Abstract
Aim
When peritoneal fibrosis (PF) causes ultrafiltration failure in peritoneal dialysis (PD) patients, PD has to be discontinued. Currently, there is no effective way to relieve PF. In this study, we aimed to determine whether miR-15a-5p is involved in PF and to determine the underlying mechanism.
Methods
Six normal rats were used as the control group. A uremic rat model was constructed using 5/6 nephrectomy in a Sprague–Dawley model. The uremic rats were randomly divided into PD, lentivirus-transfected, negative control, VEGFR-inhibited and gavage control groups. Except for the control group, all uremia rats received continuous PD for 28 days. In the lentivirus-transfected group, the miR-15a-5p plasmid was injected into the peritoneal cavity to upregulate miR-15a-5p expression. Axitinib was used to block vascular endothelial growth factor receptor (VEGFR) in the peritoneum. The mRNA levels of miR-15a-5p and VEGF were detected by qRT-PCR and FISH. Protein levels of VEGF, E-cadherin, collagen IV, fibronectin and α-SMA were detected by western blot and immunohistochemistry.
Results
PD leads to peritoneal thickening and fibrosis. The expression level of miR-15a-5p decreased and that of VEGF increased in the PD group than in the controls. Additionally, E-cadherin was significantly reduced while collagen IV, fibronectin and α-SMA were obviously increased in the PD group compared to controls. FISH showed that VEGF might be the target gene of miR-15a-5p. Overexpression of miR-15a-5p or inhibition of VEGFR could reverse PF.
Conclusion
miR-15a-5p may participate in the endothelial to mesenchymal transition of PF caused by PD through VEGF.
Introduction
The number of end-stage renal disease (ESRD) patients caused by chronic kidney diseases (CKD), such as glomerular disease, diabetic nephropathy, hypertension and obesity, is rapidly increasing [Citation1]. ESRD patients must rely on kidney transplant, hemodialysis (HD) and peritoneal dialysis (PD) as renal replacement therapy (RRT). PD has become an important method of RRT because of its weak influence on hemodynamics and protection of residual renal function [Citation2,Citation3]. Biological incompatibility of peritoneal dialysate, oxidative stress, activation of the renin-angiotensin-aldosterone system (RAAS), and recurrent peritonitis can result in changes in peritoneal morphology and function in patients undergoing long-term PD. Patients have to withdraw from PD when peritoneal fibrosis (PF) causes ultrafiltration failure (UFF) [Citation4–8]. Peritoneal angiogenesis, epithelial-to-mesenchymal transition (EMT), and inflammatory cell infiltration are the pathophysiological causes of PF in patients with PD. EMT and angiogenesis are the major causes of UFF [Citation9–11].
Vascular endothelial growth factor (VEGF) is a specific mitogen of vascular endothelial cells. It can promote the growth of vascular endothelial cells in vitro and induce vascular hyperplasia in vivo. VEGF can also increase vascular permeability to facilitate the migration of inflammatory cells to inflammatory sites [Citation12]. VEGF is closely related to the EMT. VEGF can participate in myocardial fibrosis through the VEGF/MAPK signaling pathway [Citation13]. VEGF can also mediate the EMT of cancer cells via the VEGF/PI3K/Akt/mTOR signaling pathway [Citation14]. The expression of VEGF in peritoneal tissues of PD patients with PF is significantly higher than those of PD patients without PF [Citation15]. Overexpression of VEGF can promote peritoneal neovascularization and the occurrence of UFF [Citation16].
MicroRNAs (miRNAs, miRs) are a class of non-coding small RNAs with a length of 20-22 nucleotides. miRNAs were first identified in nematodes [Citation17,Citation18]. They can bind to target genes via short nucleotide sequences [Citation19]. Each miRNA can have multiple target genes or several miRNAs can regulate a gene together. The miRNAs of different species show high homology in gene position and their own sequence [Citation20,Citation21]. We hypothesized that a high level of miRNA conservation is closely related to the importance of its function, as they play important roles in cell differentiation, apoptosis and tissue development. miRNAs are involved in the EMT and PF. For example, miR-21 can promote fibrosis. Additionally, miR-30a has anti-fibrotic effects [Citation22,Citation23]. However, the function of most miRNAs remains unclear.
In previous experiments, we observed a significantly decreased expression of miR-15a-5p in human peritoneal mesothelial cells. VEGF may be the target gene of miR-15a-5p [Citation24]. Here, we performed further studies using Sprague–Dawley (SD) rats. SD rats were transfected with lentivirus to overexpress miR-15a-5p, or they were given axitinib to inhibit vascular endothelial growth factor receptor (VEGFR). We found that miR-15a-5p participated in the PF caused by PD.
