Abstract
Objectives
Carpal tunnel syndrome (CTS) is a common complication in maintenance hemodialysis (MHD) patients and leads to disabilities and increased risk of mortality. Hepatitis C virus (HCV) infection is associated with inflammatory and oxidative stress, and HCV infection can be cured. This study aimed at evaluating the association of HCV infection with CTS.
Methods
Using a cross-sectional design, anthropometric and laboratory data were collected. Serum β2-microglobulin, HCV antibody and HCV-RNA were measured. CTS was diagnosed according to clinical manifestation, electrophysiological test or ultrasonography. The related factors for CTS were analyzed by multivariate logistic regression.
Results
This study included 113 participants, of whom 33 (29.2%) patients were positive for HCV antibody and 18 (15.9%) were positive for HCV antibody and HCV-RNA. Thirty-two (28.3%) patients were diagnosed with CTS. There were significant differences in the dialysis vintage, age of onset of MHD, high-sensitivity C-reactive protein, serum β2M, anti-HCV-positive, HCV-RNA-positive, HCV load values and urine volume category between the CTS group and non-CTS group (p < 0.05). High-sensitivity C-reactive protein (OR: 1.238, 95% CI: 1.071–1.431, p = 0.004), dialysis vintage (OR: 1.017, 95% CI: 1.008–1.026, p < 0.001) and HCV-RNA-positive (OR: 5.929, 95% CI: 1.295–27.132, p = 0.022) rather than anti-HCV-positive were related factors for CTS.
Conclusions
High-sensitivity C-reactive protein, dialysis vintage and HCV-RNA replication but not previous HCV-infection were related factors for CTS in MHD patients. Further studies are needed to clarify whether intervention is beneficial for preventing and delaying the progression of CTS in MHD patients with HCV-RNA replication.
Introduction
In healthy individuals, most β2-microglobulin (β2M) is filtrated by the glomerular membrane and then reabsorbed and catabolized in the proximal tubules. Therefore, the serum levels of β2M are inversely related to the glomerular filtration rate. In end-stage renal disease (ESRD) patients, the serum levels of β2M are elevated, especially in maintenance hemodialysis (MHD) patients. The deposition of β2M amyloid in osteoarticular sites, particularly synovial membranes, is involved in the pathologic process of many diseases, such as carpal tunnel syndrome (CTS), joint osteoarthropathy and spondyloarthropathy [Citation1]. CTS is the most common type of dialysis-related amyloidosis [Citation2]. Recently, a study suggested that the incidence of CTS in MHD patients was 8.8% [Citation3] and increased to greater than 50% when the dialysis vintage was over 10 years [Citation4]. The major risk factors for CTS in MHD patients are age, dialysis vintage, residual renal function, chronic inflammation, oxidant stress, advanced glycation end products, and some genetic factors [Citation4–6]. As CTS may cause severe disability and effective therapies remain lacking, the early diagnosis and prevention of CTS are considered important.
Hepatitis C virus (HCV) infection is common in dialysis patients and is associated with increased morbidity and mortality. In 2012–2015, HCV prevalence was nearly 10% overall among MHD patients and ranged from 4.1% to 20.1% in the DOPPS [Citation7]. HCV incidence was 1.2 per 100 patient years in 2012–2015 and ranged from 0 to 2.9 [Citation7]. Due to the medicine side effect, treatment of HCV infection was restricted in past. The ensuing decade bore witness to remarkable advances in the treatment of HCV infection following the approval of direct-acting antiviral agents that deliver cure rates routinely >95% [Citation8]. 2018 KDIGO Clinical Practice Guideline Treatment recommended that HCV infection should be treated with a ribavirin-free DAA-based regimen in MHD [Citation8].
