?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To investigate the diagnostic performances of renal resistive index (RRI) and semiquantitative power Doppler ultrasound (PDU) scores in predicting acute kidney injury (AKI) stage 3 in critically ill patients.
Methods
This prospective observational study included 148 patients (80 with reduced cardiac index [CI], 68 with maintained CI). RRI and semiquantitative PDU scores were measured within 6 h after intensive care unit admission. AKI was defined according to Kidney Disease Improving Global Outcomes criteria.
Results
A negative correlation between RRI and PDU score (r = −0.517, p < 0.001) and a positive correlation between PDU score and CI (r = 0.193, p = 0.019) were found, whereas RRI was not correlated with CI (r = 0.131, p = 0.121). The predictive value of RRI for AKI stage 3 was similar between CI-reduced (area under the curve [AUC] 0.761, 95% confidence interval 0.650–0.851, p < 0.001) and CI-maintained (AUC 0.786, 95% confidence interval 0.665–0.878, p < 0.001) patients. Conversely, PDU score could effectively predict AKI stage 3 in CI-reduced patients (AUC 0.872, 95% confidence interval 0.778–0.936, p < 0.001) but not in CI-maintained patients (AUC 0.669, 95% confidence interval 0.544–0.778, p = 0.071). The predictive value of PDU score for AKI stage 3 was statistically different between CI-reduced and CI-maintained patients (p = 0.021).
Conclusions
PDU scores could effectively predict AKI stage 3 in CI-reduced patients but not in CI-maintained patients. RRI is a poor predictor of AKI stage 3 in patients with reduced or maintained CI.
1. Introduction
Acute kidney injury (AKI) is a heterogeneous group of conditions characterized by a sudden decrease in glomerular filtration rate (GFR), with major complications including volume overload, electrolyte disorders, uremic complications, and drug toxicity [Citation1], and remains associated with a dismal prognosis [Citation2]. In studies in adults, the pooled incidence rate of AKI was reported to be 21.6%. Approximately 10% of patients with AKI require dialysis. The highest pooled AKI rate was observed in critical care settings (32%) [Citation3]. Serum creatinine (SCr) and urine volume are used as diagnostic and staging markers for AKI [Citation4,Citation5]. However, oliguria is not specific to acute tubular necrosis, and SCr elevation is delayed and only occurs after a prolonged decrease in GFR. Furthermore, the diagnosis of AKI stage 3 usually takes 12–24 h. Hence, an indicator that could predict AKI stage 3 within 6 h of admission may help in providing necessary medical attention to patients, thus promoting improved outcomes.
Many biomarkers, such as serum cystatin C [Citation6], neutrophil gelatinase-associated lipocalin [Citation7], and urinary kidney injury molecule-1 [Citation8], and imaging modalities, such as magnetic resonance imaging [Citation9], are potentially useful in assessing kidney injury. However, most of these indicators are time-consuming, not immediately available, and have not been widely used in clinical practice. Doppler-based renal resistive index (RRI) calculations and semiquantitative power Doppler ultrasound (PDU) scores are rapid, noninvasive, and repeatable tools that were proposed for early AKI detection in patients confined to the intensive care unit (ICU) [Citation10]. RRI is derived from the Doppler spectrum of intrarenal (segmental/interlobar) arteries and refers to the percentage reduction of end-diastolic blood flow in renal vessels in relation to the maximum systolic blood flow. Several studies have demonstrated that RRI has a promising performance to detect early renal dysfunction [Citation11–13]. PDU displays the total power in the Doppler signal, which is not frequency-dependent and does not display directional or velocity information. PDU is useful in organs or areas where blood flow is relatively slow, such as the prepubertal testes, placenta, and renal cortex. In most kidneys, PDU reveals a diffused, homogeneous blush color of nearly the entire renal cortex, which results from the sum of numerous weak signals from small vessels and their branches distal to the arcuate arteries [Citation14]. PDU depicts more vessels than does color Doppler ultrasound, particularly at the renal poles and in the superficial cortex [Citation14]. Several studies have found that PDU correlates well with invasively measured renal blood flow [Citation15,Citation16]. Recent studies have used semiquantitative PDU scores as an indicator of renal perfusion and found that it could help predict delayed graft function after renal transplantation [Citation17] and could help evaluate the severity and prognosis of AKI [Citation18].
