Abstract
Background
We aimed to evaluate the acute kidney injury (AKI) incidence and its associated risk of mortality in patients with implantable left ventricular assist devices (LVAD).
Methods
A systematic literature search in Ovid MEDLINE, EMBASE, and Cochrane Databases was conducted through January 2020 to identify studies that provided data on the AKI incidence and AKI-associated mortality risk in adult patients with implantable LVADs. Pooled effect estimates were examined using random-effects, generic inverse variance method of DerSimonian-Laird.
Results
Fifty-six cohort studies with 63,663 LVAD patients were enrolled in this meta-analysis. The pooled incidence of reported AKI was 24.9% (95%CI: 20.1%–30.4%) but rose to 36.9% (95%CI: 31.1%–43.1%) when applying the standard definition of AKI per RIFLE, AKIN, and KDIGO criteria. The pooled incidence of severe AKI requiring renal replacement therapy (RRT) was 12.6% (95%CI: 10.5%–15.0%). AKI incidence did not differ significantly between types of LVAD (p = .35) or indication for LVAD use (p = .62). While meta-regression analysis did not demonstrate a significant association between study year and overall AKI incidence (p = .55), the study year was negatively correlated with the incidence of severe AKI requiring RRT (slope = −0.068, p < .001). The pooled odds ratios (ORs) of mortality at 30 days and one year in AKI patients were 3.66 (95% CI, 2.00–6.70) and 2.22 (95% CI, 1.62–3.04), respectively. The pooled ORs of mortality at 30 days and one year in severe AKI patients requiring RRT were 7.52 (95% CI, 4.58–12.33) and 5.41 (95% CI, 3.63–8.06), respectively.
Conclusion
We found that more than one-third of LVAD patients develop AKI based on standard definitions, and 13% develop severe AKI requiring RRT. There has been a potential improvement in the incidence of severe AKI requiring RRT for LVAD patients. AKI in LVAD patients was associated with increased 30-day and 1 year mortality.
Introduction
Implantable left ventricular assist devices (LVADs) are increasingly utilized as a bridge to heart transplantation or destination therapy for patients with end-stage heart failure [Citation1–7]. The use of LVADs is shown to be associated with reduced mortality in patients on heart transplantation waiting lists, and they improve quality of life and functional status in advanced heart failure patients [Citation8]. LVADs alleviate the cardiovascular load on a failing heart and have shown notable advantages in treating patients with advanced heart failure, providing prolonged survival and improvement in the quality of life [Citation9,Citation10]. Clinical outcomes after LVAD implantation have significantly improved over the past decade, with 1 year and 2 year survival of 83% and 73%, respectively [Citation11, Citation12]. In the United States, the number of LVAD implantations rose, from only 459 implants in 2008 to a total of 2,118 implants in 2017 [Citation11].
Despite the LVAD benefits mentioned above, several studies have reported persistent adverse complications following LVAD implantation, such as bleeding, cardiac arrhythmias, hypertension, sepsis, disabling stroke, and acute kidney injury (AKI) [Citation8,Citation13]. Post-implantation AKI has been associated with negative impacts on patient outcomes, including right ventricular failure, arrhythmia, and reduced survival [Citation14,Citation15]. The reported AKI incidence among LAVD patients widely ranged from 4–70%. This variability is likely due to the use of non-standardized AKI definitions in previous studies [Citation15–70]. Furthermore, the mortality associated with AKI and current trends of AKI occurrence in LVAD patients are unclear [Citation18,Citation21,Citation22,Citation24,Citation26,Citation40,Citation44,Citation46,Citation52,Citation54,Citation57,Citation63,Citation65].
This systematic review and meta-analysis were conducted to summarize the AKI incidence and mortality risk among adult patients with LVADs.
Methods
The protocol for this meta-analysis is registered with PROSPERO (no. CRD42020134592). The meta-analyses were conducted in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [Citation71].
Search strategy
Two investigators (CT and PL) independently searched for published clinical trials or observational studies indexed in MEDLINE, EMBASE and the Cochrane databases from inception to January 2020 using a search strategy (S1 in online Supplementary Data 1) that included the terms “left ventricular assist device”, “LVAD”, “ventricular assist device”, “acute kidney failure”, “acute kidney injury” and “renal replacement therapy”. No language restrictions were applied in this systemic review and meta-analysis. A manual search for additional pertinent studies and review articles using references from the retrieved articles was also completed.