Materials and methods
Establishment of a rat model of uremia
Thirty-eight male SD rats weighing 180–220 g at eight weeks old were purchased (Qinglong mountain animal breeding ground, Nanjing, China). The animal experiments in this study have been ethically approved and the ethics number is 2020-KY-024. After seven days of adaptive feeding, all rats except the control group underwent 5/6 nephrectomy. 10% chloral hydrate (0.3 mL/100 g) was used to anesthetize rats. Penicillin was administered after surgery. At the eighth week, blood samples from the inner canthus vein were taken from all rats. If serum creatinine and urea nitrogen of the uremia rats were 2–3 times higher than the normal rats, the modeling of uremia rats was considered successful [Citation25].
Recombinant lentivirus
The 293T cells, which were obtained from the Chinese Academy of Sciences Cell Bank, were cultured in DMEM containing 10% FBS. The cells were cultured in a 15 cm dish in an incubator (37 °C, 5% CO2) and to 90% confluence. An aliquot of 1.5 mL serum-free DMEM was added into two sterile centrifuge tubes marked A. Then, LV3 (H1/GFP Puro) plasmids that contained either the target sequence or negative control sequence, and package plasmids (pGag/Pol, pRev, pvsv-g) (GenePharma, Shanghai, China), were added and subsequently mixed. In two aseptic centrifuge tubes marked B, 1.5 mL serum-free DMEM and 300 μL RNAi-Mate were added and mixed. After a 5 min incubation period, the solution in tubes A and B was mixed and incubated at room temperature for a further 25 min. The sequence of rat miR-15a-5p was 5′-UAGCAGCACAUAAUGGUUU-3′. The miR-15a-5p plasmid sequence was 5′-TAGCAGCACATAATGGTTT-3′, and the negative control sequence was 5′-TTCTCCGAACGTGTCACGT-3′.
Medium in petri dishes was replaced with serum-free medium. The transfection mixture (DMEM, LV3 (H1/GFP Puro) plasmids, package plasmids, RNAi-Mate) was added to the dish using a drop-by-drop technique. Then, it was gently mixed and incubated (37 °C, 5% CO2) for 4-6 h. After the transfer solution was discarded, DMEM containing 10% FBS was added into the dish and cultured for a further 72 h. The supernatant in the dish was removed and centrifuged (4 °C, 4000 rpm, for 4 min). The supernatant was filtered using a 0.45 µm filter and centrifuged again (4 °C, 20,000 rpm, for 2 h). Finally, we sub-packaged the recombinant lentivirus liquid and stored it at −80 °C. The virus titer test was 1 × 109 TU/mL.
Experimental conditions for each group
Six SD rats that did not undergo surgery were used as the control group (control, n = 6). Uremia rats were randomly divided into a PD group (PD, n = 6), a lentivirus-transfected group (PD + LV3-miR-15a-5p, n = 6), a negative control group (PD + LV3-shNC, n = 6), a VEGFR-inhibited group (PD + CMC + axitinib, n = 6), and a gavage control group (PD + CMC, n = 6). All uremic rats received continuous PD for 28 days. The PD method was as follows: each uremic rat was given 20 mL 4.25% peritoneal dialysis solution (Fresenius Medical Care, Baden Humboldt, Germany) by intraperitoneal injection every day. To overexpress miR-15a-5p, rats in the PD + LV3-miR-15a-5p group were given 100 µL miR-15a-5p plasmid with a titer of 1 × 109 TU/mL by intraperitoneal injection on the first and fourteenth day of PD. The same dose of LV3NC plasmid was administered to the PD + LV3-shNC group at the same time. Axitinib (0.5 mg/100 g, Beyotime Biotechnology, Shanghai, China) was dissolved in 0.5% carboxymethyl cellulose (CMC) and administered to the PD + CMC + axitinib group by gavage every day. The same dose of CMC was simultaneously administered to the PD + CMC group. Rats were sacrificed on the 28th day. The visceral peritoneum of the rats was collected for fluorescence in situ hybridization (FISH), quantitative real-time PCR (qRT-PCR) and western blot analysis. Parietal peritoneum was used for hematoxylin–eosin (HE), Masson and immunohistochemistry staining.
