Abstract
Objectives
A meta-analysis and systematic review was conducted on kidney-related outcomes of three recent pandemics: SARS, MERS, and COVID-19, which were associated with potentially fatal acute respiratory distress syndrome (ARDS).
Methods
A search of all published studies until 16 June 2020 was performed. The incidence/prevalence and mortality risk of acute and chronic renal events were evaluated, virus prevalence, and mortality in preexisting hemodialysis patients was investigated.
Results
A total of 58 eligible studies involving 13452 hospitalized patients with three types of coronavirus infection were included. The reported incidence of new-onset acute kidney injury (AKI) was 12.5% (95% CI: 7.6%–18.3%). AKI significantly increased the mortality risk (OR = 5.75, 95% CI 3.75–8.77, p < 0.00001) in patients with coronavirus infection. The overall rate of urgent-start kidney replacement therapy (urgent-start KRT) use was 8.9% (95% CI: 5.0%–13.8%) and those who received urgent-start KRT had a higher risk of mortality (OR = 3.43, 95% CI 2.02–5.85, p < 0.00001). Patients with known chronic kidney disease (CKD) had a higher mortality than those without CKD (OR = 1.97, 95% CI 1.56–2.49, p < 0.00001). The incidence of coronavirus infection was 7.7% (95% CI: 4.9%–11.1%) in prevalent hemodialysis patients with an overall mortality rate of 26.2% (95% CI: 20.6%–32.6%).
Conclusions
Primary kidney involvement is common with coronavirus infection and is associated with significantly increased mortality. The recognition of AKI, CKD, and urgent-start KRT as major risk factors for mortality in coronavirus-infected patients are important steps in reducing future mortality and long-term morbidity in hospitalized patients with coronavirus infection.
Introduction
The novel coronavirus disease-2019 (COVID-19), first reported in Wuhan, China, has become a worldwide pandemic and has caused over 28,918,900 confirmed cases of COVID-19 globally, including 922,252 deaths reported to the World Health Organization (WHO) as of 3:28 pm CEST, 14 September 2020 [Citation1]. Apart from the rapid development of acute respiratory distress syndrome (ARDS), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as well as the previously identified severe acute respiratory syndrome coronavirus (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS-CoV), which are members of the coronavirus family [Citation2], also have major associated but under-recognized extrapulmonary manifestations [Citation3–5]. These three types of coronaviruses have caused catastrophic coronavirus pandemics in human history, namely SARS, MERS, and COVID-19. Among the organs affected, the kidneys are often involved due to the organ cross-talk between alveolar and tubular damage, i.e. the lung–kidney axis in ARDS [Citation6]; the occurrence of kidney involvement usually indicates a worse prognosis [Citation7,Citation8]. Although the etiology of coronavirus-associated AKI is likely to be multifactorial, all three coronaviruses can directly invade renal cells through hijacking native surface receptors: angiotensin-converting enzyme 2 (ACE2) serves as a receptor for SARS-CoV-1 and SARS-CoV-2 [Citation9,Citation10], while MERS-CoV enters target cells via binding to dipeptidyl-peptidase 4 (DDP4) [Citation11]. However, it is unclear how the virus causes cellular damage following the entry. If maintained during the course of infection, the kidney could function as a viral reservoir and urine become a potential source of viral transmission.
Acute kidney injury (AKI) is the most frequent extra-pulmonary organ dysfunction associated with ARDS and is an independent risk factor for mortality [Citation12,Citation13]. However, the reported prevalence and mortality of AKI for all three coronavirus infection differs between studies. All patients with chronic kidney disease (CKD), including those with end-stage kidney disease (ESKD) or on kidney replacement therapy (KRT), are immunosuppressed making them more susceptible to infection and potentially a more severe course [Citation14–16]. The potential increased risk related to preexisting CKD and urgent-start KRT treatment is presently unclear. The influence of SARS-CoV-2/COVID-19 on sustained dialysis patients is also unknown.