Study eligibility criteria
Two main criteria were used for study inclusion. First, the study had to report the incidence of AKI or severe AKI requiring renal replacement therapy (RRT), and AKI associated mortality risk in adult patients with LVADs aged at least 18 years. Second, the study had to include data assessing AKI incidence or mortality risk with 95% confidence intervals (CIs) (or sufficient raw data for the calculation). Patients were excluded if they only used a temporary, short-term, non-implantable LVAD during a hospitalization. Study eligibility was independently determined by two investigators (CT and PL). Differences were resolved by mutual consensus.
A standardized data collection form was used to obtain the following information from each study: title, name of the first author, year of study, year of publication, country of origin, number of participants, demographic data of participants, the method used to diagnose the outcomes of interest (AKI incidence and associated mortality), the average duration of follow-up, adjusted and unadjusted risk ratios and their corresponding 95% CI, and list of confounders that were adjusted for in the multivariate analyses. To ensure accuracy, both investigators independently performed this data extraction process. Any data discrepancy was resolved by referring back to the original articles. The Newcastle-Ottawa quality assessment scale was utilized to appraise the quality of observational studies [Citation72].
Statistical analysis
The meta-analysis of combined data was performed using a random-effects, generic inverse variance method of DerSimonian and Laird [Citation73]. We assessed the overall incidence of AKI, which was defined by the consensus definitions provided by the Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) [Citation74], Acute Kidney Injury Network (AKIN) [Citation75], and Kidney Disease: Improving Global Outcomes) (KDIGO) [Citation76] classifications. We did not impute missing values for any outcomes in our analyses. A random-effect model was used to pool AKI incidence and AKI-associated mortality risk due to the possibility of between-study variance. Heterogeneity among included studies was statistically evaluated by the Cochrane’s Q test and the I2 statistic. Heterogeneity was considered insignificant when I2 of ≤25%, low when I2 of 26–50%, moderate when I2 of 51–75%, and high when I2 of ≥75% [Citation77]. Per Cochrane, publication bias was assessed using a funnel plot. Funnel plot asymmetry was further confirmed with Egger’s test if there were >10 available studies [Citation78]. All analysis was performed using The Comprehensive Meta-Analysis software version 3.3.070 (Biostat Inc, New Jersey, USA). The data underlying the results presented in the study are available through the Open Science Framework (https://osf.io/8hk35/)
Results
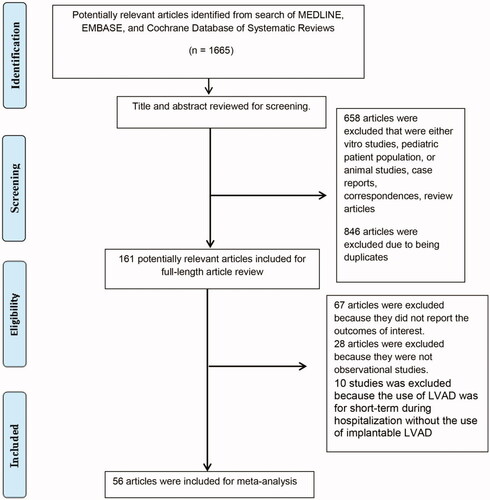
Our search approach identified a total of 1,665 potentially eligible articles. We initially excluded 846 articles because they were case reports, correspondences, review articles, or studies involving in-vitro, animal, or pediatric patients. Six hundred fifty-eight duplicated articles were additionally excluded. After the review of 161 full-length articles, we subsequently excluded 67 articles because the data on AKI incidence and its associated mortality was not available, 28 articles because they were not observational studies or clinical trials, and 10 articles because they investigated AKI in short-term LVAD use, not implantable LVAD [Citation79–88]. Therefore, 56 cohort studies [Citation15–70] with a total of 63,663 adult patients were included in this meta-analysis. demonstrates by flowchart the systematic review of the literature. shows the characteristics of the included studies. The kappa for systematic searches, selection of studies and data extraction were 1.00, 0.91 and 0.98, respectively
Table 1. Main characteristic of the included studies assessing the incidence of acute kidney injury in LVAD patients.
Incidence of AKI in LVAD patients
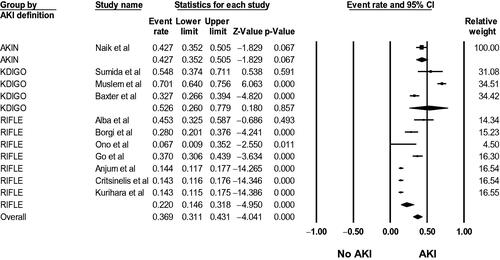
Fifty-six studies [Citation15–70] evaluated AKI incidence in LVAD patients. The pooled incidence of reported AKI was 24.9% (95%CI: 20.1%–30.4%, I2 = 99%, Supplementary Figure S1), and the pooled incidence of severe AKI requiring RRT was 12.6% (95%CI: 10.5%–15.0%, I2 = 95%, ). Using standard AKI definitions (RIFLE, AKIN, and KDIGO criteria), the pooled incidence of AKI was 36.9% (95%CI: 31.1%–43.1%, I2 = 97%, ).