Recently, numerous studies have shown that HCV infection causes greater oxidative stress than any other inflammatory liver disease [Citation9–14]. However, few studies have focused on the relationship between HCV infection and CTS in MHD patients [Citation3,Citation4,Citation15]. HCV infection is a common problem associated with increased risk of cardiovascular events [Citation16] and lower quality of life in MHD patients. If HCV infection was related with CTS in MHD patients, delaying progression of CTS will become possible. This present study evaluated the associations among serum β2M, CTS and HCV infection.
Materials and methods
Patients
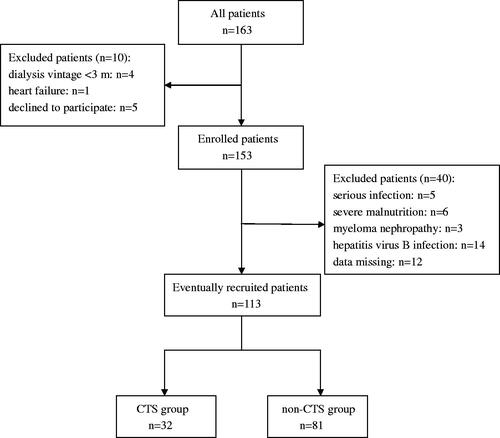
This observational cross-sectional study of MHD patients was conducted at the Department of Blood Purification, Beijing Chao-Yang Hospital, Capital Medical University, from July 2017 to September 2017. The inclusion criteria were (1) duration of dialysis >3 months, (2) patient older than 18 years, and (3) patient in stable condition and consenting to participate in the study. The exclusion criteria were (1) active infection within the past month, (2) severe malnutrition (serum albumin <35 g/L), (3) hepatitis virus B infection, (4) myeloma nephropathy, and (5) data missing. shows a flow diagram of patient selection.
All enrolled patients underwent hemodialysis 3 times per week for 4 h per dialysis session. Standard bicarbonate-based ultrapure dialysate was used, the dialysate flow was 500 mL/min, and the blood flow ranged from 200 to 300 mL/min. The dialysate ingredients were as follows: sodium, 138-140 mmol/L; potassium, 2.5 mmol/L; calcium, 1.25 mmol/L, and magnesium, 0.5 mmol/L. Hemodialysis was performed using low-flux membranes (F7, Fresenius, Germany). Water for hemodialysis and related therapies meet the People's Republic of China pharmaceutical industry standards YY 0572-2015.
The study was approved by the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University, and written informed consent was obtained from each patient (Number: 12-ke-41).
Data collection
The recorded demographic data of patients included gender; age; dialysis vintage; age of onset of MHD; cause(s) of renal failure; urine volume; vascular access (arteriovenous fistula or central venous catheterization); history of smoking; waiting for transplant and complications, including hepatitis, diabetes, hypertension, cardiovascular disease, and cerebrovascular disease. All patients were divided into two groups by interdialytic urine volume (≤400 mL/d and >400 mL/d).
All laboratory parameters, including serum alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), serum creatine (sCr), uric acid (UA), albumin (Alb), low-density lipoprotein cholesterol (LDL), high-sensitivity C-reactive protein (hs-CRP), and intact parathyroid hormone (iPTH), were measured by automated and standardized methods before hemodialysis. Blood samples were collected after an over-night fast.
Serum β2M was measured before hemodialysis by scattering turbidimetry (Siemens Medical Diagnostic Products Co., Ltd., Shanghai, China). HCV antibody was measured by chemiluminescence microparticle immunoassay (Architect i2000SR, Abbott Diagnostics, USA), and HCV-RNA was measured by real-time fluorescence quantitative PCR (Daan Gene Co., Ltd., Guangzhou, China). The cutoff value for HCV-RNA was 103. Anti-HCV-positive was defined to HCV previous infection and HCV-RNA-positive was defined HCV present infection.
Diagnostic criteria of CTS
CTS was diagnosed according to (1) signs or symptoms verified by nerve conduction examination; (2) clinical CTS diagnosis of nocturnal pain, numbness in the median nerve distribution, and a positive Tinel sign/Phalen sign; (3) prolonged sensory and/or motor latencies from the wrist to the digits innervated by the median nerve in the electrophysiological test; or (4) the median nerve cross-sectional area exceeding 10 mm3 or compression rate over 20% by ultrasonography [Citation17,Citation18]. Meeting each one of the four criteria supported the diagnosis of CTS.