In view of our previous experience with SARS, MERS, and more recent experiences of the COVID-19 outbreaks, we conducted a meta-analysis and systematic review to investigate the kidney involvement and patients’ outcomes in hospitalized coronavirus-infected patients.
Methods
Data sources and search
This systematic review was performed following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The registration of this review was published in PROSPERO (CRD42020200941). A search for published studies was performed using the PubMed database, EMBASE, and Cochrane library until 16 June 2020. Research articles on coronavirus (SARS-CoV, MERS-CoV, and SARS-CoV2) infected patients with information on kidney disease, AKI, dialysis, or kidney function were eligible and included. Keywords (‘COVID-19′ OR ‘SARS-CoV2’) or ‘SARS-CoV’ or ‘MERS-CoV’ and (‘chronic kidney disease’ or ‘CKD’ or ‘kidney disease’ or ‘end-stage kidney disease’ or ‘ESKD’) or (‘acute kidney injury’ or ‘AKI’) or (‘kidney replacement therapy’ or ‘KRT’ or ‘blood purification’) or (‘dialysis’ or ‘hemodialysis’ or ‘blood purification’) or (‘mortality’ or ‘death’) were combined to construct corresponding search formulas in databases. We used a combination of subject terms with free-text terms during the search, supplemented by a manual search and citation search. We also screened the latest relevant articles about COVID-2019 and met the inclusion criteria, through the "https://www.biorxiv.org/search/covid-19" website.
Inclusion and exclusion criteria
Studies that met the following PICOS criteria were included: (1) articles were original reports including patients infected with coronavirus; (2) studies with outcomes of interest consisting of mortality or kidney-related outcomes, i.e. urgent-start KRT, AKI or dialysis; (3) types of articles were cohort studies, case series, and case-control studies. The exclusion criteria of the study included articles reporting patients infected with coronavirus other than SARS-CoV, MERS-CoV, and SARS-CoV2; articles without relevant outcomes of interest; commentaries or reviews; research articles with patient numbers below five.
Study selection and data extraction
Two reviewers (ZS and XC) independently screened the titles and abstracts and then checked the full text of all the articles that might be eligible. Differences were resolved through discussion or consultation with the third reviewer (XJ). The two researchers separately extracted data from the included studies, including first author of the article, year, study design, follow-up, number of reported cases, mortality, CKD, AKI, use of urgent-start KRT, ESKD, incidence or mortality of infected dialysis patients and related baseline characteristics. Mortality was defined as the death of patients during hospitalization.
The quality rating for each study was evaluated by the NOS (Newcastle-Ottawa scale). For the evaluation of case reports and case series included, we applied a generally recommended standard similar to NOS, based on the domains of selection, ascertainment, causality and reporting and provide signaling questions [Citation17].
Patient and public involvement
No patient involved.
Certainty rating of evidence
The GRADE instrument (Grading of Recommendation, Assessment, Development, and Evaluation) was applied to rate the certainty of evidence and the strength of recommendations generated in our study [Citation18–20]. The certainty of evidence was rated for kidney-related complications and underlying kidney diseases prevalence and associations with patients’ prognosis. The five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) were taken into account to assess the confidence in effect estimates. Quality of evidence was characterized as high, moderate, low, or very low [Citation20,Citation21]. GRADE was assessed per http://gradepro.org.