Figure 2. Forest plots of the included studies evaluating the incidence of severe AKI requiring RRT among LVAD patients. A diamond data marker represents the overall rate from the individual studies (square data marker) and 95% CI.
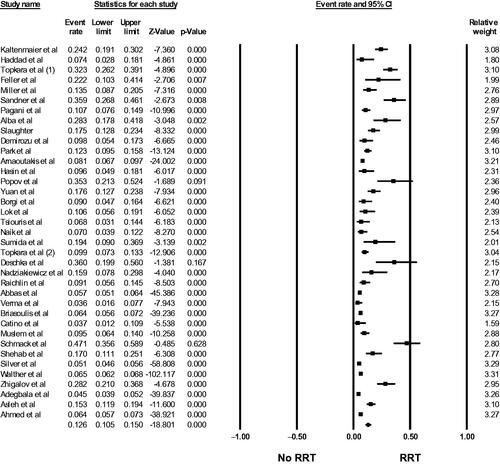
Figure 3. Forest plots of the included studies evaluating the incidence of AKI using standard AKI definitions (RIFLE, AKIN, and KDIGO criteria) among LVAD patients. A diamond data marker represents the overall rate from the individual studies (square data marker) and 95% CI.
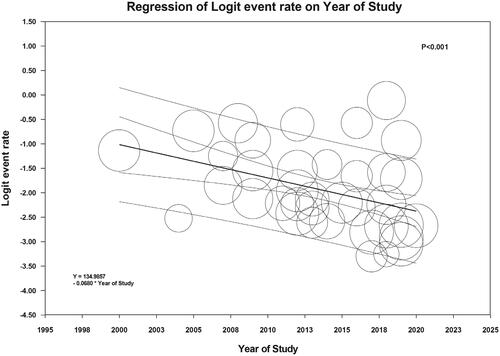
AKI incidence did not differ significantly between types of LVAD (pulsatile vs. continuous flow) (p = .35) or indication of LVAD use (bridge to transplant vs. destination therapy) (p = 0.62). While meta-regression analysis did not demonstrate a significant association between study year and overall AKI incidence (p = .55) (Supplementary Figure S2), the study year was negatively correlated with the incidence of severe AKI requiring RRT (slope = −0.068, p < .001, ).
Mortality associated with AKI in LVAD patients
Thirteen studies [Citation18,Citation21,Citation22,Citation24,Citation26,Citation40,Citation44,Citation46,Citation52,Citation54,Citation57,Citation63,Citation65] evaluated mortality associated with AKI in LVAD patients, as shown in . The pooled odds ratio (OR) of 30-day mortality was 3.66 (95% CI, 2.00–6.70, I2 = 71%, Supplementary Figure S3) and the pooled OR of 1 year mortality was 2.22 (95% CI, 1.62–3.04, I2 = 0%, Supplementary Figure S4) in LVAD patients with AKI, compared with no AKI. The pooled OR of 30-day mortality was 7.52 (95% CI, 4.58–12.33, I2 = 73%, Supplementary Figure S5) and the pooled OR of 1-year mortality was 5.41 (95% CI, 3.63–8.06, I2 = 0%, Supplementary Figure S6) in LVAD patients with severe AKI requiring RRT, compared with no RRT.
Table 2. AKI associated Mortality in LVAD Patients.
Publication bias evaluation
Using funnel plots (Supplementary Figure S7–10) and Egger’s regression asymmetry tests, there was no significant publication bias found in this meta-analysis (p-values = .78, .25, .53, and .59, respectively).
Discussion
This meta-analysis supports that AKI is a common complication after LVAD implantation. The pooled incidence of post-LVAD AKI (using standard AKI definitions) and severe AKI requiring RRT was 37% and 13%, respectively. We found no significant difference in AKI incidence after adjusting for LVAD indication (bridging vs. destination therapy). Moreover, our analysis did not show any difference in AKI incidence between pulsatile and continuous flow LVAD devices. It was also noted that the incidence of AKI was higher (37% vs. 25%) when using standard AKI criteria, such as RIFLE, AKIN, and KDIGO. This may indicate that defining AKI using consensus criteria may improve the sensitivity of detecting AKI in LVAD patients. This meta-analysis further identified that AKI incidence remained constant over time, while the need for RRT due to AKI decreased significantly in more recent studies.