Statistical analyses
The SPSS 17.0 statistics package for Windows was used for statistical analysis. Normally distributed continuous variables were expressed as the mean ± standard deviation (SD) and were compared between two groups using Student's t-tests. Non-normally distributed variables are expressed as the median (interquartile range) and were compared between two groups by Mann–Whitney U-tests. Categorical variables are expressed as numbers and percentages, and chi-square tests were used for analysis. Spearman analysis was used to examine the univariate correlations between serum β2M and other variables. Binary logistic multivariate regression analysis was used to identify related factors of CTS. p < 0.05 was considered statistically significant.
Results
Patient characteristics
One hundred and thirteen patients were included in this study, comprising 70 males and 43 females. The mean age (SD) was 58.5 (15.4) years, and median (interquartile range) dialysis vintage was 92 (31,136.5) months. The primary diseases of these MHD patients were as follows: chronic glomerulonephritis, 53 (46.9%); diabetic renal disease, 24 (21.2%); hypertensive-related nephropathy, 16 (14.2%); chronic interstitial nephritis, 6 (5.3%); and other, 14 (12.4%). Thirty-three (29.2%) patients were anti-HCV-positive, and 18 (15.9%) patients were positive for both HCV antibody and HCV-RNA. None of patients was negative for HCV antibody but positive for HCV-RNA. The range of viral load values detected was 1.16 × 105 to 9 × 107 in HCV-RNA-positive patients. There were 103 patients using arteriovenous fistula, and 10 patients using central venous catheterization. Due to various reasons, such as age, complications, insufficient kidney donors, and so on, twenty-seven patients were on wait list for the transplant in this study. presents the demographic characteristics of the participants.
Table 1. The demographic characteristics of all participants (n = 113).
Serum β2M and associated variables
The level of serum β2M was approximately normally distributed, and the mean was 46.2 mg/L (normal range: 1.09–2.53 mg/L). Serum β2M was positively related to age (r = 0.233, p = 0.004), dialysis vintage (r = 0.255, p = 0.006) and age of onset of MHD (r = 0.196, p = 0.038) and negatively related to urine volume category (r= −0.224, p = 0.017). However, there was no relationship between serum β2M and gender, vascular access, ALT, AST, ALP, sCr, UA, Alb, LDL, or hs-CRP (p > 0.05). The serum β2M level in anti-HCV-positive patients was higher than that in anti-HCV-negative patients, but the difference was not statistically significant (48.13 ± 12.79 vs. 45.42 ± 9.99, t = 1.208, p = 0.230). However, there was difference between the HCV-RNA-positive and HCV-RNA-negative patients (51.59 ± 13.68 vs. 45.19 ± 10.05, t = 2.329, p = 0.022). Among the anti-HCV-positive patients, serum β2M was higher in the HCV-RNA-positive patients than in the HCV-RNA-negative patients, but the difference was not statistically significant (51.59 ± 13.68 vs. 43.99 ± 10.64, t = 1.754, p = 0.089).
Related factors for CTS
Among the 113 MHD patients, the prevalence of CTS was 28.3% (32 cases). Among the 33 anti-HCV-positive patients, the prevalence of CTS was 54.5% (18 cases), which was significantly higher than that in the anti-HCV-negative patients (χ2=8.621, p = 0.004). lists the anthropometric and laboratory data for the CTS and non-CTS groups. There were significant differences in dialysis vintage, age of onset of MHD, hs-CRP, serum β2M and urine volume category between the CTS group and non-CTS group (p < 0.05, ). The patients in the CTS group were more likely to be positive for HCV antibody and HCV-RNA than were those in the non-CTS group (p < 0.001, ). There were no significant differences in gender, age, previous history, vascular access pattern, smoking, AST, ALT, ALP, sCr, UA, Alb, LDL, and iPTH between these two groups (p > 0.05).