FISH
After dewaxing and hydrating the peritoneal paraffin sections, the slices were boiled in retrieval solution for 10 min and allowed to cool naturally. Then, the sections were digested with protease K (20 µg/mL) at 37 °C for 20 min. Endogenous peroxidase was blocked using 3% methanol–H2O2 at room temperature for 30 min. The slices were rinsed with PBS and then incubated with prehybridization solution at 37 °C for 1 h. After removing the prehybridization solution, sections were incubated overnight with the VEGF probe hybrid solution (5 ng/µL) (Wuhan Servicebio Technology Co. Ltd., Wuhan, China) at 37 °C. Then, the sections were washed with SSC detergent. BSA serum was used to seal the sections at room temperature for 30 min. After removing the sealant, the sections were treated with rat anti-digoxin that was conjugated to horseradish peroxidase (anti-DIG-HRP) at 37 °C for 50 min. Then sections were rinsed with PBS. FITC-TSA was used to incubate the sections at room temperature, in the dark, for 5 min. After washing with TBST and PBS, the sections were incubated with prehybridization solution at 37 °C for 1 h. Sections were incubated overnight with rno-miR-15a-5p probe hybrid solution (5 ng/µL) (Wuhan Servicebio Technology Co. Ltd.). After treatment with SSC and BSA, the sections were incubated with rat anti-DIG-HRP and CY3-TSA as described above. After washing the sections with TBST and PBS, DAPI was added for nuclear staining. Then, the sections were sealed with an anti-fluorescence quench sealant and observed under the microscope. The sequence of the VEGF probe was 5′-DIG-GCATTAGGGGCACACAGGACGGCTTGA-DIG-3′. The rno-miR-15a-5p probe sequence was 5′-DIG-ACAAACCATTATGTGCTGCTA-DIG-3′.
Histopathological and immunohistochemical examinations
Parietal peritoneum tissues were prepared into 4 µm thick paraffin sections, HE and Masson staining were performed using an HE staining kit and a Masson three color dyeing kit (Beijing Solarbio Science & Technology Co. Ltd., Beijing, China), according to the manufacturer's instructions.
Immunohistochemical staining of peritoneum tissue sections was performed using a rabbit two-step detection kit and a DAB developing kit (Goldenbridge Biotechnology Co. Ltd., Beijing, China). Sections were incubated in primary antibody overnight at 4 °C in a wet box. The primary antibodies used were as follows: anti-VEGF, anti-E-cadherin, anti-smooth muscle actin (1:200, ProteinTech Group, Chicago, IL), anti-fibronectin, anti-collagen IV (1:200, Abcam, Cambridge, MA), anti-CD31 and anti-CD34 (1:3000, Abcam, Cambridge, MA). The catalog numbers of antibodies were as follows: anti-VEGF (19003-1-AP), anti-E-cadherin (20874-1-AP), anti-smooth muscle actin (55135-1-AP), anti-fibronectin (ab2413), anti-collagen IV (ab6586), anti-CD31 (ab182981), and anti-CD34 (ab81289). Primary antibodies of CD31 and CD34 were monoclonal antibodies, and the others were polyclonal antibodies. Sections were incubated with secondary antibody (1:200, Goldenbridge Biotechnology Co.) for 2 h on the next day. Immunohistochemical positive controls and negative controls were set up at the same time. The positive controls were as follows: rat brain tissue for collagen IV, mouse colon tissue for fibronectin, mouse liver tissue for E-cadherin and α-SMA, mouse kidney tissue for VEGF, rat kidney tissue for CD34, and mouse lung tissue for CD31. The negative control was administered with PBS. Immunohistochemistry of CD31 and CD34 was used to observe the peritoneal microvascular density (MVD). MVD was evaluated according to the method proposed by Weidner et al. [Citation26].