The mechanisms of AKI among LVAD patients are complex and can be multifactorial [Citation5,Citation89,Citation90]. Mechanical stress on red blood cells traveling through the LVAD leads to constant low-level hemolysis, potentially resulting in pigment nephropathy [Citation5]. These patients also tend to have acquired von Willebrand disease, as the von Williebrand factor multimers suffer fragmentation when passing through the LVAD pump, leading to subsequent increased risk of AKI due to decreased effective blood volume secondary to bleeding from arteriovenous malformations or severe epistasis [Citation5,Citation8]. An additional concern is, the development of right heart failure following LVAD implantation, which is observed in approximately 20–50% of patients [Citation14,Citation91–94]. This right heart failure could further potentiate renal venous congestion, compromised net effective renal perfusion pressure and decrease GFR [Citation95,Citation96]. Hemodynamic instability in the immediate post-operative period could exacerbate kidney ischemia and lead to acute tubular necrosis. Accelerated thrombogenicity secondary to the LVAD pump and blood stasis may trigger renal microemboli, as evidenced by the presence of kidney infarctions [Citation97]. Yet an another proposed hypothesis for the development of worsening kidney function in LVAD patients is that the continuous flow of the LVAD might lead to a proliferation of afferent arteriolar smooth muscle cells and periarteriolitis, which causes an eventual decline in eGFR [Citation98]. However, in our study, the incidence of AKI was similar between pulsatile-flow and continuous-flow LVADs, suggesting that the lack of pulsatility from continuous-flow LVADs might not be the cause of associated AKI. On the other hand, currently only a limited amount of pulsatility can be generated by LVADs using periodic speed steps, and it is considerably smaller in both flow increase and rate than what is found with natural pulsatile circulation [Citation99]. Given the ongoing efforts to advance LVAD technology, future studies are needed to evaluate whether or not improvements in pulsatile-flow LVADs can reduce the incidence of post-LVAD implantation AKI.
The findings from our study demonstrated that LVAD patients who developed AKI had greater odds of 30-day and 1-year mortality. The pooled odds ratios were even higher in patients with severe AKI requiring RRT. It is emphasized that even an occurrence of AKI following LVAD implantation has long-lasting negative clinical impacts, especially if dialysis is required [Citation40, Citation100]. Post-implantation AKI is associated with right ventricular failure and arrhythmias, both of which are, in turn, associated with increased mortality [Citation28]. Our study shows that LVAD patients with severe AKI requiring RRT are associated with 7.5-fold and 5.4-fold increased risks of 30-day and 1-year mortality, respectively. While the findings of our study suggested no significant changes in overall AKI incidence over the study years, the incidence of severe AKI requiring RRT appeared to decrease over study year significantly. This finding suggests potential improvements in the prevention, mitigation, and clinical management of severe AKI in LVAD patients. Interventions proposed to mitigate the incidence and severity of post-LVAD implantation AKI include maintenance of high mean arterial pressures (MAP) and coronary perfusion rates [Citation96], inotropic support when needed, frequent monitoring of MAP via audible doppler ultrasound in combination with calibrated blood pressure measurement devices, and maintaining central venous pressures between 8 and 12 mm Hg via diuretics or extracorporeal ultrafiltration. Avoiding nephrotoxic medications postoperatively until hemodynamic stability is achieved has also been recommended. In patients with severe right heart failure, right ventricular assist devices may help decrease right-sided venous congestion and improve renal perfusion [Citation96]. Future studies are required to assess whether these measures can significantly help to reduce AKI incidence or promote AKI recovery among LVAD patients, to improve patient survival rates ultimately.
Our systematic and meta-analysis is subject to certain limitations. First, all studies were observational in design, making them susceptible to potential selection bias. The potential sources of this heterogeneity included differences in variation in baseline characteristics (e.g., age, sex, ethnicity, and underlying chronic kidney disease), LVAD types, indications for LVAD, and outcome ascertainments. Second, the incidence of AKI is predisposed to several confounding factors. Our meta-analysis had a high degree of heterogeneities. However, we performed subgroup analyses after applying standardized AKI definitions and conducted meta-regression analyses assessing the effects of the study year, LVAD types (pulsatile vs. continuous flow), and indications for LVAD implantation (bridge to transplant vs. destination therapy) on AKI incidence. The results from these additional analyses provided clinical insights that may highlight and stimulate the need for additional research to intervene on AKI in LVAD patients. Third, the data on the use of peritoneal dialysis as a modality of RRT in LVAD patients is limited, and all of the included studies defined RRT as either non-peritoneal continuous or intermittent renal replacement therapies. Lastly, AKI diagnoses in the included studies were solely based on the change in serum creatinine, which might underestimate the incidence of AKI [Citation101–105]. Data on urine output or other AKI biomarkers data were limited [Citation104, Citation106] Furthermore, future studies using artificial intelligence to predict AKI among LVAD patients are needed [Citation107].