Table 2. Patients’ characteristics between CTS and non-CTS.
We used a binary logistic multivariate regression model for analysis. CTS group was included as the dependent variable, and dialysis vintage, age of onset of MHD, hs-CRP, serum β2M, urine volume category, HCV antibody status and HCV-RNA replication were included as independent variables. These independent variables were included because they were found to differ significantly between the two groups in the univariate analyses. We found that HCV-RNA replication, hs-CRP, and dialysis vintage were related factors for CTS (p < 0.05, ).
Table 3. Binary classification logistic multivariate regression model of CTS.
Discussion
Our single-center, observational, cross-sectional study of 113 MHD patients indicated that being positive for HCV-RNA, rather than for HCV antibody, was a related factor for CTS. Evidence-based data in the relation of HCV infection and CTS were limited [Citation3,Citation4,Citation15], with most studies investigating the correlation of HCV antibody and CTS [Citation19–21]. Since HCV-RNA replication can be inhibited now, it’s possible to delay to CTS if CTS is related to HCV present infection. Therefore, it is important to investigate the relationship between HCV-RNA replication and CTS.
CTS is a common complication of DRA (dialysis-related amyloidosis), and it is recognized as the most evident marker of DRA development in MHD patients. CTS could lead to disability (thenar eminence muscles atrophy and finger dysfunction) and reduced the quality of life (hand pain, numbness and sleeping disorder) in MHD patients [Citation22,Citation23]. The incidence of CTS in MHD patients is 8–32% [Citation4,Citation6]. In our research, the incidence of CTS was 28.3%. However, in previous work, the incidence of DRA after 15 years of dialysis was found to be more than 95% in the US, and 100% of patients in a European study were found to have DRA after 13 years of dialysis [Citation24]. The incidence in the present study is significantly lower than these previous estimates. Possible reasons for this difference are as follows: (1) CTS is a kind of DRA, which includes bilateral shoulder pain, bone cysts and so on; (2) the proportion of patients with dialysis vintage <5 years was higher in our study than in the previous studies; and (3) the diagnosis and treatment of CTS were later in our study than in the previous studies.
To further investigate the influences of clinical features on CTS in MHD patients, multivariate binary logistic regression analysis was performed. The identified related factors for CTS were HCV-RNA replication, hs-CRP, and dialysis vintage. The effect of an elevated concentration of circulating β2M on CTS remains unclear, and the precise mechanism of pathogenesis of CTS has yet to be fully elucidated. Although marked elevation of serum β2M was identified previously as a prerequisite for the formation of β2M fibrils [Citation25,Citation26], the K/DOQI clinical guidelines suggest that there is no direct correlation between serum β2M level and the onset of CTS. Furthermore, a study of more than 290,000 Japanese MHD patients reported that neither pretreatment serum β2M level nor β2M clearance was associated with the risk of CTS [Citation5]. CTS is characterized by the deposition of amyloid fibrils, principally composed of β2M, in the osteoarticular structures [Citation27]. The process of amyloid deposition “in a target tissue requires that the fibrillogenic protein attains the amyloidogenic conformation at the right site, at the right time, and at the right concentration” [Citation28]. Although urine volume category was negatively correlated with the level of β2M and was a related factor of CTS in univariate analyses, it was not statistically significant in multivariate analysis. Increased serum β2M, due to decreased urine volume, key to developing CTS, but elevation of circulating β2M levels may not be the only cause of β2M derived amyloid [Citation28]. Other factors have been clearly incriminated such as longer dialysis vintage [Citation29]. Therefore, urine volume category and serum β2M were not related factors for CTS in this study.