Qrt-PCR
Peritoneal tissue was crushed in liquid nitrogen and transferred to Trizol (Dingguo Changsheng Biotech Co. Ltd., Beijing, China). Total RNA was extracted according to the manufacturer's instructions. Total RNA was reverse-transcribed with the PrimeScript™ RT reagent Kit and gDNA Eraser (Perfect Real Time) (Takara Biomedical Technology (Beijing) Co. Ltd.). The expression of miR-15a-5p and U6 was detected by qRT-PCR. U6 was used as the internal reference. The Two Step Stemaim-it miR qRT-PCR Quantitation Kit and the U6 Endogenous Control Kit (Shanghai Novland Co. Ltd., Shanghai, China) were used for this experiment. The expression of VEGF was detected by qRT-PCR using the SYBR Premix Ex Taq II kit (Takara Biomedical Technology (Beijing) Co. Ltd.). GAPDH was used as the internal reference. Experimental data were analyzed using the 2−ΔΔCT method. The primer sequences used in this experiment were as follows:
Western blot
Total protein of the peritoneum was extracted using radio-immunoprecipitation assay (RIPA) buffer (Beijing Solarbio Science & Technology Co. Ltd., Beijing, China). The target proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes. Membranes were incubated with 5% skim milk powder for 2 h and then incubated with primary antibody at 4 °C overnight. This study used the following primary antibodies: anti-VEGF (1:500, ProteinTech Group, Chicago, IL, Catalog no. 19003-1-AP), anti-smooth muscle actin (1:500, ProteinTech Group, Catalog no. 55135-1-AP), anti-E-cadherin (1:1000, ProteinTech Group, Catalog no. 20874-1-AP), anti-fibronectin (1:1000, Abcam, Cambridge, MA, Catalog no. ab2413), anti-collagen IV (1:1000, Abcam, Catalog no. ab6586), and anti-GAPDH (1:1000, Hangzhou Goodhere Biotechnology Co. Ltd, Catalog no. AB-P-R001). All the above primary antibodies were rabbit polyclonal antibodies. On the second day, membranes were incubated in horseradish proxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:2000, Dingguo Changsheng Biotech Co. Ltd., Beijing, China) at room temperature for 2 h. An enhanced chemiluminescence reagent kit (Gen-View Scientific Inc., Alachua, FL) was used to detect luminescence. The contrast (grayscale) of the membranes was analyzed using ImageJ v1.8.0.
Statistical analysis
TargetScan was used to analyze the target genes. Image-Pro Plus 6.0 was used to analyze HE, Masson and immunohistochemical staining. SPSS 21.0 (SPSS, Chicago, IL) was used for data analysis. One-way ANOVA and least significant difference (LSD) t-tests were used for intergroup comparisons. When p < 0.05, the difference was considered statistically significant.
Results
The rat uremia model was successfully established
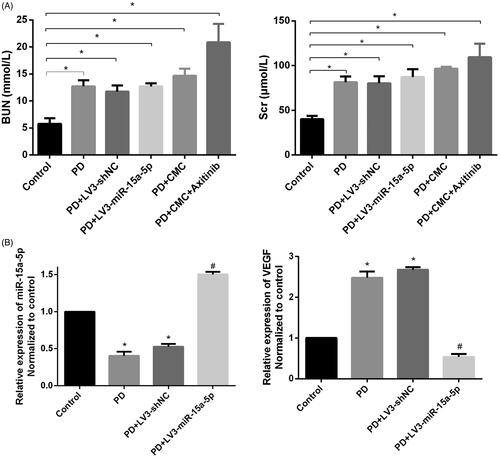
Thirty-eight rats underwent 5/6 nephrectomy. One died due to poor hemostasis on the renal section and one died from infection of the skin incision. Blood samples were collected at the eighth week to measure urea nitrogen and creatinine levels. Statistical analysis showed that these indicators of the uremic rats were significantly different from those of the control group (*p < 0.05, ).
Figure 1. The rat uremia model was successfully constructed. (A) The serum of rats in each group was collected before peritoneal dialysis. The levels of creatinine (Scr) and blood urea nitrogen (BUN) are shown (*p < 0.05). (B) Expression of miR-15a-5p and VEGF in the peritoneum, as detected by quantitative real-time PCR (qRT-PCR). *p < 0.05 versus control, #p < 0.05 versus PD.