In conclusion, AKI is a common complication among LVAD patients. There have been some potential improvements in the incidence rates of severe AKI requiring RRT in LVAD patients. AKI, while on LVAD, is associated with increased 30-day and 1-year mortality.
Authors’ contributions
All authors had access to the data, reviewed, and approved the final manuscript.
Supplemental Material
Download PDF (68.8 KB)Supplemental Material
Download PDF (213.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sen A, Larson JS, Kashani KB, et al. Mechanical circulatory assist devices: a primer for critical care and emergency physicians. Crit Care. 2016;20(1):153.
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1–S39. [20181499]
- Timms D. A review of clinical ventricular assist devices. Med Eng Phys. 2011;33(9):1041–1047.
- Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36(10):1080–1086.
- Patel AM, Adeseun GA, Ahmed I, et al. Renal failure in patients with left ventricular assist devices. Clin J Am Soc Nephrol. 2013;8(3):484–496.
- Mao H, Katz N, Kim JC, et al. Implantable left ventricular assist devices and the kidney. Blood Purif. 2014;37(1):57–66.
- Tromp TR, de Jonge N, Joles JA. Left ventricular assist devices: a kidney’s perspective. Heart Fail Rev. 2015;20(4):519–532.
- Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376(5):451–460.
- Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail. 2017;19(5):595–602.
- Maciver J, Ross HJ. Quality of life and left ventricular assist device support. Circulation. 2012;126(7):866–874.
- Singhvi A, Trachtenberg B. Left ventricular assist devices 101: shared care for general cardiologists and primary care. J Clin Med. 2019;8(10):pii:E1720.
- Kormos RL, Cowger J, Pagani FD, et al. The society of thoracic surgeons intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38(2):114–126.
- Soliman OII, Akin S, Muslem R, et al. Derivation and validation of a novel right-sided heart failure model after implantation of continuous flow left ventricular assist devices: the EUROMACS (European Registry for Patients with Mechanical Circulatory Support) right-sided heart failure risk score. Circulation. 2018;137(9):891–906.
- Yalcin YC, Bunge JJH, Guven G, et al. Acute kidney injury following left ventricular assist device implantation: contemporary insights and future perspectives. J Heart Lung Transplant. 2019;38(8):797–805.
- Genovese EA, Dew MA, Teuteberg JJ, et al. Early adverse events as predictors of 1-year mortality during mechanical circulatory support. J Heart Lung Transplant. 2010;29(9):981–988.
- Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125(25):3191–3200.
- Abbas A, Mahmoud A, Ahmed M, et al. Gastrointestinal bleeding during the index hospitalization for mechanical circulatory support devices implantation, a nationwide perspective. Dig Dis Sci. 2017;62(1):161–174.
- Adegbala O, Olakanmi O, Akintoye E, et al. Trends, outcomes, and readmissions among left ventricular assist device recipients with acute kidney injury requiring hemodialysis. ASAIO journal (American Society for Artificial Internal Organs: 1992). 2019.
- Ahmed A, Adegbala O, Akintoye E, et al. Gender differences in outcomes after implantation of left ventricular assist devices. Ann Thorac Surg. 2020;109(3):780–786.
- Aissaoui N, Morshuis M, Schoenbrodt M, et al. Temporary right ventricular mechanical circulatory support for the management of right ventricular failure in critically ill patients. J Thorac Cardiovasc Surg. 2013;146(1):186–191.
- Alba AC, Rao V, Ivanov J, et al. Predictors of acute renal dysfunction after ventricular assist device placement. J Card Fail. 2009;15(10):874–881.
- Anjum A, Kurihara C, Critsinelis A, et al. Acute kidney injury after implantation of a left ventricular assist device: a comparison of axial-flow (HeartMate II) and centrifugal-flow (HeartWare HVAD) devices. J Artif Organs. 2018;21(3):285–292.
- Arnaoutakis GJ, George TJ, Kilic A, et al. Risk factors for early death in patients bridged to transplant with continuous-flow left ventricular assist devices. Ann Thorac Surg. 2012;93(5):1549–1555. discussion 1555.
- Asleh R, Schettle S, Briasoulis A, et al. Predictors and outcomes of renal replacement therapy after left ventricular assist device implantation. Mayo Clin Proc. 2019;94(6):1003–1014.
- Baxter RD, Tecson KM, Still S, et al. Predictors and impact of right heart failure severity following left ventricular assist device implantation. J Thorac Dis. 2019;11(S6):S864–s870.