Statistical analysis
Assessment of risk of bias was performed by two authors (XC and XJ) independently using the Newcastle–Ottawa Quality Assessment Scale (NOS) [Citation22]. Studies were scored up to a maximum of 9 points by NOS. Study quality was classified into three categories: 0–3 (low), 4–6 (moderate), and 7–9 (high). Statistical analyses were performed using Revman software V5.4 (Cochrane). Sensitivity analysis on the results of pooled analysis was performed by method of excluding all the included preprinted literatures and method of one-by-one elimination to verify the stability of the results. Rates for dichotomous data were analyzed using the Stata 16.0 (Stata Corp LP, TX, USA) Metaprop package. Since most studies were of retrospective design and heterogeneity between studies expected, the random-effects model was chosen for data synthesis [Citation23]. Odds Ratio (ORs) and 95% confidence intervals (CIs) were used for dichotomous variables as effect measures and were graphically visualized using Forest plots. Besides, T-statistic using Hartung–Knapp–Sidik–Jonkman method was performed for the degrees of freedom in the random-effects analysis, when the number of studies was < 10 by R version 4.03 (Metafor package) [Citation24]. Heterogeneity across studies was evaluated using the Cochrane Q test and I2 test (I2 = 100% ((Q-df)/Q). An I2 value of 0–49%, 50–74% and >75% indicated low, moderate or high heterogeneity, respectively [Citation25]. A two-sided P value < 0.10 was considered statistically significant. Subgroup analysis was performed for each individual virus. If the number of studies was <9, publication bias was not investigated. Publication bias was evaluated with Begg’s test, Egger’s test and Funnel-plot. Meta-regression analysis was used to find potential heterogeneity. A two-sided P-value <0.05 except heterogeneity was considered statistically significant.
Results
Search results and study selection
Our initial search strategy identified 42 papers on SARS, 32 papers on MERS and 125 papers on COVID-19 (199 records) displayed in . The search strategy was listed in Supplementary 1. A further search was then performed on citation and online preprint servers identifying 15 further papers, resulting in a total of 214 records. Following a thorough assessment, 58 articles with 13452 patients were included for quantitative synthesis and further investigation ( and ). Most COVID-19 studies were performed in Asia (68%, n = 21) [Citation6,Citation26–45], followed by Europe (19%, n = 6) [Citation46–51] and North America (13%, n = 4) [Citation52–55]. Most SARS studies were performed in Asia (88%, n = 7) [Citation5,Citation56–61] except for one study from Canada[Citation62], and almost all the MERS studies were performed in Saudi Arabia (95%, n = 18) [Citation3,Citation63–79] with the exception of one study from South Korea. Follow-up duration ranged from 1 to 46 months. The publication years of these studies ranged from 2003 to 2020. All studies on COVID-19 were published within the first 6 months of 2020.
Figure 1. Flow chart of the diagram. SARS: severe acute respiratory syndrome; MERS: Middle East respiratory syndrome; COVID-19: novel coronavirus disease 2019.
Table 1. Summary of the characteristics of the enrolled studies.
Table 2. Summary of the characteristics of the enrolled studies in patients with ESKD.
Quality rating
The quality rating for each study was evaluated by the NOS (Newcastle-Ottawa scale) (Supplementary Table 1). The average score of the included studies was 5.6 indicating moderate quality. The average score of studies for SARS, MERS, and COVID-19 were 5.8, 6.2, and 5.2, respectively. In domains of comparability, 52 studies with single arms did not fulfill the selection of the non-exposed cohort and only six studies [Citation43,Citation45,Citation59,Citation61,Citation71,Citation80] received scores. The GRADE tool was used to summarize pooled evidence for the main outcomes, as shown in Supplementary Table 2. Except several subgroup comparisons were rated as low, the majority of recommendations generated from this systematic review and meta-analysis were evaluated as very low.
AKI incidence and mortality risk in patients with coronavirus infection
The overall incidence of AKI was 12.5% (95% CI: 7.6%–18.3%, Heterogeneity I2 = 97.8%, p < 0.001; Supplementary Figure 1) adjusted for sample size with an overall mortality rate of 80.9% (95% CI: 57.6%–97.4%, Heterogeneity I2 = 96.1%, p < 0.001, Supplementary Figure 2). AKI significantly increased the risk of mortality (OR 5.73, 95% CI 3.75 to 8.77, p < 0.00001; I2 = 92%, p < 0.00001, ) in patients with coronavirus infection.
Figure 2. Mortality risk of AKI in three types of coronavirus diseases compared with non-AKI. AKI: acute kidney injury; SARS: severe acute respiratory syndrome; MERS: Middle East respiratory syndrome; COVID-19: novel coronavirus disease 2019.