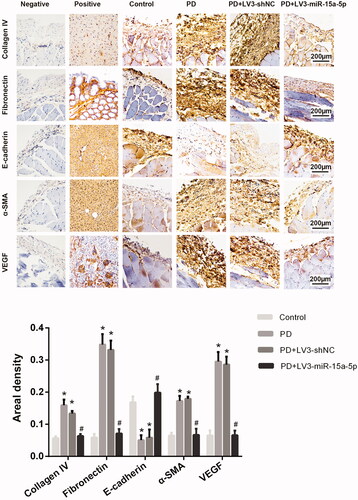
PD results in peritoneal thickening and fibrosis
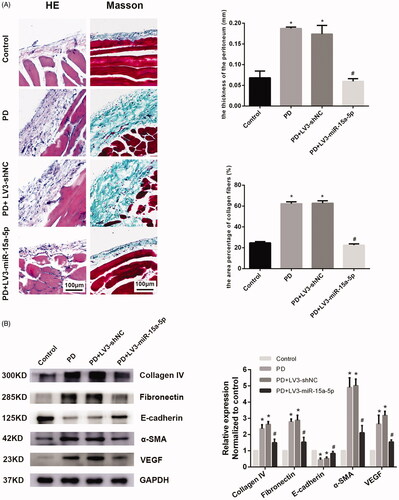
Our previous experiments showed that the reduced expression of miR-15a-5p in peritoneal mesothelial cells could up-regulate the expression of VEGF [Citation24]. In our animal experiment, the expression of miR-15a-5p was reduced and the expression of VEGF was increased in the PD group compared with the control group (*p < 0.05, ). HE and Masson staining showed that the peritoneum was thicker and the deposition of subcutaneous collagen fibers was significantly increased in PD rats (). Compared with normal rats, the peritoneal tissues of PD rats showed increased expression of collagen IV, fibronectin and α-SMA and decreased expression of E-cadherin by western blotting. This was related to mesothelial cell injury and thickening of the mesothelial subcutaneous layer. Expression of VEGF, which is reported to be closely related to neovascularization, was also significantly increased (). The results of immunohistochemistry were consistent with those of western blot (). This indicated that long-term PD could lead to PF.
Figure 2. Overexpression of miR-15a-5p can alleviate PF caused by PD. (A) Hematoxylin–eosin (HE) and Masson staining are shown for peritoneal observation. (B) Expression of collagen IV, fibronectin, α-SMA, E-cadherin, and VEGF in the control group, PD group, PD + LV3-shNC group, and PD + LV3-miR-15a-5p group, as detected by western blot. GAPDH was used as an internal control. Expression levels of proteins were normalized by the control group. *p < 0.05 versus control, #p < 0.05 versus PD.
Overexpression of miR-15a-5p could alleviate PF caused by PD
When miR-15a-5p was overexpressed, the expression of VEGF decreased. However, no significant changes were observed in the PD + LV3-shNC group (). The peritoneum of rats in the PD + LV3-miR-15a-5p group was obviously thinner and the deposition of collagen fibers was significantly reduced compared with that of the PD group (). Immunohistochemical analysis of rat peritoneal tissues from the PD + LV3-miR-15a-5p group showed a decreased expression of collagen IV, fibronectin and α-SMA and increased expression of E-cadherin compared with the PD group. By upregulating miR-15a-5p, expression of VEGF in the peritoneum was also significantly inhibited. There was no obvious difference between the PD + LV3-shNC group and the PD group. The western blot result of the PD + LV3-miR-15a-5p group was similar to that of the immunohistochemical analysis ( and Citation3). These data suggest that overexpression of miR-15a-5p could alleviate PD-associated PF.
VEGF might be the target of miR-15a-5p in the peritoneum
Analysis of targetScan found that VEGF might be the target gene of miR-15a-5p (). Green fluorescence represents VEGF. The red fluorescence represents miR-15a-5p. FISH showed that VEGF and miR-15a-5p were expressed in the cytoplasm. The two genes shared a common expression site. The location of gene co-expression is orange in the merged image (). We hypothesized that miR-15a-5p targets VEGF in the peritoneum.
Figure 4. miR-15a-5p may targeted mRNA of VEGF. (A) Analysis of argetScan found that VEGF might be the target gene of miR-15a-5p. (B) FISH targeted at miR-15a-5p and VEGF were carried out. The magnification of the picture was 200× and 400×. (C) Hematoxylin–eosin (HE) and Masson staining are shown to analyze the paraffin sections of peritoneal tissues. *p < 0.05 versus control, #p < 0.05 versus PD.