In our previous study, we found that PDU scores could effectively predict AKI stage 3 in patients with cardiac failure but not in patients with sepsis [Citation19]. However, the main reason for admission to the ICU may be unclear or there may be a combination of various reasons in the beginning, while the hemodynamic status of patients can be rapidly determined using bedside ultrasound and other parameters. Moreover, the diagnostic value of RRI and PDU score for AKI may be based on their evaluation of renal perfusion. Therefore, we expanded the sample size and obtained the cardiac index by transthoracic echocardiography at the same time as the renal ultrasound, with the aim to analyze the correlations among RRI, PDU score, and cardiac index (CI), and to investigate the diagnostic performance of RRI and semiquantitative PDU scores in predicting AKI stage 3 in critically ill patients.
2. Materials and methods
2.1. Patients
This study was approved by the ethics committee of Cangzhou Central Hospital in Cangzhou City, Hebei Province, China (ethical approval no. 2017-078-01), and all patients or their next of kin were informed in writing that the collected data will be used for research purposes. Critically ill patients admitted to the emergency ICU of Cangzhou Central Hospital from January 2018 to August 2019 were included. The inclusion criteria were as follows: admission for sepsis (according to the Sepsis-3 criteria [Citation3]), cardiac failure (defined as Killip classification grade III or IV in patients with acute myocardial infarction or as New York Heart Association Functional class IV in patients with acute heart failure), polytrauma (defined as an Injury Scale Severity score ≥25 [Citation20]), and critical conditions due to other causes. Patients with age <18 years, survival time <24 h, intra-abdominal pressure >15 mmHg (bladder pressure measurement), suspected or confirmed obstructive renal failure, known renal artery stenosis, severe chronic renal failure and a basal creatinine clearance of <30 mL/min, cardiac arrhythmia precluding renal Doppler measurement, patients recovering from previously diagnosed AKI at the time of inclusion, and pregnant patients were not included. AKI was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria (). The lowest SCr concentration within a month was chosen as the baseline creatinine value. If previous SCr concentrations within a month were unavailable, baseline creatinine was estimated based on the Modification of Diet in Renal Disease equation assuming a low normal value for baseline GFR (75 mL·min−1·[1.73 m2]−1) [Citation21]. The equation was as follows (creatinine is in mg/dL):
Table 1. Staging of AKI.
2.2. Study protocol and data collection
Height, body weight (estimated using the information provided by the patient or relatives), cause for admission, and accompanying diseases were recorded on admission. SCr concentration, 6-h urine output, arterial lactate concentration, use of mechanical ventilation, and use of vasoactive drugs were obtained within 6 h from admission. Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores were calculated 24 h after admission. The highest AKI stage within 5 days after admission assessed according to the KDIGO criteria (the need for renal replacement therapies was not considered) was used as the main outcome. Patients with AKI stage 3 as the highest AKI stage within 5 days from admission were classified into the AKI 3 group, whereas the other patients were classified into the AKI 0–2 group. Data on mortality and use of continuous renal replacement therapy (CRRT) were obtained on day 28.
2.3. RRI and semiquantitative PDU score measurements
Renal echography was performed with CX30 and HD15 (both from Philips Healthcare, Bothell, WA, USA) within the first 6 h of admission and after achieving a mean arterial pressure (MAP) of ≥65 mmHg with fluid therapy or vasoactive drugs, by an intensivist with about 3 years of experience in ultrasound operations and with a training certificate from the Chinese Critical Ultrasound Study Group. RRI and Semiquantitative PDU were measured as described in our previous study [Citation19]. Briefly, renal RI was measured from on the interlobar arteries and calculated using the following formula: (peak systolic velocity—end-diastolic velocity)/peak systolic velocity. Three measurements were taken and the mean value was recorded for further analysis. Semiquantitative PDU was conducted using the best blood flow image of energy Doppler ultrasound. Renal perfusion was assessed using semiquantitative PDU scores () [Citation22]. During the renal ultrasound examination, MAP, heart rate (HR), type and dose of catecholamine infusion, and oxygenation index were recorded.
