (OLT).
Methods
The high-risk recipients that underwent OLT were divided into two groups according to ulinastatin dose: low-dose (LD) ulinastatin group, 0.8 million U/d; high-dose (HD) ulinastatin group, 1.6 million U/d. The primary outcome was the incidence of LARF, which was defined the newly onset acute kidney injury (AKI) stage III (KDIGO, 2012) within 7–28 post-transplant days. The second outcomes were early multiple organ retrieval assessments, length of hospital stay and safety events.
Results
A total of 174 recipients were included (LD ulinastatin group, n = 55; HD ulinastatin group, n = 119). There was no significant difference in the incidence of LARF between LD (8/55, 14.50%) and HD (9/119, 7.56%) ulinastatin groups (HD vs. LD, HR, 0.49; 95%CI, 0.17–1.37; p = .1295). Multivariate Cox proportion risk regression model revealed HD ulinastatin (HR, 0.57; 95%CI, 0.38–0.98; p = .0464) was an independent protective factor for LARF. Early lactate level, oxygenation, AKI stage, graft function, and sequential organ failure assessment [SOFA] score were significantly improved in HD ulinastatin group versus LD ulinastatin group. No significant adverse events were observed in either group.
Conclusions
Higher dose of ulinastatin (1.6 million U/d) might be preferable to prevent LARF after OLT, and it may contribute to the enhancement of early multiple organ recovery and thus attenuate the incidence of LARF.
Introduction
Orthotopic liver transplantation (OLT) has become one of the most effective treatment methods for end-stage liver diseases and acute liver failure [Citation1]. Multiple organ dysfunction syndrome (MODS) often occurs after OLT accompanied by increasing postoperative morbidity and reducing patient survival rates, which may be associated with poor initial graft function, serious infections, and chronic and acute rejection [Citation2,Citation3]. Acute kidney injury (AKI) is one most common type of MODS after OLT, its occurrence is associated with various primary injuries such as ischemia reperfusion injury (IRI) and postoperative traumatic blood loss which may lead to systemic inflammatory response syndrome (SIRS) [Citation4,Citation5]. The preventive strategies of AKI after OLT mainly focus on supportive cares such as volume stabilization or renal toxic drugs avoid, presently the broad-spectrum anti-inflammatory therapy has attracted much attention [Citation6].
Ulinastatin, a urinary trypsin inhibitor with a molecular weight of 67 kDa that is purified from human urine, is a Kunitz-type and broad-spectrum protease inhibitor that inhibits endogenous proteases such as plasmin, trypsin, hyaluronidase, and α-chymotrypsin [Citation7,Citation8]. Previous studies reported that it could protect against various types of shock, ischemia-reperfusion organ injury, multi-organ dysfunction, and severe pancreatitis and suppress the deterioration of renal function associated with surgical procedures [Citation8–10]. Although the maximum recommended daily dose of ulinastatin is 3 × 105 U [Citation11], the doses required to achieve therapeutic concentrations for the treatment of acute or chronic pancreatitis, severe infection, and acute organ failure severe acute diseases are much higher. In fact, an increasing number of studies have reported the use of high dose (HD) ulinastatin (1–2 million U) [Citation12,Citation13]. Ulinastatin has a very wide treatment window and shows good tolerance and good relationship in sepsis and other diseases [Citation13,Citation14]. A previous study has shown that blood transfusion and blood loss are risk factors of AKI after OLT and that ulinastatin could reduce the incidence of AKI [Citation15]. Therefore, this study aimed to investigate the preventive effect of HD ulinastatin (1.6 million U/d) on a severe stage of AKI, referred as late-onset acute renal failure (LARF) after OLT in high-risk patients (intraoperative blood loss ≥2000 mL).