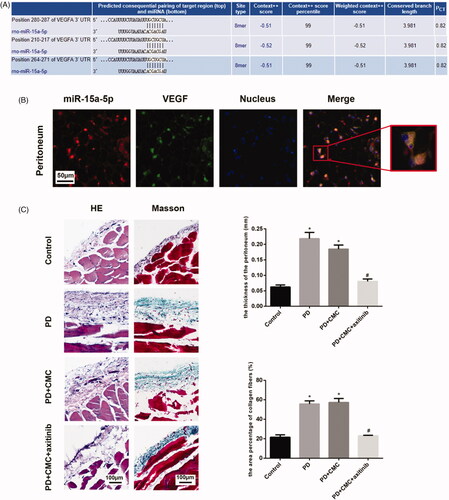
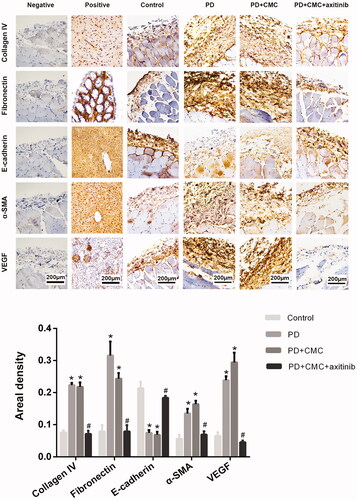
Axitinib, a VEGFR inhibitor, can alleviate PF
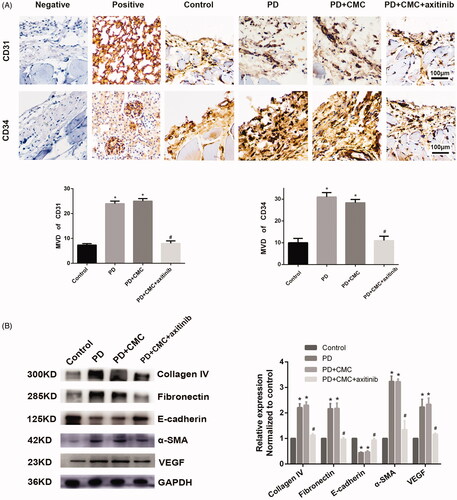
Axitinib inhibits the effect of VEGFR. After administering rats with regular oral axitinib by gastric perfusion, we found that the peritoneum in PD + CMC + axitinib group was thinner than that in PD group, and that there was less peritoneal neovascularization in PD + CMC + axitinib group ( and ). Western blot analysis showed a decreased expression of VEGF, collagen IV, fibronectin and α-SMA in the peritoneum of the PD + CMC + axitinib group, while the expression of E-cadherin increased (). Immunohistochemical analysis showed similar changes, i.e. in the PD + CMC + axitinib group, the expression of collagen IV, fibronectin, α-SMA, and VEGF in peritoneal tissues decreased, while E-cadherin expression increased (). The results of the PD + CMC group were similar to those of the PD group. These data showed that direct inhibition of VEGFR could also alleviate PF caused by PD.
Figure 5. Axitinib can reverse PF caused by long-term PD. (A) Levels of CD31 and CD34 in vascular endothelial cells, as detected by immunohistochemistry. Microvascular density (MVD) is shown, as counted by two observers. *p < 0.05 versus control, #p < 0.05 versus PD. (B) Western blot analysis depicts the expression of collagen IV, fibronectin, α-SMA, E-cadherin and VEGF in groups are shown. GAPDH is the internal control. Protein expression is normalized by the control group. *p < 0.05 versus control, #p < 0.05 versus PD.
Discussion
PD is a widely used renal replacement therapy. It has advantages over hemodialysis as it is much more convenient because patients can perform PD at home, and the risk of infectious diseases is much lower. However, PD must be terminated if PF leads to UFF in long-term PD patients. Because the mechanism of PF is currently unclear, little can be done to slow this process. miRNAs are a class of non-coding small ribonucleic acids (RNAs). They play an important role in various cells by binding to target nuclear sequences. Abnormal expression is associated with a variety of diseases. It has been reported that miR-29c is involved in the occurrence and development of diabetic nephropathy through Tristetraprolin [Citation27]. miR-200c, which has very strong oncogenic activity, is associated with tumor genesis, metastasis and invasion [Citation28]. miR-200c is also related to the occurrence and development of visceral fibrosis. The mechanism may be related to its role in regulating the EMT of peritoneal cells [Citation29]. It was first reported in 2002 that the miR-15a gene is located on region 1 of the short arm of chromosome 13, and its downregulated expression is closely related to chronic lymphoblastic leukemia [Citation30]. Other studies have found that miR-15a-5p is involved in diabetes atherosclerosis, endometrial cancer and other diseases [Citation31,Citation32]. We also investigated the role of miRNAs in PF.
