Abstract
Background
High IS level has been demonstrated to be associated with vascular calcification and lymphocyte functional disorders, which are both risk factors of CVD. Low HDL-c level is a risk factor of CVD in CKD patients. This study was designed to explore the potential relationship between IS and HDL-c levels in early stages of CKD population.
Methods
Patients of CKD stage 1-3 were enrolled in this cross-sectional study. Correlations between HDL-c and IS levels were investigated among various clinicopathological variables through independent samples t test and multivariate logistic regression.
Results
A total of 205 CKD patients (96 men) aged 43.27 ± 13.80 years old were included in this research. There were 96 patients (46 men) in CKD stage1 and 109 (50 men) in CKD stage 2 or stage 3. IS levels were significantly higher in CKD 2 + 3 group (1.50 ± 1.74 μg/ml vs. 0.94 ± 0.66 μg/ml, p = 0.007), while HDL-c levels were lower (1.19 ± 0.39 mmol/L vs. 1.33 ± 0.45 mmol/L, p = 0.017) compared to CKD 1 group. Among all the patients, a negative correlation was observed between IS and HDL-c levels (r = −0.244, p = 0.001). IS level was an independent risk factor for low HDL-c (<1.04 mmol/L) incidence even after controlling for potential confounders including concomitant disease, age, sex, blood pressure, BMI and laboratory biochemical test including eGFR (OR = 1.63, 95% CI: 1.11–2.39, p = 0.013). IS and HDL-c were both risk factors for predicting CKD stage 3.
Conclusions
In early CKD stages, low HDL-c level is associated with increased IS levels, which may be an important contributor in the development of dyslipidemia in CKD patients.
Background
Chronic kidney disease (CKD) is associated with higher mortality of cardiovascular disease (CVD) [Citation1]. Indoxyl sulfate (IS), a protein-bound uremic toxin, is one of the organic anions that results from the metabolism of dietary tryptophan and after intestinal absorption is further converted to IS in the liver [Citation2]. Microbiome and intestinal permeability changes induced by hypervolemia may lead to increased IS, inflammation and endothelial dysfunction [Citation3,Citation4]. IS is excreted via proximal tubular secretion in the kidney and it accumulates in the blood of patients with declined renal function. As one of the most extensively studied uremic toxins, IS may predict CKD progression [Citation5]. Cao et al. [Citation6] from our group reported that high serum IS level was associated with higher risk of first heart failure event in patients under hemodialysis. Previous studies [Citation7,Citation8] performed by Xiang and Chen at al. from our group have revealed the regulatory mechanism of IS on vascular calcification and lymphocyte functional disorders, which are both risk factors of CVD.
Epidemiological studies have shown that high-density lipoprotein cholesterol (HDL-c) level is independently and inversely correlated with CVD [Citation9]. Reduced kidney function is associated with disruptions in the morphology and lipid metabolism [Citation10–12]. Dyslipidemia in CKD is typically characterized by high triglyceride (TG) and low HDL-c levels [Citation13]. A recently published study demonstrated that lower HDL-c is associated with atherosclerosis cardiovascular disease (ASCVD) in persons with CKD [Citation14].
So there aroused the question that whether IS has an effect on HDL metabolism like on vascular calcification and lymphocyte functional disorder in CKD. This study was then designed to explore that if increased IS level was an independent risk factor for low HDL-c levels in early CKD stages, the results of which may provide a new intervention target on CKD dyslipidemia.
Materials and methods
Study population
From October 2012 to January 2014, stages of CKD1,CKD2 and CKD3 patients aged 18-70 years were enrolled from Department of Nephrology, Zhongshan Hospital, Fudan University.