Materials and methods
Patients
A total of 451 patients who underwent OLT at the Organ Transplant Center of the Third Affiliated Hospital of Sun Yat-sen University between January 2015 and December 2017 were studied. All the transplantations were performed in accordance with the WHO guiding principles on human cell, tissue and organ transplantation (2010) and Regulations on Human Organ Transplantation of China (2007) [Citation16–18]. The study was approved by the Regional Human Research Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (no. 2019-02-277-01). Written Informed consent was obtained from all individual participants (including donors and recipients) in the study. All the data were retrospectively collected from the China Liver Transplantation Registry follow-up System and Hospital Information System (HIS) by the Research Electronic Data Capture tool. No organ was recruited from executed prisoners in this cohort. Donation program was supervised by institutional transplant ethics committee and Guangdong Red Cross Society.
In this study, high-risk patients aged ≥18 years with massive intraoperative blood loss (≥2000 mL [Citation19]) during liver transplantation were included. Intraoperative blood loss was estimated by the surgeon, anesthesiologist, and nurse according to previous studies [Citation20,Citation21]. Exclusion criteria were as follows: (1) multiple organ transplantation or re-transplantation; (2) survival time less than 7 days after transplantation; (3) early-onset acute renal failure (EARF) [Citation22] which was defined as newly developed acute renal failure (ARF, see the definition in primary outcome) within 7 days post-transplantation; and (4) missing renal assessment data 28 days after surgery.
Ulinastatin administration
All liver transplant recipients underwent the piggy-back OLT performed by the same team. After anesthesia induction, the patients who underwent piggy-back OLT from January 2015 to June 2016 were intravenously administered at a low dose of 0.8 million U/d ulinastatin (Techpool Bio-Pharma Co. Ltd., Guangzhou, China) (low-dose [LD] ulinastatin group). Due to the wide therapeutic window of ulinastatin treatment, an increasing number of studies have demonstrated a potential dose-dependent effect in the treatment of inflammatory response [Citation13]. The patients who underwent the piggy-back OLT from July 2016 to December 2017 were intravenously administered at a high dose of 1.6 million U/d (high-dose [HD] ulinastatin group). All patients were informed of additional safety data and the potential efficacy of ulinastatin before treatment and provided informed consent. After surgery, they were routinely transferred to the transplant intensive care unit, where they underwent management according to institutional protocols as follows. Methylprednisolone (10 mg/kg) and basiliximab (20 mg) were intravenously injected for immune induction intraoperatively and 4 days postoperatively. Third-generation cephalosporins and echinocandins were used to prevent postoperative bacterial and fungal infections, respectively. Subsequent immunosuppression was maintained by calcineurin inhibitors (CNIs), such as oral tacrolimus 0.1–0.2 mg/kg/d or cyclosporine 10–15 mg/kg/d divided twice a day. The blood concentrations of CNIs were monitored once or twice weekly to reach a stable level in the first post-transplantation month.
Outcome evaluation
In this study, since ulinastatin dose was the main exposure factor and the treatment course was 3–7 days, the EARF might not be associated with ulinastatin treatment. Therefore, the primary endpoint was the incidence of LARF. Herein, LARF were defined as newly onset of ARF between days 7 and 28 after transplantation. We also defined ARF in this study as the most severe stage of AKI — AKI Stage III. AKI was diagnosed and classified according to KDIGO Clinical Practice Guidelines [Citation22]. AKI Stage III should meet any of the following criteria: 1. Oliguria (urine output <0.3 mL/kg/h over 24 h) or anuria over 12 h; 2. Serum creatine (Scr) increased to three times baseline level or elevated 354 μmol/L or greater; 3. Initiation of renal replacement therapy (RRT). AKI Stage II is defined as Scr increase to 2–3 times baseline or urine output <0.5 mL/kg/h over 12 h. AKI Stage I is defined as Scr increase over 26.5 μmol/L in 48 h or to 1.5–2 times baseline, or urine output <0.5 mL/kg/h in 6–12 h. Any above change of Scr increase or urine output decrease manifested in 7 days will be diagnosed as AKI. The second endpoints were sequential organ failure assessment (SOFA) score changes and oxygenation index (OI) and blood lactate level (1, 3, and 7 days post-transplantation), liver function, ulinastatin-related adverse events, and others.