Using the dual luciferase reporter system, lentivirus transfection, western blot, qRT-PCR, and other methods, we found that miR-15a-5p participated in the EMT of human peritoneal mesenchymal cells through VEGF in vitro. Upregulation of miR-15a-5p can reduce the EMT of human peritoneal mesothelial cells induced by high glucose [Citation24]. In animal experiments, the peritoneum of the rats in the PD group was significantly thickened and the connective tissue was markedly increased than in the control group. The expression of miR-15a-5p was decreased and VEGF expression related to angiogenesis was increased. In addition, the expression of collagen IV, fibronectin and α-SMA related to the EMT was significantly increased, but the expression of E-cadherin was decreased in the PD group than in the normal control group. After lentivirus transfection, upregulation of miR-15a-5p expression was observed. The peritoneum of the PD + LV3-miR-15a-5p group was dramatically thinner and the deposition of collagen fibers was reduced. The expression of E-cadherin was significantly increased and that of VEGF, collagen IV, fibronectin and α-SMA was reduced. All these results demonstrated the reversal of PF. These results indicated that miR-15a-5p is a protective factor for PF and upregulation of miR-15a-5p could alleviate PD-associated PF.
Previous studies have shown that VEGF has a close relationship with angiogenesis and peritoneal mesothelial cell transdifferentiation. Transdifferentiated mesothelial cells can produce more VEGF [Citation33]. The phenomenon of EMT in peritoneal mesothelial cells was first proposed in 2003. Recent studies suggest that EMT might initiate PF [Citation34]. In the peritoneum of patients with PD, the expression of VEGF was obviously higher than that of uremic patients without PD [Citation35]. In our experiment, FISH indicated that miR-15a-5p might target VEGF in the peritoneum.
Axitinib has been used as an important anti-tumor drug in the clinical treatment of some tumors. To explore whether blocking the action of VEGFR could effectively slow the progression of PF, we administered axitinib daily to uremic rats undergoing PD. The results indicated that axitinib could successfully alleviate PF. Compared with the normal control group, the peritoneum of the rats in the PD + CMC + axitinib group was thinner, collagen fiber deposition was reduced, and the expression of E-cadherin related to the EMT was increased; collagen IV, fibronectin, and α-SMA levels were also significantly reduced. This was consistent with the results of previous studies [Citation36,Citation37]. Our results demonstrated that the VEGFR inhibitor may also be a potent drug for patients with PD.
In summary, we found that miR-15a-5p could regulate the occurrence and process of EMT in PF by regulating VEGF. Upregulation of miR-15a-5p or inhibition of VEGFR could effectively alleviate PF. Thus, strategies targeting miR-15a-5p or VEGFR are expected to provide new methods for the treatment of PD-associated PF.
Disclosure statement
The authors report that they have no conflicts of interest.
Additional information
Funding
References
- Liu ZH. Nephrology in China. Nat Rev Nephrol. 2013;9(9):523–528.
- Hufnagel G, Michel C, Queffeulou G, et al. The influence of automated peritoneal dialysis on the decrease in residual renal function. Nephrol Dial Transplant. 1999;14(5):1224–1228.
- Chang JH, Yoon SJ, Han SH, et al. The impact of dialysis modality on arterial stiffness in patients with end-stage renal disease. Ren Fail. 2010;32(8):947–953.
- Ha H, Lee HB. Effect of high glucose on peritoneal mesothelial cell biology. Perit Dial Int. 2000; 20 Suppl 2(suppl 2):S15–S18.
- Witowski J, Korybalska K, Wisniewska J, et al. Effect of glucose degradation products on human peritoneal mesothelial cell function. J Am Soc Nephrol. 2000;11(4):729–739.
- Mortier S, De Vriese AS, Lameire N. Recent concepts in the molecular biology of the peritoneal membrane – implications for more biocompatible dialysis solutions . Blood Purif. 2003;21(1):14–23.
- Noh H, Kim JS, Han KH, et al. Oxidative stress during peritoneal dialysis: implications in functional and structural changes in the membrane. Kidney Int. 2006;69(11):2022–2028.
- Bozkurt D, Cetin P, Sipahi S, et al. The effects of renin-angiotensin system inhibition on regression of encapsulating peritoneal sclerosis. Perit Dial Int. 2008;28(5_suppl):38–42.
- Margetts PJ, Gyorffy S, Kolb M, et al. Antiangiogenic and antifibrotic gene therapy in a chronic infusion model of peritoneal dialysis in rats. J Am Soc Nephrol. 2002;13(3):721–728.
- Yáñez-Mó M, Lara-Pezzi E, Selgas R, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348(5):403–413.
- Hishida E, Ito H, Komada T, et al. Crucial role of NLRP3 inflammasome in the development of peritoneal dialysis-related peritoneal fibrosis. Sci Rep. 2019;9(1):10363.