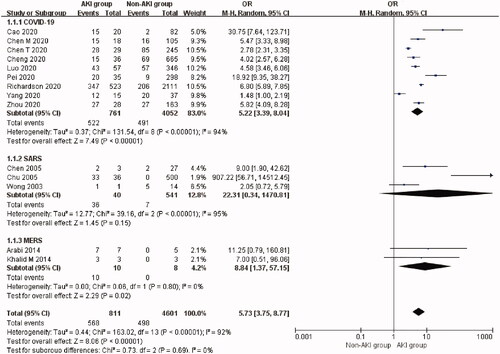
The incidence of AKI was 9.0% (95% CI: 4.2%–15.2%) in patients with COVID-19 but varied from 8.3% to 28.85% in the four studies reporting COVID-19 related AKI on ICU [Citation81]. The mortality rate of AKI patients with COVID-19 was 72.3% (95% CI: 47.1%–92.0%) in nine studies [Citation27–29,Citation32–34,Citation43,Citation45,Citation55]. AKI was associated with a higher risk of mortality compared with non-AKI patients (Nine studies [Citation27–29,Citation32–34,Citation43,Citation45,Citation55], OR 5.22, 95% CI 3.39 to 8.04, p < 0.00001; I2 = 94%, p < 0.00001, ) in COVID-19 patients. T-statistic was performed to check the stability of this result: t = 6.015, p = 0.0003. There was no significant publication bias (Begg’s test: p = 0.251, and Egger’s test: p = 0.304). Funnel plot was nearly symmetrical (Supplementary Figure 3).
The incidence of AKI was 9.6% (95% CI: 3.9%–17.2%) in SARS patients. AKI occurred in 51 out of 641 SARS patients. The mortality rate of AKI patients with SARS was 98.9% (95% CI: 86.9%–100.0%) in three studies [Citation56,Citation57,Citation59]. However, AKI itself was not associated with a significantly higher risk of mortality (three studies [Citation56,Citation57,Citation60], OR 22.31, 95% CI 0.34 to 1470.81, p = 0.15; I2 = 95%, p < 0.00001, ) in these studies. T-statistic was performed to check the stability of this result: t = 1.459, p = 0.282.
The incidence of AKI was the highest in MERS, which was 42.0% (95% CI: 29.8%–54.7%). The mortality rate of AKI patients with MERS was 100.0% (95% CI: 82.4%–100.0%) based on two studies [Citation3,Citation72]. AKI was associated with a significantly higher risk of mortality (Two studies [Citation3,Citation72], OR 8.84, 95% CI 1.37 to 57.15, p = 0.02; I2 = 0%, p = 0.80, ) in MERS patients. However, T-statistic showed that t = 9.202 and p = 0.069.
Urgent-start KRT application in patients with coronavirus infection
The overall rate of urgent-start KRT use was 8.9% (95% CI: 5.0%–13.8%, Heterogeneity I2 = 97.2%, p < 0.001, Supplementary Figure 4) but only one [Citation56] study reported the use of urgent-start KRT in SARS patients with a rate of 1.87% and no deaths.
Urgent-start KRT-treated patients with coronavirus infection had an overall mortality of 80.7% (95% CI: 58.8%–96.6%, Heterogeneity I2 = 92.9%, p < 0.001, Supplementary Figure 5). The use of urgent-start KRT was significantly associated with increased mortality (OR 3.43, 95% CI 2.02 to 5.82, p < 0.00001; I2 = 97%, p < 0.00001, ) although this applied only to patients with MERS and COVID-19.
Figure 3. Mortality risk of urgent-start KRT use in three types of coronavirus diseases. Urgent-start KRT: urgent-start renal replacement therapy; MERS: Middle East respiratory syndrome; COVID-19: novel coronavirus disease 2019.