Exclusion criteria included: (1) Dialysis therapy; (2) Obesity (BMI ≥ 30kg/m2); (3) Recent 3 months usage of drugs known to influence lipid metabolism; (4) Recent 3 months usage of drugs that scavenging toxins through the intestines, such as Coated Aldehyde Oxystarch; (5) Recently 3 months usage of glucocorticoid or immunosuppressants; (6)History of New York Heart Association class III/IV heart failure; (7) Acute infection; (8) Liver cirrhosis; (9) Severely elevated serum alanine aminotransferase(ALT) or aspartate aminotransferase (AST) levels (1.5 times higher than normal upper limit); (10) Malignant tumor; (11) Human immunodeficiency virus infection.
All patients provided written informed consent for participation in accordance with the Declaration of Helsinki. The study was approved by the hospital ethical review board (Zhongshan Hospital, Fudan University, Shanghai, China).
Anthropometric measurements, blood sampling and clinical data collection
All patients were examined and blood sampling was performed in the morning after an overnight fast of 10–12 h. The date of birth, underlying kidney disease, past medical history were recorded. Height and weight (light clothes and without shoes), and resting blood pressure were determined by an experienced physician. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared.
24h urine sample was collected for urine protein quantification under aseptic precautions from the day before interview.
Biochemical measurements
Serum albumin, prealbumin, hemoglobin, blood urea nitrogen (BUN), serum creatinine (SCr), uric acid (UA), glycated hemoglobin (HbA1c), TG, total cholesterol (TC), HDL-c, low-density lipoprotein cholesterol (LDL-c) and high-sensitivity C-reactive protein (hsCRP) were measured using standard methods in the clinical laboratory.
Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Plasma IS concentration was detected using modified high-performance liquid chromatography (HPLC) tandem mass spectrometry method as described in our previous article [Citation5].
Statistical analysis
All variables were expressed as means ± SDs, or medians (interquartile ranges).
Comparisons between the 2 groups (CKD1 vs. CKD2 + 3) were assessed by independent samples t tests and X2-test (for categorical variables). Pearson/Spearman analysis was used to examine the correlation between IS and lipids levels and other biochemical variables.
Values of IS quartiles were defined as follows: (1) Q1: <0.54 μg/ml; (2) Q2: 0.54 μg/ml–0.88 μg/ml; (3) Q3: 0.88 μg/ml–1.59 μg/ml; (4) Q4: ≥1.59 μg/ml. Difference of HDL-c levels in the four IS quartile groups was tested by One-way ANOVA.
Odds ratios of low HDL-c (HDL-c < 1.04 mmol/L) occurrence with increased IS level were explored through multivariate longitudinal logistic regression model, in which IS values were all Ln transferred.
Factors predicting CKD stage 3 were also explored through multivariate longitudinal logistic regression model.
A two-tailed p < 0.05 was considered statistically significant. For all statistical analyses, SPSS Statistics 22.0 (IBM, Armonk, NY, USA) was used.
Results
Characteristics of study population
According to eGFR levels, 205 patients were divided into 2 groups: (1) CKD1 (eGFR ≥ 90 mL/min/1.73m2, n = 96); (2) CKD2 + 3 (30 mL/min/1.73m2≤eGFR < 89 mL/min/1.73m2, n = 109). Comparisons of clinical and biochemical characteristics between the 2 groups were shown in .
Table 1. Patients characteristics.
Age and sex were equally matched (both with p > 0.05). There was no significant difference of blood pressure and BMI between the study groups. Prevalence of hypertension was higher in group CKD2 + 3 (58.7 vs. 34.4%, p = 0.001). Compared to CKD1 group, CKD2 + 3 group presented higher levels of HbA1c (5.79 ± 0.99 vs. 5.54 ± 0.87%), UA (389.62 ± 100.69 vs. 328.04 ± 86.56 μmol/L), IS (1.50 ± 1.74 vs. 0.94 ± 0.66 μg/ml) and lower levels of hemoglobin (126.16 ± 21.29 vs. 136.45 ± 14.77 g/L) and HDL-c (1.19 ± 0.39 vs. 1.33 ± 0.45 mmol/L) (all with p < 0.05).