Statistical analysis
All analyses were performed using Empower (R) (http://www.empowerstats.com; X & Y Solutions Inc., Boston, MA) and R (http://www.Rproject.org) software. R version 3.4.3. Data are expressed as mean ± SD, number (%), or median (interquartile range). The cumulative incidence of LARF in the LD and HD ulinastatin groups were estimated by Kaplan–Meier method and compared by the log-rank tests. The characteristics were compared between groups using Student’s t-test or the Mann–Whitney U test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. Cox proportion regressions were used to find associated factors in univariate analysis. Significant variables were added to the multivariate Cox proportion risk regression model to determine the independent effect of ulinastatin dose and early kidney injury stage on LARF after OLT. A generalized additive mixed model (GAMM) was used to analyze the different changes of SOFA score in two groups 1, 3, and 7 days after OLT. Values of p < .05 were considered statistically significant.
Results
Among the 451 patients who underwent OLT, an intraoperative blood loss >2000 mL occurred in 219 cases. Six who required re-transplantation, three with combined liver-kidney transplantations, and one who underwent living donor liver transplantation, one case with no report of post-transplant kidney function, 12 cases death or primary graft loss, and 22 cases EARF were excluded. Finally, a total of 174 patients were included (LD ulinastatin group, n = 55; HD ulinastatin group, n = 119), among them 17 (9.8%) patients (eight cases in LD ulinastatin group, nine cases in HD ulinastatin group) developed LARF and underwent RRT. shows the demographic information and clinical characteristics of two groups. There were significant differences in history of pneumonia (1 week before), preoperative hemoglobin and serum creatinine levels and model for end-stage liver disease with serum sodium level (MELD-Na) score, use of cyclosporine, donor age, and cold ischemic time, which might due to patients in the HD ulinastatin group being sicker with a high risk of LARF.
Table 1. Comparison of demographic information between the two groups of patients undergoing liver transplantation.
The Kaplan–Meier analysis showed there was no significant difference in the incidence of LARF between the LD (8/55, 14.5%) and HD (9/119, 7.56%) ulinastatin groups after OLT (HD ulinastatin group vs. LD ulinastatin group, HR, 0.49; 95%CI, 0.17–1.37; p = .1295, ). However, after the adjustment for preoperative serum creatinine levels, Acute Physiologic Assessment and Chronic Health Evaluation-II (APACHE-II) score, MELD-Na score, preoperative anemia, graft type, operation time, intraoperative blood loss, intraoperative blood transfusion, anhepatic phase, postoperative complications, and tacrolimus concentration, the AKI II at day 7 after OLT was an independent risk factor for the occurrence of LARF (HR, 5.52; 95%CI, 1.61–18.92; p = .0065; ); HD ulinastatin was an independent protective factor for the occurrence of LARF (HR, 0.57; 95%CI, 0.38–0.98; p = .0464; ). The common etiologies of AKI are shown in , including preoperative status of renal function, intraoperative blood loss, cold ischemic time, and anhepatic time, among which there were significant differences in serum creatinine levels and cold ischemic time. After adjustment for these indicators, there were significant differences in the composition ratio of AKI grades at 7 days after OLT between the HD and LD ulinastatin groups, which might mean that HD ulinastatin could significantly prevent the occurrence of AKI (HD vs. LD, p < .001; ) and the strong relationship between dose and efficacy, although the patients in the HD group were sicker.
Figure 1. The cumulative incidence of LARF in LD ulinastatin group and HD ulinastatin group with Kaplan–Meier method.
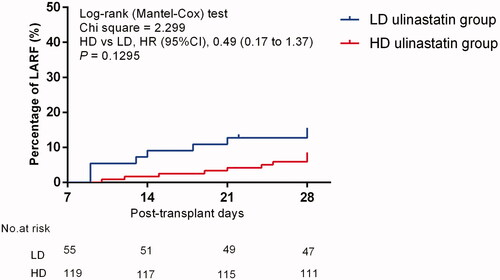
Table 2. Hazard ratio (95%CI) of primary outcomes according to the different doses of ulinastatin by Cox regression model.