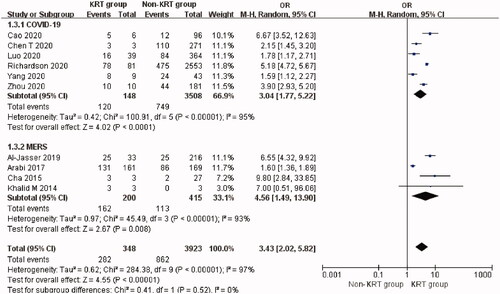
So far, 13 studies [Citation6,Citation26,Citation27,Citation29,Citation31–35,Citation39,Citation40,Citation45,Citation55] have reported the rate of urgent-start KRT use (3.4%, 95% CI: 1.9%–5.4%) in hospitalized patients with COVID-19. The mortality rate of urgent-start KRT-treated patients with COVID-19 was 74.2% (95% CI: 45.8%–95.5%) in six studies [Citation27,Citation29,Citation32–34,Citation55]. The use of urgent-start KRT was associated with a higher risk of mortality compared with non-KRT patients (Six studies [Citation27,Citation29,Citation32–34,Citation55], OR 3.04, 95% CI 1.77 to 5.22, p < 0.0001; I2 = 95%, p < 0.00001, ) in COVID-19. T-statistic showed that t = 4.597 and p = 0.006. Sensitivity analysis by removal of Richardson et al.’s study [Citation55] resulted in a 10% reduction of heterogeneity for mortality.
Seven studies [Citation3,Citation63,Citation65,Citation67,Citation72,Citation77,Citation80] reported the highest rate of urgent-start KRT use (35.0%, 95% CI: 16.8%–55.4%) in hospitalized patients with MERS. The mortality rate of urgent-start KRT patients with MERS was 85.5% (95% CI: 78.9%–91.2%) in four studies [Citation65,Citation67,Citation72,Citation80]. The use of urgent-start KRT was also associated with a higher risk of mortality in MERS patients (Four studies [Citation65,Citation67,Citation72,Citation80], OR 4.56, 95% CI 1.49 to 13.90, p = 0.008; I2 = 93%, p < 0.00001, ). T-statistic showed that t = 3.365 and p = 0.044.
Pre-dialysis CKD prevalence and mortality risk in patients with coronavirus infection
The overall prevalence of CKD was 14.2% (95% CI: 9.6%–19.6%, Heterogeneity I2 = 97.6%, p < 0.001, Supplementary Figure 6). The mortality rate of CKD patients with coronavirus infection was 65.4% (95% CI: 46.3%–82.7%, Heterogeneity I2 = 87.3%, p < 0.001, Supplementary Figure 7). CKD significantly increased the risk of mortality (OR 1.97, 95% CI 1.56 to 2.49, p < 0.00001; I2 = 65%, p < 0.0001, ) in patients with coronavirus infection.
Figure 4. Mortality risk of non-dialytic preexisting CKD in three types of coronavirus diseases compared with non-CKD. CKD: chronic kidney disease; SARS: severe acute respiratory syndrome; MERS: Middle East respiratory syndrome; COVID-19: novel coronavirus disease 2019.
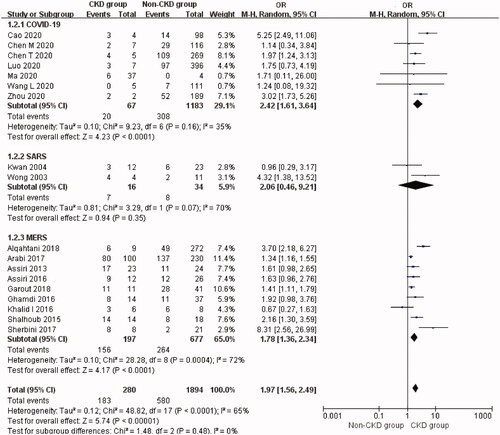
The prevalence of CKD comorbidity was 11.0% (95% CI: 5.6%–17.8%) in COVID-19 patients with an associated mortality rate of 38.7% (95% CI: 16.8%–62.7%) in eight studies [Citation27–29,Citation33,Citation34,Citation37,Citation38,Citation51]. CKD was associated with a significantly higher risk of mortality compared with non-CKD patients with COVID-19 (Seven studies [Citation27–29,Citation33,Citation34,Citation37,Citation38], OR 2.42, 95% CI 1.61 to 3.64, p < 0.0001; I2 = 35%, p = 0.16, ). T-statistic showed that t = 4.605 and p = 0.004. Sensitivity analysis by removal of a single-study showed that Cao et al.’s study [Citation34] contributed about 22% of the heterogeneity.