- Lee KS, Park SJ, Kim SR, et al. Inhibition of VEGF blocks TGF-beta1 production through a PI3K/Akt signalling pathway. Eur Respir J. 2008;31(3):523–531.
- Tao H, Chen ZW, Yang JJ, et al. MicroRNA-29a suppresses cardiac fibroblasts proliferation via targeting VEGF-A/MAPK signal pathway. Int J Biol Macromol. 2016;88:414–423.
- Yin T, Wang G, He S, et al. Malignant pleural effusion and ascites induce epithelial-mesenchymal transition and cancer stem-like cell properties via the vascular endothelial growth factor (VEGF)/phosphatidylinositol 3-kinase (PI3K)/Akt/mechanistic target of rapamycin (mTOR) pathway. J Biol Chem. 2016;291(52):26750–26761.
- Abrahams AC, Habib SM, Dendooven A, et al. Patients with encapsulating peritoneal sclerosis have increased peritoneal expression of connective tissue growth factor (CCN2), transforming growth factor-beta1, and vascular endothelial growth factor. PLoS One. 2014;9(11):e112050.
- Catar R, Witowski J, Wagner P, et al. The proto-oncogene c-Fos transcriptionally regulates VEGF production during peritoneal inflammation. Kidney Int. 2013;84(6):1119–1128.
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75(5):855–862.
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854.
- Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev. 2015;81:75–93.
- Pasquinelli AE, Reinhart BJ, Slack F, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408(6808):86–89.
- Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294(5543):862–864.
- Gao Q, Xu L, Yang Q, et al. MicroRNA-21 contributes to high glucose-induced fibrosis in peritoneal mesothelial cells in rat models by activation of the Ras-MAPK signaling pathway via *Sprouty-1. J Cell Physiol. 2019;234(5):5915–5925.
- Zhou Q, Yang M, Lan H, et al. miR-30a negatively regulates TGF-β1-induced epithelial-mesenchymal transition and peritoneal fibrosis by targeting Snai1. Am J Pathol. 2013;183(3):808–819.
- Shang J, He Q, Chen Y, et al. miR-15a-5p suppresses inflammation and fibrosis of peritoneal mesothelial cells induced by peritoneal dialysis via targeting VEGFA. J Cell Physiol. 2019;234(6):9746–9755.
- Zareie M, De Vriese AS, Hekking LH, et al. Immunopathological changes in a uraemic rat model for peritoneal dialysis. Nephrol Dial Transplant. 2005;20(7):1350–1361.
- Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36(2):169–180.
- Guo J, Li J, Zhao J, et al. MiRNA-29c regulates the expression of inflammatory cytokines in diabetic nephropathy by targeting tristetraprolin. Sci Rep. 2017;7(1):2314.
- Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11(12):1487–1495.
- Zhang L, Liu F, Peng Y, et al. Changes in expression of four molecular marker proteins and one microRNA in mesothelial cells of the peritoneal dialysate effluent fluid of peritoneal dialysis patients. Exp Ther Med. 2013;6(5):1189–1193.
- Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(24):15524–15529.
- Liu Y, Liu LY, Jia Y, et al. Role of microRNA-15a-5p in the atherosclerotic inflammatory response and arterial injury improvement of diabetic by targeting FASN. Biosci Rep. 2019;39(7):BSR20181852.
- Wang ZM, Wan XH, Sang GY, et al. miR-15a-5p suppresses endometrial cancer cell growth via Wnt/β-catenin signaling pathway by inhibiting WNT3A. Eur Rev Med Pharmacol Sci. 2017;21(21):4810–4818.
- Tomino Y. Mechanisms and interventions in peritoneal fibrosis. Clin Exp Nephrol. 2012;16(1):109–114.
- Aroeira LS, Aguilera A, Sánchez-Tomero JA, et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18(7):2004–2013.
- Gao D, Zhao ZZ, Liang XH, et al. Effect of peritoneal dialysis on expression of vascular endothelial growth factor, basic fibroblast growth factor and endostatin of the peritoneum in peritoneal dialysis patients. Nephrology (Carlton). 2011;16(8):736–742.
- Masola V, Granata S, Bellin G, et al. Specific heparanase inhibition reverses glucose-induced mesothelial-to-mesenchymal transition. Nephrol Dial Transplant. 2017;32(7):1145–1154.
- Zhang N, Hong B, Zhou C, et al. Cobalt chloride-induced hypoxia induces epithelial-mesenchymal transition in renal carcinoma cell lines. Ann Clin Lab Sci. 2017;47(1):40–46.