- Borgi J, Tsiouris A, Hodari A, et al. Significance of postoperative acute renal failure after continuous-flow left ventricular assist device implantation. Ann Thorac Surg. 2013;95(1):163–169.
- Briasoulis A, Inampudi C, Akintoye E, et al. Trends in utilization, mortality, major complications, and cost after left ventricular assist device implantation in the United States (2009–2014). Am J Cardiol. 2018;121(10):1214–1218.
- Brisco MA, Kimmel SE, Coca SG, et al. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail. 2014;7(1):68–75.
- Catino AB, Ferrin P, Wever-Pinzon J, et al. Clinical and histopathological effects of heart failure drug therapy in advanced heart failure patients on chronic mechanical circulatory support. Eur J Heart Fail. 2018;20(1):164–174.
- Critsinelis A, Kurihara C, Volkovicher N, et al. Model of end-stage liver Disease-eXcluding International Normalized Ratio (MELD-XI) scoring system to predict outcomes in patients who undergo left ventricular assist device implantation. Ann Thorac Surg. 2018;106(2):513–519.
- Demirozu ZT, Etheridge WB, Radovancevic R, et al. Results of HeartMate II left ventricular assist device implantation on renal function in patients requiring post-implant renal replacement therapy. J Heart Lung Transplant. 2011;30(2):182–187.
- Deng MC, Edwards LB, Hertz MI, et al. Mechanical circulatory support device database of the International Society for Heart and Lung Transplantation: third annual report-2005. J Heart Lung Transplant. 2005;24(9):1182–1187.
- Deschka H, Holthaus AJ, Sindermann JR, et al. Can perioperative right ventricular support prevent postoperative right heart failure in patients with biventricular dysfunction undergoing left ventricular assist device implantation? J Cardiothorac Vasc Anesth. 2016;30(3):619–626.
- Feller ED, Sorensen EN, Haddad M, et al. Clinical outcomes are similar in pulsatile and nonpulsatile left ventricular assist device recipients. Ann Thorac Surg. 2007;83(3):1082–1088.
- Frazier OH, Rose EA, Oz MC, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122(6):1186–1195.
- Go PH, Hodari A, Nemeh HW, et al. Effect of preoperative albumin levels on outcomes in patients undergoing left ventricular device implantation. ASAIO J (Am Soc Artif Int Organs: 1992). 2015;61(6):734–737.
- Haddad M, Hendry PJ, Masters RG, et al. Ventricular assist devices as a bridge to cardiac transplantation: the Ottawa experience. Artif Organs. 2004;28(2):136–141.
- Hasin T, Topilsky Y, Schirger JA, et al. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol. 2012;59(1):26–36.
- John R, Naka Y, Smedira NG, et al. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg. 2011;92(4):1406–1413; discussion 1413.
- Kaltenmaier B, Pommer W, Kaufmann F, et al. Outcome of patients with ventricular assist devices and acute renal failure requiring renal replacement therapy. ASAIO J (Am Soc Artif Int Organs: 1992). 2000;46(3):330–333.
- Kurihara C, Critsinelis AC, Kawabori M, et al. Frequency and consequences of right-sided heart failure after continuous-flow left ventricular assist device implantation. Am J Cardiol. 2018;121(3):336–342.
- Lok SI, Martina JR, Hesselink T, et al. Single-centre experience of 85 patients with a continuous-flow left ventricular assist device: clinical practice and outcome after extended support. Eur J Cardiothorac Surg. 2013;44(3):e233–238.
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885–896.
- Muslem R, Caliskan K, Akin S, et al. Acute kidney injury and 1-year mortality after left ventricular assist device implantation. J Heart Lung Transplant. 2018;37(1):116–123.
- Nadziakiewicz P, Szygula-Jurkiewicz B, Niklewski T, et al. Effects of left ventricular assist device support on end-organ function in patients with heart failure: comparison of pulsatile- and continuous-flow support in a single-center experience. Transplant Proc. 2016;48(5):1775–1780.
- Naik A, Akhter SA, Fedson S, et al. Acute kidney injury and mortality following ventricular assist device implantation. Am J Nephrol. 2014;39(3):195–203.
- Ono M, Joshi B, Brady K, et al. Cerebral blood flow autoregulation is preserved after continuous-flow left ventricular assist device implantation. J Cardiothorac Vasc Anesth. 2012;26(6):1022–1028.
- Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54(4):312–321.
- Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail. 2012;5(2):241–248.
- Popov AF, Hosseini MT, Zych B, et al. Clinical experience with HeartWare left ventricular assist device in patients with end-stage heart failure. Ann Thorac Surg. 2012;93(3):810–815.