Table 2. Semiquantitative PDU scores for evaluating intra-renal perfusion.
2.4. CI Measurements
Transthoracic echocardiography was performed at the same time as RRI and semiquantitative PDU score measurements. Cardiac output was calculated from the left ventricular outflow tract (LVOT), as described by McLean et al. [Citation23]. The diameter of the LVOT was taken to be the distance between the bases of the aortic valve cusp during systole, as seen from the long parasternal view. Pulsated wave Doppler samples were then obtained in the center of the LVOT from the apical view, paying close attention to obtaining an angle of Doppler signal to aortic blood flow close to 0° (<20°). The leading edge of five consecutive Doppler velocity curves was traced, and the average velocity time integral (VTI) was calculated. The body surface area (BSA) was calculated as follows (height was in cm and weight was in kg): BSA (m2) = 0.0061 × height + 0.0128 × weight − 0.1529. Thus, CI was thereafter calculated as follows (LVOT diameter and VTI were in cm): CI (L·min−1·[m2]−1) = (LVOT diameter/2)2×3.14 × VTI × HR × BSA−1 × 0.001. Reduced CI was defined as <3 L·min−1·(m2) −1.
2.5. Statistical analysis
Data are presented as median and interquartile range (IQR), mean and standard deviation, or number and percentage, as appropriate. The normality of all numeric continuous variables was examined by Kolmogorov–Smirnov test. Continuous variables without a normal distribution were compared using Non-parametric tests, continuous variables with a normal distribution using independent-sample t-tests, and categorical data using the chi-square test. Correlations among RRI, PDU score, and CI were evaluated using Spearman correlation coefficients. The diagnostic performance of RRI and PDU score in predicting AKI stage 3 was analyzed by Receiver operator characteristic (ROC) curves. Statistical tests were performed using IBM SPSS, version 26.0. All tests were two-sided, and P values <0.05 (α = 0.05) were considered statistically significant.
3. Results
3.1. General patient characteristics
A total of 209 patients initially participated in this study, of whom 15 died within 24 h, 10 discontinued the treatment during hospitalization, 6 were unsuitable for RRI assessment because of the occurrence of arrhythmia or abdominal hypertension, and 30 could not be evaluated for CI using transthoracic echocardiography. Thus, 148 patients were included in the final analysis. In this study, 65 patients were overlapped with our previous study [Citation19].
We analyzed the correlations among RRI, PDU score, and CI. In all patients, a negative correlation between RRI and PDU score (r = −0.517, p < 0.001) and a positive correlation between PDU score and CI (r = 0.193, p = 0.019) were found, whereas RRI was not correlated with CI (r = 0.131, p = 0.121). Furthermore, the correlation between PDU score and CI was better in CI-reduced patients (r = 0.283, p = 0.011) but was not statistically significant in CI-maintained patients (r = −0.008, p = 0.948). Thus, we divided all patients into the CI-reduced group (CI < 3 L·min−1·[m2] −1) and the CI-maintained group (CI ≥ 3 L·min−1·[m2]−1) and analyzed them to investigate the diagnostic performances of RRI and semiquantitative PDU scores in predicting AKI stage 3. The descriptive results of KDIGO stage assessment performed on day 5 are shown in . The patient characteristics are shown in . APACHE-II score, SOFA score, and mortality on day 28 significantly differed between the AKI 3 and AKI 0–2 groups in all patients and in patients with reduced CI (p < 0.05) but not in patients with normal CI (p > 0.05). The arterial lactate concentration, use of mechanical ventilation, and use of vasoactive drugs differed between the AKI 3 and AKI 0–2 groups in patients with reduced CI (p < 0.05) but not in all patients and in patients with normal CI (p > 0.05). Urine output, SCr, RRI, PDU score, and use of CRRT significantly differed between the AKI 3 and AKI 0–2 groups in all patients (p < 0.05) as well as in patients with reduced CI (p < 0.05) and in patients with normal CI (p < 0.05). Only admission for sepsis, admission for acute cardiac failure, and RRI significantly differed between CI-reduced and CI-maintained patients (p < 0.05).