This study identified dialysis vintage and hs-CRP as related factors of the onset of CTS, which is consistent with previous reports [Citation5,Citation15,Citation30,Citation31]. However, few studies have examined the relationship between HCV infection and CTS. Incidence of CTS was statistically significantly higher in patients with anti-HCV-positive or HCV infection by univariate analysis [Citation3,Citation4,Citation15,Citation21,Citation32]. However, after adjustment for dialysis vintage by multivariate logistic regression, this association disappeared [Citation3,Citation15,Citation32], which is inconsistent with our findings. In our multivariate logistic regression analysis, unexpectedly, HCV-RNA replication was related to CTS, whereas anti-HCV-positive was not. We speculate that this result might reflect the fact that the appearance of anti-HCV is not evidence of present infection; it is regularly used in preliminary screening of HCV infection as an indicator of previous HCV infection. After the moment of the HCV infection, the serological tests allow for anti-HCV antibodies detection on 7–8 weeks [Citation33]. Anti-HCV antibodies may persist indefinitely in chronic infection patients.
The mechanisms underlying the correlation between HCV present infection and CTS are unclear; however, the correlation may be due to micro-inflammation and oxidative stress induced by HCV present infection, which are related factors for CTS [Citation34]. The core nucleocapsid protein of HCV is the main contributor to the increased oxidative stress in the liver. Additionally, previous work has suggested that HCV infection itself causes greater inflammation and oxidative stress than do other inflammatory liver diseases [Citation35]. It can be speculated that the inflammatory process induces cytokine production and complement activation in the liver. The released cytokines, including interleukin-1, tumor necrosis factor-α and interleukin-6, stimulate the synthesis and release of β2M by macrophages and/or augment the expression of MHC class I antigens and β2M. These processes play pivotal roles in the pathogenesis of CTS. Furthermore, the antioxidant capacity in liver and blood is reduced in chronic hepatitis C patients. In addition, the generation of reactive oxygen species is increased in chronic hepatitis C patients [Citation10] due to the production of viral protein by HCV [Citation13,Citation36–39]. HCV-infected patients have been shown to exhibit increased lipid hydroperoxide levels and advanced oxidation protein products (AOPP) levels [Citation40]. AOPP are derived from oxidation-modified albumin, fibrinogen and lipoproteins during oxidative stress induced by the action of chloraminated oxidants, which are produced by myeloperoxidase in activated neutrophils [Citation41]. AOPP are similar to advanced glycation end-products (AGEs) in structure and biological activities, that is, the induction of proinflammatory cytokines and adhesion molecules [Citation41]. The presence of β2M modified by AGEs or AOPP lead to an inflammatory response and the recruitment of inflammatory cells around amyloid deposits. The above processes may compose the key mechanism underlying the higher incidence of CTS in MHD patients with HCV-RNA replication than in those without.
Some limitations of this study merit consideration. One limitation is that our study had a small sample size and employed a cross-sectional design, which might have introduced some bias and hampered inference of cause and effect. The effects of viral therapy on inflammation, oxidative stress and the symptoms of CTS will be studied in our future work. Another limitation is that because CTS is clinically silent in the early period, most of the studied patients were diagnosed with CTS after obvious symptoms and signs appeared. The development of noninvasive methods for screening to facilitate early diagnosis is needed. In addition, we measured hs-CRP but did not consider other inflammatory and oxidative stress markers, such as interleukin-6 and AGEs. Despite these limitations, this study is informative for CTS prevention and treatment.
Conclusions
CTS, a serious long-term complication of MHD, caused disability and reduced the quality of life. Although previously traditional related factors, such as age, dialysis vintage, genetic factors and so on, were naturally unchangeable, in this study, we also identified a relationship between CTS and HCV-RNA replication. Given the advent of effective direct-acting antiviral regimens, research is needed to determine whether treatment of HCV, in addition to combating hepatitis, can delay the occurrence and development of CTS in MHD patients with HCV-RNA replication.
Disclosure statement
The authors have no conflicts of interest to declare.
References
- Drüeke TB. Beta2-microglobulin and amyloidosis. Nephrol Dial Transplant. 2000;15(suppl_1):17–24.