The prevalence of CKD was 4.4% (95% CI: 0.0%–19.0%) in SARS patients. The mortality rate of CKD patients with SARS was 46.5% (95% CI: 20.6%–73.2%) in two studies [Citation57,Citation61]. CKD was not associated with a significantly higher risk of mortality in SARS patients although this analysis was based on only two studies (Two studies [Citation57,Citation61], OR 2.06, 95% CI 0.46 to 9.21, p = 0.35; I2 = 70%, p = 0.07, ). T-statistic showed that t = 0.998 and p = 0.501.
The prevalence of CKD was 23.8% (95% CI: 15.8%–32.7%) in MERS patients. The mortality rate of CKD patients with MERS was very high, i.e. 83.6% (95% CI: 69.4%–94.7%). As for the nine articles [Citation63,Citation65,Citation66,Citation68,Citation69,Citation73,Citation74,Citation76,Citation79] describing the prognosis of 197 CKD patients versus 677 non-CKD patients with MERS, pooled analysis of the mortality revealed a significantly higher risk of mortality in MERS patients with CKD (OR 1.78, 95% CI 1.36 to 2.34, p < 0.0001; I2 = 72%, p = 0.0004, ). T-statistic showed that t = 3.244 and p = 0.012. There was no significant publication bias (Begg’s test: p = 0.118, and Egger’s test: p = 0.075) but the Funnel plot was not so symmetrical (Supplementary Figure 8).
ESKD prevalence and mortality risk in patients with coronavirus infection
The overall prevalence of ESKD was 16.4% (95% CI: 7.2%–27.9%, Heterogeneity I2 = 98.2%, p < 0.001, Supplementary Figure 9). The overall mortality rate of ESKD patients with coronavirus infection was 51.7% (95% CI: 27.0%–76.1%, Heterogeneity I2 = 83.3%, p < 0.001, Supplementary Figure 10). Overall analysis showed that ESKD significantly increased the risk of mortality (OR 1.81, 95% CI 1.44 to 2.27, p < 0.00001; I2 = 0%, p = 0.62, ) in patients with coronavirus infection.
Figure 5. Mortality risk of preexisting ESKD in three types of coronavirus diseases compared with non-ESKD. ESKD: end-stage renal disease; SARS: severe acute respiratory syndrome; MERS: Middle East respiratory syndrome; COVID-19: novel coronavirus disease 2019.
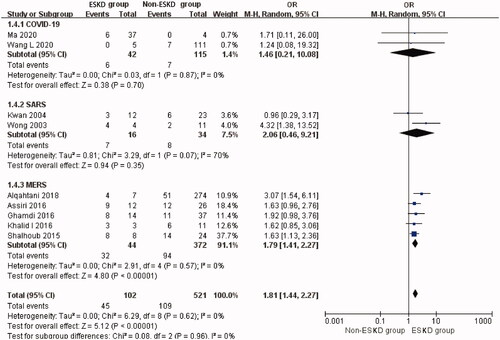
The prevalence of ESKD was 30.9% (95% CI: 4.6%–66.8%) in the COVID-19-related studies. The mortality rate of ESKD patients with COVID-19 was 17.6% (95% CI: 8.2%–29.2%). Compared with non-ESKD patients, ESKD was not associated with a higher risk of mortality although this was based on two studies (two studies [Citation37,Citation38], OR 1.46, 95% CI 0.21 to 10.08, p = 0.70; I2 = 0%, p = 0.87, ) in SARS-CoV-2. T-statistic showed that t = 2.339 and p = 0.257.
The prevalence of ESKD was 4.4% (95% CI: 0.0%–19.0%) in SARS related studies. The mortality rate of ESKD patients with SARS was 46.5% (95% CI: 20.6%–73.2%). ESKD was also not associated with a higher risk of mortality (Two studies [Citation57,Citation61], OR 2.06, 95% CI 0.46 to 9.21, p = 0.35; I2 = 70%, p = 0.07, ) in SARS patients. T-statistic showed that t = 0.999 and p = 0.500.