Table 3. Descriptive analysis of KDIGO stage assessed on day 5.
Table 4. Patient characteristics according to KDIGO stage assessed on day 5.
3.2. Comparison of predictive values for AKI 3
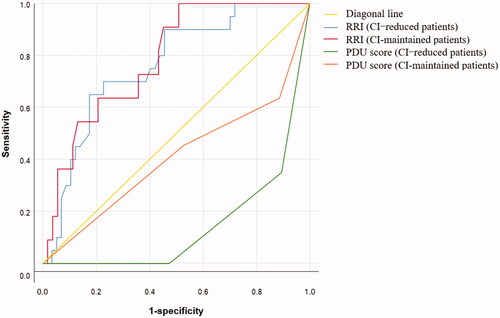
ROC curves were plotted to examine the role of RRI and PDU score in predicting AKI stage 3. Seven patients (three with reduced CI and four with maintained CI) had a failed RRI determination. The ROC curves of these indicators are shown in . The predictive value of RRI for AKI stage 3 was similar among all patients (area under the curve [AUC] 0.753, 95% confidence interval 0.674–0.822, p < 0.001), CI-reduced patients (AUC 0.761, 95% confidence interval 0.650–0.851, p < 0.001), and CI-maintained patients (AUC 0.786, 95% confidence interval 0.665–0.878, p < 0.001). PDU score could effectively predict AKI stage 3 in CI-reduced patients (AUC 0.872, 95% confidence interval 0.778–0.936, p < 0.001), and the optimal cutoff for PDU score was ≤1 (sensitivity 69.6%, specificity 89.5%, Youden index 0.590, accuracy in our population 83.8%). However, PDU score could not predict AKI stage 3 in in CI-maintained patients (AUC = 0.669, 95% confidence interval 0.544–0.778, p = 0.071).
Table 5. ROC curves for RRI and PDU score as predictors of AKI 3.
We compared the AUCs of RRI and PDU score for predicting AKI stage 3 in CI-reduced and CI-maintained patients (). In CI-reduced patients, the difference between RRI and PDU score was not statistically significant (p = 0.120). In CI-maintained patients, the predictive value of RRI for AKI 3 was better that of PDU score (p = 0.018). The predictive value of PDU score for AKI stage 3 was better in CI-reduced patients than in CI-maintained patients (p = 0.021). The predictive value of RRI for AKI stage 3 was not statistically different between CI-reduced and CI-maintained patients (p = 0.781).
4. Discussion
In this study, we found that PDU score and CI were statistically correlated with each other in critically ill patients, especially in those with reduced CI. Furthermore, PDU scores could effectively predict AKI stage 3 in CI-reduced patients but not in CI-maintained patients. Reductions in CI result in inadequate renal blood flow leading to renal ischemia, which plays a significant role in the progression of AKI in critically ill patients, especially in those with acute cardiac failure, massive blood or fluid loss, and other critical conditions. However, renal blood flow is maintained or actually increases in AKI induced by nephrotoxic substances or in sepsis-associated AKI [Citation24]. The assessment of renal injury using PDU is based on the assessment of renal perfusion. Therefore, the hemodynamic status of patients should be considered when evaluating renal function using semiquantitative PDU scores. However, admission for sepsis or admission for acute cardiac failure significantly differed between CI-reduced and CI-maintained patients in our study. Thus, it is unclear whether the difference of the predictive value of PDU score for AKI 3 between CI-reduced and CI-maintained patients was based on hemodynamic differences or etiological differences, and this issue needs further research. Given the good predictive value of PDU score for AKI 3 in CI-reduced patients and its advantages of convenience and repeatability of measurements, PDU score may useful as a tool for assessing AKI in patients with reduced CI. However, several disadvantages of semiquantitative PDU scores should be considered. For example, the results may be affected by breathing movement because the technique has significant soft-tissue flash artifacts. In addition, gain settings and obesity may affect the imaging results, and semiquantitative PDU scores are categorical and may show a certain degree of subjectivity.