The association between is and HDL-c as well as other variables
As shown in , serum IS levels were positively correlated with systolic BP, diastolic BP, hypertension history, CVD history, levels of albumin, BUN, Scr and hsCRP and negatively correlated with eGFR, hemoglobin, HDL-c and urine protein levels (all with p < 0.05).
Table 2. Correlation of high IS levels with other variables.
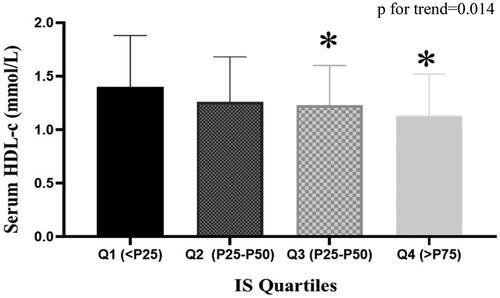
The subjects were then divided into four groups according to the quartile values of IS (Q1: IS < P25, Q2: P25 ≤ IS < P50, Q3:P50 ≤ IS < P75, Q4:IS ≥ P75). showed that as IS levels increased, HDL-c levels decreased from group to group. Serum HDL-c level in each group was 1.40 ± 0.48, 1.26 ± 0.42, 1.23 ± 0.37, 1.13 ± 0.39 mmol/L respectively (p for trend = 0.014).
Impact of is levels on risk of low HDL-c levels incidence
lists the risk of low HDL-c levels (defined as HDL-c < 1.04 mmol/L) incidence as IS levels increased [OR = 1.56, 95%CI (1.07–2.27), p = 0.018] (Model 1). After adjustment for medical history of hypertension, diabetes, gout and CVD, age, sex, Systolic BP, Diastolic BP and BMI (Model 2), IS showed an odds ratio of 1.55 [95%CI (1.05–2.30), p = 0.028]. After further adjustment for hemoglobin, HbA1c, albumin, hsCRP, eGFR, BUN, Scr, UA and 24 h urine protein(Model 4), IS still showed a significant OR of 1.57[95%CI (1.02–2.41), p = 0.039].
Table 3. Logistic regression of low HDL-c incidence with IS levels increment.
Risk factors predicting for CKD stage 3
shows that HDL-c [OR:0.15, 95%CI (0.04–0.57)], IS[OR:1.67,95%CI (1.06–2.63)], systolic BP [OR:1.04 (1.01–1.06)], hemoglobin [OR:0.98 (0.95–1.00)] and urine protein [OR:1.23 (1.08-1.40)] levels were risk factors predicting for CKD stage 3.
Table 4. Risk factors predicting for CKD stage 3.
Discussion
CKD is correlated with an increased risk of CVD as disease progresses [Citation13,Citation14]. Patients under dialysis have an extremely high risk of cardiovascular events [Citation1]. Actually, relationship between CKD and CVD is present even under minor renal injury. However, most studies have focused on CVD risks mostly when eGFR is lower than 60 mL/min/1.73 m2 [Citation1,Citation15,Citation16], In all relevant studies published to date, CVD is the predominant cause of increased mortality, accounting for over 50% of all deaths[Citation1,Citation15,Citation17,Citation18].
In general population, every 1 mmol/L (40 mg/dl) elevation in LDL-c level may result in an increased risk of CVD by 40% [Citation19,Citation20]. While in CKD patients, levels of residual renal function and proteinuria as well as comorbidities (especially type 2 diabetes) and treatment can all affect lipid metabolism [Citation21,Citation22]. However, the relationship between lipid profiles and CVD risks in CKD patients remains uncertain. In dialysis patients, serum LDL-c level has a negative association with all-cause mortality [Citation23,Citation24], the phenomenon of which is called ‘reverse epidemiology’. Low serum HDL-c levels are common among patients with CKD and ESRD [Citation25–27]. Archna Bajaj et al. [Citation14] recently reported that HDL-c was associated with increased risk for ASCVD beyond LDL-c among individuals with CKD.