Table 3. Comparison of secondary outcomes between the two groups of patients.
The 28-days graft loss and reintubation rates were significant lower and hospital stays were significant shorter in the HD group than in the LD group (all p < .001; ). The reduction of serum alanine transaminase (ALT) and total bilirubin (TBIL) levels were more significant in the HD ulinastatin group than that of LD ulinastatin group on day 3 after OLT (p < .0001, ). In the HD ulinastatin group, the OI on days 3 and 7 after OLT were significantly improved compared to day 1 after OLT (all p < .001), but not in the LD ulinastatin group; furthermore, there were significant intergroup differences in OI on days 3 and 7 (HD vs. LD: day 3, p = .0292; day 7, p < .001) (). Different doses of ulinastatin significantly reduced the blood lactate level and SOFA score on days 1 and 3 after OLT, and there was a significant intergroup difference in SOFA score on day 7 (all p < .001, ). In addition, the International Normalized Ratio (INR) in the two groups on day 3 after OLT was significantly higher than that on day 1 after OLT (day 3 vs. day 1: LD group, p = .0326; HD, p = .0039), while there was no significant intergroup difference ().
Figure 2. The secondary outcomes of the two groups on days 0, 1, 3, 7. (A) TBIL, (B) ALT, (C) OI, (D) SOFA score, (E) LAC level, (F) INR. TBIL: Total bilirubin; ALT: alanine aminotransferase; SOFA: sequential organ failure assessment; LAC: lactate; INR: International Normalized Ratio. *LD ulinastatin group vs. HD ulinastatin group, p < .05.
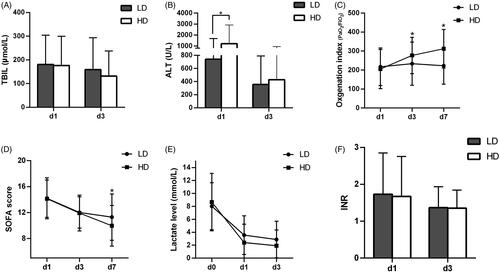
The GAMM model () showed the following: when comparison between the days 7 and day 1 after OLT, the SOFA score in HD ulinastatin group was 1.28 lower than that in LD ulinastatin group (95%CI, (−2.30, −0.25), p = .0015); when comparison between the days 3 and day 0 (immediate outcome), or comparison between the days 7 and day 0 after OLT, the lactate level in the HD ulinastatin group respectively decreased by 1.80 mmol/L (95%CI, (−2.99, −0.60), p = .0003) and 1.60 mmol/L (95%CI, (−2.79, −0.41), p = .0009) more than that in the LD ulinastatin group; when comparison between the days 3 and day 1, or comparison between the days 7 and day 1 after OLT, the OI in HD ulinastatin group were 54.88 (95%CI, (15.65, 94.10) p = .006) and 100.87 (95%CI, (61.62, 140.13), p < .001) higher than that in LD ulinastatin group.
Table 4. GAMM model analysis of changes of SOFA, Lac and OI in different doses of ulinastatin early after operation.
In addition, treatment in the HD ulinastatin group did not significantly increase the adverse reactions compared with that in the LD ulinastatin group, and most adverse reactions were mild and controllable. The mean length of hospital stay in the HD ulinastatin group was significantly shorter than that in the LD ulinastatin group ().