- Jadoul M, Drüeke TB. β2 microglobulin amyloidosis: an update 30 years later. Nephrol Dial Transplant. 2016;31(4):507–509.
- Weng CH, Hu CC, Yen TH, et al. Association between environmental particulate matter and carpal tunnel syndrome in patients undergoing hemodialysis. Kidney Blood Press Res. 2017;42(5):827–836.
- Kopeć J, Gadek A, Drozdz M, et al. Carpal tunnel syndrome in hemodialysis patients as a dialysis-related amyloidosis manifestation–incidence, risk factors and results of surgical treatment. Med Sci Monit. 2011;17(9):CR505–R509.
- Hoshino J, Yamagata K, Nishi S, et al. Carpal tunnel surgery as proxy for dialysis-related amyloidosis: results from the Japanese society for dialysis therapy. Am J Nephrol. 2014;39(5):449–458.
- Busch M, Schwenzky A, Franke S, et al. Advanced glycation end products and β(2)-microglobulin as predictors of carpal tunnel syndrome in hemodialysis patients. Blood Purif. 2012;34(1):3–9.
- Jadoul M, Bieber BA, Martin P, et al. Prevalence, incidence, and risk factors for hepatitis C virus infection in hemodialysis patients. Kidney Int. 2019;95(4):939–947.
- Roth D, Bloom RD, Molnar MZ, et al. KDOQI US commentary on the 2018 KDIGO clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C. Am J Kidney Dis. 2020;75(5):665–683.
- Takeda H, Takai A, Inuzuka T, et al. Genetic basis of hepatitis virus-associated hepatocellular carcinoma: linkage between infection, inflammation, and tumorigenesis. J Gastroenterol. 2017;52(1):26–38.
- de Mochel NS, Seronello S, Wang SH, et al. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52(1):47–59.
- Machida K, McNamara G, Cheng KT, et al. Hepatitis C virus inhibits DNA damage repair through reactive oxygen and nitrogen species and by interfering with the ATM-NBS1/Mre11/Rad50 DNA repair pathway in monocytes and hepatocytes. J Immunol. 2010;185(11):6985–6998.
- Pal S, Polyak SJ, Bano N, et al. Hepatitis C virus induces oxidative stress, DNA damage and modulates the DNA repair enzyme NEIL1. J Gastroenterol Hepatol. 2010;25(3):627–634.
- Dionisio N, Garcia-Mediavilla MV, Sanchez-Campos S, et al. Hepatitis C virus NS5A and core proteins induce oxidative stress-mediated calcium signalling alterations in hepatocytes. J Hepatol. 2009;50(5):872–882.
- Irshad M, Mankotia DS, Irshad K. An insight into the diagnosis and pathogenesis of hepatitis C virus infection. World J Gastroenterol. 2013;19(44):7896–7909.
- Huang WH, Hsu CW, Weng CH, et al. Association of a high normalized protein catabolic rate and low serum albumin level with carpal tunnel syndrome in hemodialysis patients. Medicine (Baltimore). 2016;95(26):e4050.
- Oyake N, Shimada T, Murakami Y, et al. Hepatitis C virus infection as a risk factor for increased aortic stiffness and cardiovascular events in dialysis patients. J Nephrol. 2008;21(3):345–353.
- Klauser AS, Halpern EJ, De Zordo T, et al. Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. 2009;250(1):171–177.
- Yamazaki T, Kawahara N, Arai K, et al. Utility of ultrasonography of the median nerve with a high-frequency probe for the diagnosis of dialysis-related carpal tunnel syndrome. Ther Apher Dial. 2016;20(5):483–491.
- Rahnavardi M, Hosseini MS, Alavian SM. Hepatitis C in hemodialysis patients: current global magnitude, natural history, diagnostic difficulties, and preventive measures. Am J Nephrol. 2008;28(4):628–640.