In this study, we found that RRI was not correlated with CI and has poor predictive value for AKI stage 3. The results were similar to the findings of recent studies [Citation25,Citation26]. Besides the renal perfusion state, RRI could be directly influenced by intrinsic kidney diseases characterized by increased pressure of the renal interstitium or urinary tract [Citation27]. RRI, which is elevated in patients with hypertension with preserved renal function, also reflects systemic vascular changes [Citation28]. In critically ill patients, RRI was influenced by several other factors, including MAP, lactate concentration, and age [Citation29]. Thus, RRI is an integrative parameter whose determination involves renal and extra-renal factors, and is often difficult to discriminate in common clinical practice.
A prospective multicentre study found that the performances of RRI (AUC 0.58, 95% confidence interval 0.52–0.64) and semiquantitative renal perfusion (AUC 0.59, 95% confidence interval 0.54–0.65) in predicting persistent AKI were poor [Citation30]. In this study, the predictive values of RRI and PDU score for AKI 3 were also poor in all critically ill patients. However, we grouped critically ill patients according to CI and found that PDU scores could effectively predict AKI stage 3 in CI-reduced patients but not in CI-maintained patients. The clinical status of critically ill patients is complex and changeable. These results suggest that the hemodynamic status of patients should be considered when evaluating renal function using PDU scores. This is the unique of this study compared to other studies discuss the same issue.
This study has several limitations that may deserve to be discussed. First, Chronic renal lesions may cause elevated RRIs without changes in Scr or urine volume. Second, RRI and PDU scores were measured after the interventions with fluid therapy or vasoactive drugs aiming to avoid the influence of hemodynamic conditions on results. Third, owing to the small sample size in this study, the ROC results may be unreliable [Citation31]. Moreover, the study was not blinded. Further studies should include larger populations.
Ethics approval
This study was approved by the ethics committee of Cangzhou Central Hospital in Cangzhou City, Hebei Province, China (ethical approval no. 2017-078-01). The patients or their next of kin were informed in writing that the collected data will be used for research purposes.
| Abbreviations | ||
| AKI | = | acute kidney injury |
| APACHE | = | Acute Physiology and Chronic Health Evaluation |
| AUC | = | area under the curve |
| BSA | = | body surface area |
| CI | = | cardiac index |
| CRRT | = | continuous renal replacement therapy |
| GFR | = | glomerular filtration rate |
| HR | = | heart rate |
| ICU | = | intensive care unit |
| KDIGO | = | Kidney Disease Improving Global Outcomes |
| LVOT | = | left ventricular outflow tract |
| MAP | = | mean arterial pressure |
| PDU | = | power Doppler ultrasound |
| ROC | = | receiver operator characteristic |
| RRI | = | renal resistive index |
| SCr | = | serum creatinine |
| SOFA | = | Sequential Organ Failure Assessment |
| VTI | = | velocity time integral. |
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Levey AS, James MT. Acute kidney injury. Ann Intern Med. 2017;167(9):Itc66–80.
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762–774.
- Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. CJASN. 2013;8(9):1482–1493.
- Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10(3):R73.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–84.
- Zhang Z, Lu B, Sheng X, et al. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58(3):356–365.
- Albeladi FI, Algethamy HM. Urinary neutrophil gelatinase-associated lipocalin as a predictor of acute kidney injury, severe kidney injury, and the need for renal replacement therapy in the intensive care unit. Nephron Extra. 2017;7(2):62–77.
- Xie Y, Wang Q, Wang C, et al. High urinary excretion of kidney injury molecule-1 predicts adverse outcomes in acute kidney injury: a case control study. Crit Care. 2016;20(1):286.
- Razek A, Al-Adlany M, Alhadidy AM, et al. Diffusion tensor imaging of the renal cortex in diabetic patients: correlation with urinary and serum biomarkers. Abdom Radiol. 2017;42(5):1493–1500.
- Schnell D, Darmon M. Bedside Doppler ultrasound for the assessment of renal perfusion in the ICU: advantages and limitations of the available techniques. Crit Ultrasound J. 2015;7(1):24.