- Raichlin E, Baibhav B, Lowes BD, et al. Outcomes in Patients with Severe Preexisting Renal Dysfunction After Continuous-Flow Left Ventricular Assist Device Implantation. ASAIO J (Am Soc Artif Int Organs: 1992). 2016;62(3):261–267.
- Sandner SE, Zimpfer D, Zrunek P, et al. Renal function after implantation of continuous versus pulsatile flow left ventricular assist devices. J Heart Lung Transplant. 2008;27(5):469–473.
- Schechter MA, Daneshmand MA, Patel CB, et al. Outcomes after implantable left ventricular assist device replacement procedures. ASAIO J (Am Soc Artif Int Organs: 1992). 2014;60(1):44–48.
- Schmack B, Grossekettler L, Weymann A, et al. Prognostic relevance of hemodialysis for short-term survival in patients after LVAD implantation. Sci Rep. 2018;8(1):8546.
- Shehab S, Macdonald PS, Keogh AM, et al. Long-term biventricular HeartWare ventricular assist device support-Case series of right atrial and right ventricular implantation outcomes. J Heart Lung Transplant. 2016;35(4):466–473.
- Shehab S, Rao S, Macdonald P, et al. Outcomes of venopulmonary arterial extracorporeal life support as temporary right ventricular support after left ventricular assist implantation. J Thorac Cardiovasc Surg. 2018;156(6):2143–2152.
- Silver SA, Long J, Zheng Y, et al. Outcomes after left ventricular assist device implantation in patients with acute kidney injury. J Thorac Cardiovasc Surg. 2019. 2019;S0022–5223(19)30742–1.
- Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;32(7):675–683.
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251.
- Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol. 2011;57(19):1890–1898.
- Strueber M, Larbalestier R, Jansz P, et al. Results of the post-market Registry to Evaluate the HeartWare Left Ventricular Assist System (ReVOLVE). J Heart Lung Transplant. 2014;33(5):486–491.
- Strueber M, HeartWare Investigators, O'Driscoll G, Jansz P, Khaghani A. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol. 2011;57(12):1375–1382.
- Sumida M, Doi K, Kinoshita O, et al. Perioperative plasma neutrophil gelatinase-associated lipocalin measurement in patients who undergo left ventricular assist device implantation surgery. Circ J. 2014;78(8):1891–1899.
- Topkara VK, Coromilas EJ, Garan AR, et al. Preoperative proteinuria and reduced glomerular filtration rate predicts renal replacement therapy in patients supported with continuous-flow left ventricular assist devices. Circ-Heart Fail. 2016;9(12):e002897.
- Topkara VK, Dang NC, Barili F, et al. Predictors and outcomes of continuous veno-venous hemodialysis use after implantation of a left ventricular assist device. J Heart Lung Transplant. 2006;25(4):404–408.
- Tsiouris A, Brewer RJ, Borgi J, et al. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant. 2013;32(3):299–304.
- Verma S, Bassily E, Leighton S, et al. Renal function and outcomes with use of left ventricular assist device implantation and inotropes in end-stage heart failure: a retrospective single center study. J Clin Med Res. 2017;9(7):596–604.
- Walther CP, Winkelmayer WC, Niu J, et al. Acute kidney injury with ventricular assist device placement: national estimates of trends and outcomes. Am J Kidney Dis. 2019;74(5):650–658.
- Yuan N, Arnaoutakis GJ, George TJ, et al. The spectrum of complications following left ventricular assist device placement. J Card Surg. 2012;27(5):630–638.
- Zhigalov K, Szczechowicz M, Mashhour A, et al. Left ventricular assist device implantation with concomitant tricuspid valve repair: is there really a benefit? J Thorac Dis. 2019;11(S6):S902–s912.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
- Bellomo R, Ronco C, Kellum JA, et al. Acute dialysis quality initiative w. acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–212.
- Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31
- Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol. 2012;8(7):423–429.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet (London, England). 1991;337(8746):867–872.
- Bresson D, Sibellas F, Farhat F, et al. Preliminary experience with Impella Recover(®) LP5.0 in nine patients with cardiogenic shock: a new circulatory support system in the intensive cardiac care unit . Arch Cardiovasc Dis. 2011;104(8–9):458–464.
- Cohen MG, Matthews R, Maini B, et al. Percutaneous left ventricular assist device for high-risk percutaneous coronary interventions: real-world versus clinical trial experience. Am Heart J. 2015;170(5):872–879.
- Flaherty MP, Pant S, Patel SV, et al. Hemodynamic support with a microaxial percutaneous left ventricular assist device (Impella) protects against acute kidney injury in patients undergoing high-risk percutaneous coronary intervention. Circ Res. 2017;120(4):692–700.