Atheroprotective functions of HDL include anti-thrombotic activities [Citation28] and endothelium regenerative capabilities [Citation29,Citation30], anti-inflammatory and anti-oxidative properties [Citation31,Citation32]. Innumerable studies have revealed that HDL metabolism is complex and involving multiple pathways. The process of HDL biogenesis is mediated primarily by the liver. ApoA-1 is the major lipoprotein on HDL which stimulates cholesterol efflux through ATP-binding cassette (ABC) transporters. The movement of cholesterol from peripheral tissues to the liver for clearance is termed reverse cholesterol transport (RCT), a pathway that represents a key atheroprotective function of HDL. Defective maturation of HDL particles, impaired Apo-A1-mediated cholesterol efflux, and limited RCT have been revealed in CKD patients [Citation33].
As one of the most extensively studied protein-bound uremic toxins, IS may be associated with CVD and mortality in CKD patients. The process of IS biogenesis is mediated mainly in the liver [Citation2,Citation34–37]. More and more attention has been focused on the relationship between IS levels and CVD incidence among CKD population in recent years [Citation38–42]. Taki et al. [Citation43] found that high serum IS level was significantly correlated with incidence of atherosclerosis. Cao et al. [Citation6] from our group reported that high serum IS was associated with higher risk of first failure event in patients on hemodialysis.
It is known that progressive decline of renal function is associated with increased IS and decreased HDL-c levels. This study firstly found an association between IS and HDL-c independent of renal function in early CKD stages. Besides the negative correlation, IS was an independent risk factor of low HDL-c incidence. Even after adjusting related conventional factors such as age, sex, BMI, history of diabetes, history of primary hypertension, history of coronary heart disease, blood pressure and so on, the OR value remained statistically significant as we expected. However, more basic researches are needed to confirm whether IS has a direct effect on any step of RCT, ApoA-1 mediated cholesterol efflux, HDL biogenesis and maturation, the results of which might bring new target on dyslipidemia therapy in CKD patients.
Smoking and obesity are known as related factors to dyslipidemia. In this study, percentage of smoking patients was small and statistically equal in the two study groups. No obvious correlation was found between smoking and IS or HDL-c levels. As for obesity, we did exclude obesity patients (BMI ≥ 30kg/m2). Though BMI in the two groups was matched, it was actually correlated with HDL-c (r = −0.232, p = 0.001). However, the association between IS and HDL-c remained meaningful after adjustment of BMI. Even after adjustment of age, sex, diabetes history, hypertension history, CVD history, HbA1c et al, the association was still meaningful. Therefore, we think that IS’s impact on HDL-c among CKD patients is independent on conventional risk factors.
There’s another important finding in this article that a negative correlation was found between proteinuria and IS (r = −0.254, p < 0.001). Definite mechanism has not been found through literature review. What has been already known is that glomerular proteinuria level decreases gradually as renal function declines with/without obviously reduced urine volume, so that there’s a positive correlation between eGFR and urine protein quantity. While IS level is negatively correlated with eGFR, so statistically we may consider that IS level may be negatively correlated with urine protein quantity. It’s not clear whether IS has a direct affect on glomerular pathological changes, which might inhibit protein excretion from kidney. More basic researches are needed to explore the mechanism of this finding.
There were still several limitations in this study. Firstly, the sample size was relatively small as a cross-sectional research. Secondly, the tested lipid contents (only TC, TG, HDL-c and LDL-c included) were not adequate to make omni-directional exploration of the relationship between lipid metabolism and IS in patients with CKD.
Our data revealed that low HDL-c level occurs in early stages of CKD, which might be resulted from increased IS level as the CKD stage worsens. This negative correlation exits between IS and HDL-c independent of GFR. Thus clinically, methods to reduce serum IS level (e.g., avoidance of hypervolemia induced microbiome and intestinal permeability changes, and use of uremic toxin adsorbent such as the oral charcoal adsorbent AST-120 [Citation44,Citation45]) might improve HDL-c metabolic disorder. Detailed mechanisms need to be further investigated.