Discussion
AKI is a frequent complication after liver transplantation, with incidences ranging from 12 to 95% [Citation23]. Many researchers prefer to use the term of ARF instead of the term AKI, which emphasizes that some may acquire long term damage despite an apparently good early recovery [Citation24]. Postoperative ARF is the most frequent complications of OLT associated with the development of chronic renal dysfunction and the increased mortality rate [Citation25,Citation26]. ARF after liver transplantation can be apparently divided into two phases [Citation1]: EARF, which occurred within the first 7 days after transplantation, was strongly associated with perioperative factors, like blood loss, anhepatic time or preoperative renal status; LARF, which occurred within 7–28 post-transplant days, might be related to early preventive interventions, such as renal toxic agents avoid and systemic organ protection. In this study, we focused on LARF, also refers to a distinct severe AKI — AKI stage III according to KDIGO Clinical Practice Guidelines [Citation22], and explored an alternative preventing strategy in clinic.
Ulinastatin, as a broad-spectrum protease inhibitor, has been widely used in China, Korea, and Japan for the treatment of postoperative organs protection, pancreatitis, shock, and inflammatory disorders; however, it is not approved in the United States [Citation27,Citation28]. It is a major goal of clinical pharmacology to understand the dose-effect relationship in therapeutics. Chen et al. [Citation11] recently evaluated the safety and tolerability of HD ulinastatin in healthy volunteers and reported a 2-h intravenous infusion of a single dose of 8 million U ulinastatin was well tolerated by healthy Chinese subjects. In addition, increasing numbers of studies have reported the therapeutics of HD ulinastatin on patients or in an animal model. In a rat model of sepsis, HD ulinastatin (2 × 105 U/kg) significantly inhibited the production of P-selectin, tumor necrosis factor-α, and thrombin-antithrombin complex compared with LD ulinastatin (0.5 × 105 U/kg) [Citation29]. Ji et al. [Citation30] reported that different doses of ulinastatin (0.5 × 104 U/kg, 1 × 104 U/kg, 1.5 × 104 U/kg) have a certain effect on cellular immunity in patients undergoing laparoscopic colorectal carcinoma surgery. Rhee et al. [Citation12] reported HD ulinastatin (10,000 U/kg followed by 5000 U/kg/h) could improve pulmonary oxygenation after cardiopulmonary bypass (CPB) and in the early stages of the intensive care unit stay in patients undergoing aortic valve surgery with CPB. In this study, the doses of 0.8 million U/d and 1.6 million U/d ulinastatin were administrated in the LD and HD ulinastatin groups, respectively. No serious adverse events were observed at either dose, and most adverse reactions were tolerable. The multivariate analysis suggested that the higher dose of ulinastatin might be a protective factor for the occurrence of LARF in comparison with low dose of ulinastatin.
AKI after OLT affects the recipient’s short- and long-term prognosis. Preoperative renal function, disease severity, intraoperative blood loss, lack of liver staging, early postoperative graft function, and use of immunosuppressive agents are risk factors for postoperative AKI. The superposition of early AKI and secondary organ injury is the main reason for recovery difficulty or deterioration of postoperative renal function [Citation31]. The level of renal injury in the early stage (within 7 days after onset) is a risk factor for unrecoverable AKI [Citation32]. In this study, multivariate analysis showed that AKI stage II in the early postoperative period (day 7) was an independent risk factor for progression to LARF, indicating that early renal injury might make patients susceptible and increase the risk of LARF. Prasa [Citation33] believed that this kidney injury was in line with clinical scenarios based on the second hit, which was consistent with what Sophia et al. observed after cardiac surgery [Citation34]. Early multiple organ injury was significantly associated with AKI prognosis. Kellumet et al. found that a distant organ injury, such as in cases requiring mechanical ventilation and vasoactive drugs, was also an independent risk factor for delayed or no AKI recovery [Citation35]. In this study, although no significant correlation was seen between early oxygenation or SOFA score and LARF, for patients treated with HD ulinastatin, multiple organ injuries (including graft, lung, and kidney and overall organ function SOFA score) in the early postoperative period was significantly improved (; ), the incidence of reintubation within 28 days was lower, the mean length of hospital stay was shorter, and the 28-day graft loss rate was improved. All of the above might mean that alleviating early organ damage could prevent the incidence of LARF. Therefore, HD ulinastatin for early multi-organ protection might be among the most effective methods to prevent the incidence of LARF.