- Cherry AD, Hauck JN, Andrew BY, et al. Intraoperative renal resistive index threshold as an acute kidney injury biomarker. J Clin Anesth. 2019; 61:109626.
- David S, Stéphane D, Anatole H, et al. Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock. 2012;38:592–597.
- Bellos I, Perrea DN, Kontzoglou K. Renal resistive index as a predictive factor of delayed graft function: a meta-analysis. Transplant Rev (Orlando). 2019;33(3):145–153.
- Bude RO, Rubin JM, Adler RS. Power versus conventional color Doppler sonography: comparison in the depiction of normal intrarenal vasculature. Radiology. 1994;192(3):777–780.
- Durick JE, Winter TC, Schmiedl UP, et al. Renal perfusion: pharmacologic changes depicted with power Doppler US in an animal model. Radiology. 1995;197(3):615–617.
- Kuwa T, Cancio LC, Sondeen JL, et al. Evaluation of renal cortical perfusion by noninvasive power Doppler ultrasound during vascular occlusion and reperfusion. J Trauma. 2004;56(3):618–624.
- Contti MM, Garcia PD, Kojima CA, et al. Quantified power Doppler as a predictor of delayed graft function after renal transplantation. Int Urol Nephrol. 2015;47(2):405–412.
- Chen XK, Huang LF, Wang XT, et al. Value of power Doppler ultrasound to evaluate acute kidney injury. Zhonghua Yi Xue Za Zhi. 2012;92(47):3354–3357.
- Zhi HJ, Zhao J, Nie S, et al. Semiquantitative power Doppler ultrasound score to predict acute kidney injury in patients with sepsis or cardiac failure: a prospective observational study. J Intensive Care Med. 2019 Nov 13:885066619887333. DOI:https://doi.org/10.1177/0885066619887333. [Epub ahead of print]
- Baker SP, Oʼneill B, Haddon W, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196.
- Group Cepc. Modification and evaluation of MDRD estimating equation for Chinese patients with chronic kidney disease. Chin J Nephrol. 2006;22:589–595.
- Barozzi L, Valentino M, Santoro A, et al. Renal ultrasonography in critically ill patients. Crit Care Med. 2007;35(Suppl):S198–S205.
- McLean AS, Needham A, Stewart D, Parkin R. Estimation of cardiac output by noninvasive echocardiographic techniques in the critically ill subject. Anaesth Intensive Care. 1997;25(3):250–254.
- Langenberg C, Wan L, Egi M, et al. Renal blood flow and function during recovery from experimental septic acute kidney injury. Intensive Care Med. 2007;33(9):1614–1618.
- Haitsma Mulier JLG, Rozemeijer S, Rottgering JG, et al. Renal resistive index as an early predictor and discriminator of acute kidney injury in critically ill patients; a prospective observational cohort study. PLoS One. 2018;13(6):e0197967.
- Muthukrishnan K, Parida S, Barathi SD, et al. Doppler resistive index to reflect risk of acute kidney injury after major abdominal surgery: a prospective observational trial. Indian J Anaesth. 2019;63:551–557.
- Boddi M, Natucci F, Ciani E. The internist and the renal resistive index: truths and doubts. Intern Emerg Med. 2015;10(8):893–905.
- Andrikou I, Tsioufis C, Konstantinidis D, et al. Renal resistive index in hypertensive patients. J Clin Hypertens. 2018;20(12):1739–1744.
- Oliveira RAG, Mendes PV, Park M, et al. Factors associated with renal Doppler resistive index in critically ill patients: a prospective cohort study. Ann Intensive Care. 2019;9(1):23.
- Darmon M, Bourmaud A, Reynaud M, et al. Performance of Doppler-based resistive index and semi-quantitative renal perfusion in predicting persistent AKI: results of a prospective multicenter study. Intensive Care Med. 2018;44(11):1904–1913.
- Hanczar B, Hua J, Sima C, et al. Small-sample precision of ROC-related estimates. Bioinformatics. 2010;26(6):822–830.