- Sułowicz W, Radziszewski A, Chowaniec E. Hepatitis C virus infection in dialysis patients. Hemodial Int. 2007;11(3):286–295.
- Dung NH, Loc ND, Quyen DBQ, et al. Association between low serum prealbumin levels and carpal tunnel syndrome in maintenance hemodialysis patients. Ren Fail. 2020;42(1):944–949.
- Padua L, Coraci D, Erra C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. 2016;15(12):1273–1284.
- Genova A, Dix O, Saefan A, et al. Carpal tunnel syndrome: a review of literature. Cureus. 2020;12(3):e7333
- Jadoul M, Garbar C, Noël H, et al. Histological prevalence of beta 2-microglobulin amyloidosis in hemodialysis: a prospective post-mortem study. Kidney Int. 1997;51(6):1928–1932.
- Liabeuf S, Lenglet A, Desjardins L, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82(12):1297–1303.
- Drueke TB, Massy ZA. Beta2-microglobulin. Semin Dial. 2009;22(4):378–380.
- Scarpioni R, Ricardi M, Albertazzi V, et al. Dialysis-related amyloidosis: challenges and solutions. Int J Nephrol Renovasc Dis. 2016;9:319–328.
- Zumrutdal A. Role of β2-microglobulin in uremic patients may be greater than originally suspected. World J Nephrol. 2015;4(1):98–104.
- Labriola L, Jadoul M. Dialysis-related amyloidosis: is it gone or should it be? Semin Dial. 2017;30(3):193–196.
- Hoshino J, Yamagata K, Nishi S, et al. Significance of the decreased risk of dialysis-related amyloidosis now proven by results from Japanese nationwide surveys in 1998 and 2010. Nephrol Dial Transplant. 2016;31(4):595–602.
- Schiffl H. Impact of advanced dialysis technology on the prevalence of dialysis-related amyloidosis in long-term maintenance dialysis patients. Hemodial Int. 2014;18(1):136–141.
- Huang WH, Hu CC, Yen TH, et al. Blood lead level: an overlooked risk of carpal tunnel syndrome in hemodialysis patients. Ren Fail. 2019;41(1):786–793.
- Timofte D, Dragos D, Balcangiu-Stroescu AE, et al. Infection with hepatitis C virus in hemodialysis patients: an overview of the diagnosis and prevention rules within a hemodialysis center (review). Exp Ther Med. 2020;20(1):109–116.
- Inoue H, Saito I, Nakazawa R, et al. Expression of inflammatory cytokines and adhesion molecules in haemodialysis-associated amyloidosis. Nephrol Dial Transplant. 1995;10(11):2077–2082.
- Ohno T, Tanaka Y, Sugauchi F, et al. Suppressive effect of oral administration of branched-chain amino acid granules on oxidative stress and inflammation in HCV-positive patients with liver cirrhosis. Hepatol Res. 2008;38(7):683–688.
- Waris G, Turkson J, Hassanein T, et al. Hepatitis C virus (HCV) constitutively activates STAT-3 via oxidative stress: role of STAT-3 in HCV replication. J Virol. 2005;79(3):1569–1580.
- Machida K, Cheng KT, Sung VM, et al. Hepatitis C virus infection activates the immunologic (type II) isoform of nitric oxide synthase and thereby enhances DNA damage and mutations of cellular genes. J Virol. 2004;78(16):8835–8843.
- Gong G, Waris G, Tanveer R, et al. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98(17):9599–9604.
- Smirnova OA, Ivanova ON, Bartosch B, et al. Hepatitis C virus NS5A protein triggers oxidative stress by inducing NADPH oxidases 1 and 4 and cytochrome P450 2E1. Oxid Med Cell Longev. 2016;2016:8341937
- de Almeida JP, Liberatti LS, Barros FE, et al. Profile of oxidative stress markers is dependent on vitamin D levels in patients with chronic hepatitis C. Nutrition. 2016;32(3):362–367.
- Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49(5):1304–1313.