- Iliodromitis KE, Kahlert P, Plicht B, et al. High-risk PCI in acute coronary syndromes with Impella LP 2.5 device support. Int J Cardiol. 2011;153(1):59–63.
- Kusa S, Miller MA, Whang W, et al. Outcomes of ventricular tachycardia ablation using percutaneous left ventricular assist devices. Circul-Arrhythmia Elec. 2017;10(6):pii:e004717.
- Miller MA, Dukkipati SR, Chinitz JS, et al. Percutaneous hemodynamic support with Impella 2.5 during scar-related ventricular tachycardia ablation (PERMIT 1). Circ Arrhythm Electrophysiol. 2013;6(1):151–159.
- O'Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126(14):1717–1727.
- Parascandola SA, Pae WE, Jr., Davis PK, et al. Determinants of survival in patients with ventricular assist devices. ASAIO Trans. 1988;34(3):222–228.
- Pieri M, Contri R, Winterton D, et al. The contemporary role of Impella in a comprehensive mechanical circulatory support program: a single institutional experience. BMC Cardiovasc Disord. 2015;15:126
- Schiller P, Hellgren L, Vikholm P. Survival after refractory cardiogenic shock is comparable in patients with Impella and veno-arterial extracorporeal membrane oxygenation when adjusted for SAVE score. Eur Heart J Acute Cardiovasc Care. 2019;8(4):329–337.
- Nigwekar SU, Kandula P, Hix JK, et al. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized and observational studies. Am J Kidney Dis: Off J Nat Kidney Found. 2009;54(3):413–423.
- Tecson KM, Lima B, Lee AY, et al. Determinants and outcomes of vasoplegia following left ventricular assist device implantation. J Am Heart Assoc. 2018;7(11):pii:e008377.
- Meineri M, Van Rensburg AE, Vegas A. Right ventricular failure after LVAD implantation: prevention and treatment. Best Pract Res Clin Anaesthesiol. 2012;26(2):217–229.
- Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260–269.
- Goldstein SL. Medication-induced acute kidney injury. Curr Opin Crit Care. 2016;22(6):542–545.
- Nickel NP, O'Leary JM, Brittain EL, et al. Kidney dysfunction in patients with pulmonary arterial hypertension. Pulm Circ. 2017;7(1):38–54.
- Kopitkó C, Gondos T, Fülöp T, et al. Reinterpreting renal hemodynamics: the importance of venous congestion and effective organ perfusion in acute kidney injury. Am J Med Sci. 2020;359(4):193–205.
- Ross DW, Stevens GR, Wanchoo R, et al. Left ventricular assist devices and the kidney. Clin J Am Soc Nephrol. 2018;13(2):348–355.
- Cooper TK, Zhong Q, Nabity M, et al. Use of urinary biomarkers of renal ischemia in a lamb preclinical left ventricular assist device model. Artif Organs. 2012;36(9):820–824.
- Ootaki C, Yamashita M, Ootaki Y, et al. Reduced pulsatility induces periarteritis in kidney: role of the local renin-angiotensin system. J Thorac Cardiovasc Surg. 2008;136(1):150–158.
- Schima H, Dimitrov K, Zimpfer D. Debate: creating adequate pulse with a continuous flow ventricular assist device: can it be done and should it be done? Probably not, it may cause more problems than benefits!. Curr Opin Cardiol. 2016;31(3):337–342.
- Sandner SE, Zimpfer D, Zrunek P, et al. Renal function and outcome after continuous flow left ventricular assist device implantation. Ann Thorac Surg. 2009;87(4):1072–1078.
- Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis. 2016;8(5):E305–E311.
- Cheungpasitporn W, Kashani K. Electronic data systems and acute kidney injury. Contrib Nephrol. 2016;187:73–83.
- Thongprayoon C, Cheungpasitporn W, Kittanamongkolchai W, et al. Prognostic importance of low admission serum creatinine concentration for mortality in hospitalized patients. Am J Med. 2017;130(5):545–554 e541.
- Thongprayoon C, Hansrivijit P, Kovvuru K, et al. Diagnostics, risk factors, treatment and outcomes of acute kidney injury in a new paradigm. J Clin Med. 2020;9(4):1104.
- Thongprayoon C, Kaewput W, Thamcharoen N, et al. Incidence and impact of acute kidney injury after liver transplantation: a meta-analysis. J Clin Med. 2019;8(3):372.
- Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med. 2017;55(8):1074–1089.
- Thongprayoon C, Kaewput W, Kovvuru K, et al. Promises of big data and artificial intelligence in nephrology and transplantation. J Clin Med. 2020;9(4):1107.