However, there were some limitations to our study. First, patients who died within 7 days were excluded; thus, the effect of ulinastatin on severe renal impairment was not observed. Second, the sample size was small, and it was unexpectedly found that average patient condition in the HD ulinastatin group was more serious than that in the LD ulinastatin group. Third, this study lacked the actual incidence of postoperative AKI in OLT without the administration of ulinastatin, and the group of patients in whom ulinastatin was not administered will be collected to study the actual incidence of postoperative AKI in OLT. Fourth, the patient data were retrospective collected, so some important data might be missing. Fifth, our study’s primary outcome of LARF was confined to 7–28 days post-OLT; other clinical outcomes beyond the postoperative period were not analyzed. Therefore, further studies with larger sample sizes and more clinical information are needed to confirm the result and detect the oxidative and inflammatory mediators to increase our understanding of the protective mechanism of different doses of ulinastatin for preventing postoperative AKI in OLT.
Conclusions
Compared to LD ulinastatin (0.8 million U/d), HD ulinastatin (1.6 million U/d) might be preferable to prevent LARF after OLT, and it may attribute the enhancement of early multiple organ recovery to attenuate the incidence of LARF.
Acknowledgements
The authors are grateful to Xiaocui Fang from China Liver Transplant Registry (CLTR) for her great contribution in data acquisition and quality control to the article.
Disclosure statement
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Cabezuelo JB, Ramirez P, Rios A, et al. Risk factors of acute renal failure after liver transplantation. Kidney Int. 2006;69(6):1073–1080.
- Thuluvath PJ, Thuluvath AJ, Hanish S, et al. Liver transplantation in patients with multiple organ failures: feasibility and outcomes. J Hepatol. 2018;69(5):1047–1056.
- Mueller AR, Platz KP, Krause P, et al. Perioperative factors influencing patient outcome after liver transplantation. Transpl Int. 2000;13 (Suppl 1):S158–S161.
- Chen CC, Liu ZM, Wang HH, He W, et al. Effects of ulinastatin on renal ischemia-reperfusion injury in rats. Acta Pharmacologica Sinica. 2004;25(10):1334–1340.
- Garcia-Fernandez N, Lavilla FJ, Rocha E, et al. Assessment of haemostatic risk factors in patients with acute renal failure associated with severe systemic inflammatory response syndrome. Development of a prognostic index. Nephron. 2002;92(1):97–104.
- McCaffrey J, Dhakal AK, Milford DV, et al. Recent developments in the detection and management of acute kidney injury. Arch Dis Child. 2017;102(1):91–96.
- Muramatu M, Mori S, Matsuzawa Y, et al. Purification and characterization of urinary trypsin inhibitor, UTI68, from normal human urine, and its cleavage by human uropepsin. J Biochem. 1980;88(5):1317–1329.
- Honore PM, Spapen HD. Ulinastatin to prevent acute kidney injury after cardiopulmonary bypass surgery: does serum creatinine tell the whole story? Crit Care. 2016;20(1):183.
- Lee B, Lee SY, Kim NY, et al. Effect of ulinastatin on postoperative renal function in patients undergoing robot-assisted laparoscopic partial nephrectomy: a randomized trial. Surg Endosc. 2017;31(9):3728–3736.
- Pang XY, Fang CC, Chen YY, Liu K, et al. Effects of ulinastatin on perioperative inflammatory response and pulmonary function in cardiopulmonary bypass patients. Am J Ther. 2016;23(6):e1680–e1689.
- Chen Q, Hu C, Liu Y, et al. Safety and tolerability of high-dose ulinastatin after 2-hour intravenous infusion in adult healthy Chinese volunteers: a randomized, double-blind, placebo-controlled, ascending-dose study. PloS One. 2017;12(5):e0177425.
- Rhee KY, Sung TY, Kim JD, et al. High-dose ulinastatin improves postoperative oxygenation in patients undergoing aortic valve surgery with cardiopulmonary bypass: a retrospective study. J Int Med Res. 2018;46(3):1238–1248.