Ethics approval and consent to participate
The study protocol complied with the ethical principles of the Declaration of Helsinki and received full approval from the institutional review boards of Shanghai Fudan University Zhongshan Hospital (no.B2017-076R). All patients provided written informed consent.
Consent to publish
Not applicable, as it does not contain an individual person’s data.
Author contributions
LW and FFX wrote the paper. JJ, JZZ and YQC designed the study. NX collected the data. LZ and XTJ did statistical analysis. XQD revised the manuscript. XSC interpreted the study. All authors have read and approved the final manuscript.
Acknowledgments
A preprint version of the paper was published before. (https://doi.org/10.21203/rs.2.18545/v1).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Additional information
Funding
References
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305.
- Bueschkens DH, Stiles ME. Escherichia coli variants for gas and indole production at elevated incubation temperatures. Appl Environ Microbiol. 1984;48(3):601–605.
- Barreto FC, Barreto DV, Liabeuf S, et al.; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(10):1551–1558.
- Zsom L, Faludi M, Fülöp T, et al. The association of overhydration with chronic inflammation in chronic maintenance hemodiafiltration patients. Hemodial Int. 2019;23(3):384–391.
- Wu IW, Hsu KH, Lee CC, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26(3):938–947.
- Cao X-S, Chen J, Zou J-Z, et al. Association of indoxyl sulfate with heart failure among patients on hemodialysis. CJASN. 2015;10(1):111–119.
- Chen J, Zhang X, Ding X, et al. Indoxyl sulfate enhance the hypermethylation of klotho and promote the process of vascular calcification in chronic kidney disease. Int J Biol Sci. 2016;12(10):1236–1246.
- Xiang F, Chen R, Cao X, et al. Monocyte/lymphocyte ratio as a better predictor of cardiovascular and all-cause mortality in hemodialysis patients: a prospective cohort study. Hemodialysis Inter. 2018;22(1):82–92.
- Santos-Gallego CG, Badimon JJ, Rosenson RS. Beginning to understand high-density lipoproteins. Endocrinol Metab Clin North Am. 2014;43(4):913–947.
- Reiss AB, Voloshyna I, De Leon J, et al. Cholesterol metabolism in CKD. Am J Kidney Dis. 2015;66(6):1071–1082.
- Florens N, Calzada C, Lyasko E, et al. Modified lipids and lipoproteins in chronic kidney disease: a new class of uremic toxins. Toxins (Basel). 2016;8(12):376.
- Vaziri ND. Disorders of lipid metabolism in nephrotic syndrome: mechanisms and consequences. Kidney Int. 2016;90(1):41–52.
- Keane WF, Tomassini JE, Neff DR. Lipid abnormalities in patients with chronic kidney disease: implications for the patho physiology of atherosclerosis. JAT. 2013;20(2):123–133.
- Bajaj A, Xie D, Cedillo-Couvert E, CRIC Study Investigators, et al. Lipids, apolipoproteins, and risk of atherosclerotic cardiovascular disease in persons with CKD. Am J Kidney Dis. 2019;73(6):827–836.
- Matsushita K, van der Velde M, Astor BC, et al. ; Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081.
- Vanholder R, Massy Z, Argiles A, et al.; European Uremic Toxin Work Group. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20(6):1048–1056.
- Steenkamp R, Rao A, Roderick P. UK Renal Registry 17th annual report: chapter 5 survival and cause of death in UK adult patients on renal replacement therapy in 2013: national and centre-specific analyses. Nephron. 2015;129(s1):99–129.
- United States Renal Data System. 2015. USRDS Annual Data Report volume 2: ESRD in the United States.
- Wilson PW, Garrison RJ, Castelli WP, et al. Prevalence of coronary heart disease in the Framingham Offspring Study: role of lipoprotein cholesterols. Am J Cardiol. 1980;46(4):649–654.
- Emerging Risk Factors Collaboration et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000.
- Hager MR, Narla AD, Tannock LR. Dyslipidemia in patients with chronic kidney disease. Rev Endocr Metab Disord. 2017;18(1):29–40.
- Zheng- Lin B, Ortiz A. Lipid management in chronic kidney disease: systematic review of PCSK9 targeting. Drugs. 2018;78(2):215–229.
- Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am. J. Kidney Dis. 1990;15(5):458–482.
- Baigent C, Landray MJ, Wheeler DC. Misleading associations between cholesterol and vascular outcomes in dialysis patients: the need for randomized trials. Semin Dial. 2007;20(6):498–503.
- Lo JC, Go AS, Chandra M, et al. GFR, body mass index, and low high-density lipoprotein concentration in adults with and without CKD. Am. J. Kidney Dis. 2007;50(4):552–558.
- Ganda A, Magnusson M, Yvan-Charvet L, et al. Mild renal dysfunction and metabolites tied to low HDL cholesterol are associated with monocytosis and atherosclerosis. Circulation. 2013;127(9):988–996.
- Anderson JL, et al. High density lipoprotein (HDL) particles from end-stage renal disease patients are defective in promoting reverse cholesterol transport. Sci. Rep. 2017;7:41481.
- Mineo C, Deguchi H, Grifn JH, et al. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98(11):1352–1364.
- Besler C, Heinrich K, Rohrer L, et.al. Mechanisms underlying adverse efects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Investig. 2011;121(7):2693–26708.
- Spieker LE, Sudano I, HüRlimann D, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105(12):1399–1402.
- Barter PJ, Nicholls S, Rye K-A, et al. Antiinfammatory properties of HDL. Circ Res. 2004;95(8):764–772.
- Navab M, Yu R, Gharavi N, et al. High-density lipoprotein: antioxidant and anti-inflammatory properties. Curr Atheroscler Rep. 2007;9(3):244–248.
- Moradi H, Vaziri ND. Molecular mechanisms of disorders of lipid metabolism in chronic kidney disease. Front Biosci. 2018;23:146–161.
- Meyer TW, Walther JL, Pagtalunan ME, et al. The clearance of protein-bound solutes by hemofiltration and hemodiafiltration. Kidney Int. 2005;68(2):867–877.
- Niwa T. Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr. 2010;20(5 Suppl):S2–S6.
- Vanholder R, Bammens B, de Loor H, et al. Warning: the unfortunate end of p-cresol as a uraemic toxin. Nephrol Dial Transplant. 2011;26(5):1464–1467.
- Banoglu E, Jha GG, King RS. Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur J Drug Metab Pharmacokinet. 2001;26(4):235–240.
- Lin CJ, Liu HL, Pan CF, et al. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch Med Res. 2012;43(6):451–456.
- Adijiang A, Goto S, Uramoto S, et al. Indoxyl sulphate promotes aortic calcification with expression of osteoblastspecific proteins in hypertensive rats. Nephrol Dial Transplant. 2008;23(6):1892–1901.
- Muteliefu G, Enomoto A, Jiang P, et al. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol Dial Transplant. 2009;24(7):2051–2058.
- Lekawanvijit S, Adrahtas A, Kelly DJ, et al. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J. 2010;31(14):1771–1779.
- Yisireyili M, Shimizu H, Saito S, et al. Indoxyl sulfate promotes cardiac fibrosis with enhanced oxidative stress in hypertensive rats. Life Sci. 2013;92(24-26):1180–1185.
- Taki K, Tsuruta Y, Niwa T. Indoxyl sulfate and atherosclerotic risk factors in hemodialysis patients. Am J Nephrol. 2007;27(1):30–35.
- Asai M, Kumakura S, Kikuchi M. Review of the efficacy of AST-120 (KREMEZIN®) on renal function in chronic kidney disease patients. Ren Fail. 2019;41(1):47–56.
- Ito S, Ohno Y, Tanaka T, et al. Neutrophil/lymphocyte ratio elevation in renal dysfunction is caused by distortion of leukocyte hematopoiesis in bone marrow. Ren Fail. 2019;41(1):284–293.