- Xu CE, Zou CW, Zhang MY, et al. Effects of high-dose ulinastatin on inflammatory response and pulmonary function in patients with type-A aortic dissection after cardiopulmonary bypass under deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth. 2013;27(3):479–484.
- Karnad DR, Bhadade R, Verma PK, et al. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med. 2014;40(6):830–838.
- Li XY, Guan JQ, Shen N, et al. Protective effects of ulinastatin during orthotopic liver transplantation on kidney function with decreasing acute renal failure after liver transplantation in patients with severe hepatitis. Zhonghua yi Xue za Zhi. 2010;90(5):315–318.
- World Health O. WHO guiding principles on human cell, tissue and organ transplantation. Transplantation. 2010;90(3):229–233.
- The Regulation on Human Organ Transplantation [Internet]. State Council of the People’s Republic of China; 2007. Available from: http://www.gov.cn/flfg/2007-04/06/content_575602.htm
- The Interim Provisions on Human Organ Procurement and Allocation. Beijing: National Health and Family Planning Commission; 2013. Available from: http://www.gov.cn/jrzg/2013-08/21/content_2471265.htm
- Stainsby D, MacLennan S, Hamilton PJ. Management of massive blood loss: a template guideline. Br J Anaesth. 2000;85(3):487–491.
- Brecher ME, Monk T, Goodnough LT. A standardized method for calculating blood loss. Transfusion. 1997;37(10):1070–1074.
- Yuasa T, Niwa N, Kimura S, et al. Intraoperative blood loss during living donor liver transplantation: an analysis of 635 recipients at a single center. Transfusion. 2005;45(6):879–884.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–184.
- Barreto AG, Daher EF, Silva GB Jr, et al. Risk factors for acute kidney injury and 30-day mortality after liver transplantation. Ann Hepatol. 2015;14(5):688–694.
- Coulthard MG. The management of neonatal acute and chronic renal failure: a review. Early Hum Dev. 2016;102:25–29.
- Bastos-Neves D, Salvalaggio PRO, Almeida MD. Risk factors, surgical complications and graft survival in liver transplant recipients with early allograft dysfunction. Hepatobiliary Pancreat Dis Int. 2019;18(5):423–429.
- Smoter P, Nyckowski P, Grat M, et al. Risk factors of acute renal failure after orthotopic liver transplantation: single-center experience. Transplant Proc. 2014;46(8):2786–2789.
- Luo GJ, Yao WF, He Y, et al. Ulinastatin prevents acute lung injury led by liver transplantation. J Surg Res. 2015;193(2):841–848.
- Jiang W, Yu X, Sun T, et al.; for the China Critical Care Clinical Trials Group (CCCCTG). ADJunctive Ulinastatin in Sepsis Treatment in China (ADJUST study): study protocol for a randomized controlled trial. Trials. 2018;19(1):133.
- Liu Y, Wu XH. Effect of ulinastatin on serum levels of tumor necrosis factor-alpha, P-selectin, and thrombin-antithrombin complex in young rats with sepsis. Zhongguo Dang Dai er ke za Zhi. 2017;19(2):237–241.
- Ji Z, Tian H, Shang J, et al. Effect of different doses of ulinastatin on cellular immunity and hepatorenal functions in patients undergoing laparoscopic colorectalcarcinoma surgery. Pak J Pharm Sci. 2018;31(5 (Special)):2311–2314.
- Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. JASN. 2016;27(3):687–697.
- Forni LG, Darmon M, Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855–866.
- Devarajan P. Acute kidney injury: acute kidney injury: still misunderstood and misdiagnosed. Nat Rev Nephrol. 2017;13(3):137–138.
- Chew ST, Ng RR, Liu W, et al. Acute kidney injury increases the risk of end-stage renal disease after cardiac surgery in an Asian population: a prospective cohort study. BMC Nephrol. 2017;18(1):60.
- Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6):784–791.