The prevalence of ESKD was 13.8% (95% CI: 5.1%–25.2%) included MERS related studies. The mortality rate of ESKD patients with MERS was the highest: 78.1% (95% CI: 51.1%–97.6%). The pooled analysis of the mortality revealed a significantly higher risk of mortality in MERS patients with ESKD (Five studies [Citation63,Citation68,Citation74,Citation76,Citation79], OR 1.79, 95% CI 1.41 to 2.27, p < 0.00001; I2 = 0%, p = 0.57, ). T-statistic showed that t = 5.682 and p = 0.005.
Patients on chronic hemodialysis and the occurrence of coronavirus infection
The overall incidence of coronavirus infection was 7.7% (95% CI: 4.9%–11.1%, Heterogeneity I2 = 97.2%, p < 0.001, Supplementary Figure 11) with a mortality rate of 26.4% (95% CI: 20.6%–32.6%, Heterogeneity I2 = 51.6%, p < 0.001, Supplementary Figure 12).
The incidence of COVID-19 was 8.0% (nine studies [Citation30,Citation38,Citation42,Citation44,Citation46–50], 95% CI: 4.7%–12.0%) in hemodialysis patients with a mortality rate of 25.7% (nine studies [Citation30,Citation38,Citation42,Citation44,Citation46,Citation49–51,Citation53], 95% CI: 21.3%–30.3%). The incidence of SARS was 1.7% (95% CI: 0.9%–3.0%) based on a single study [Citation61] with a mortality rate of 25.0% (95% CI: 5.5%–57.2%). The incidence of MERS was 3.6% (95% CI: 1.8%–5.9%) from two studies [Citation74,Citation75] in hemodialysis patients with an associated mortality rate of 75.0% (one study [Citation74], 95% CI: 42.8%–94.5%).
Sensitivity analysis and meta-regression analysis
We further conducted sensitivity analysis to evaluate the influence of case series and preprinted literatures on the stability of results. First, the results maintained significance after excluding all the preprinted literatures included in the pooled analysis [Citation27,Citation28,Citation38,Citation39,Citation41]. Second, the results also maintained stable by excluding the literatures included in the pooled analysis one by one. Moreover, too few studies were left in each subgroup after excluding all the case series, because this type of study occupied a relatively large proportion (about 50%). Thus, we kept the case series with number of patients reported equal or greater than 5 cases, and rated the quality of these literatures referring to a generally recommended standard [Citation17]. Meta-regression analysis was used to find potential heterogeneity in primary results. However, different ethnicities and study types did not contribute significantly to the heterogeneity in four results (P all > 0.05).
Discussion
The COVID-19 pandemic that is currently raging around the world is causing major disruption to health systems [Citation82]. As a member of the coronavirus family [Citation2], COVID-19 together with SARS and MERS lead to severe acute respiratory symptoms [Citation83], as well as extrapulmonary disease [Citation84]. Although the kidney is commonly affected, its contribution to patient mortality and morbidity is only belatedly being recognized. Compared with similar systematic reviews [Citation85,Citation86] that had been published so far, our research explored the impact of kidney-related events on the prognosis of patients in the face of coronavirus abuse from a more comprehensive and in-depth perspective. We conducted this systematic review to investigate the incidence of AKI, the increased risk to patients with preexisting CKD, ESKD or urgent-start KRT and differences in kidney outcomes for all three recent coronavirus pandemics.
Our results indicate that AKI occurs in around one-tenth of the infected study population with an overall mortality rate of 80.9%. The incidence of AKI was highest in MERS patients, while being similar between COVID-19 and SARS patients. The incidence in ICU-treated patients varied between 8.3% and 28.85% [Citation81]. Compared to COVID-19 patients, the mortality rate was higher in SARS and MERS patients although fewer studies were reported for the SARS [Citation56,Citation57,Citation60] and MERS [Citation3,Citation72] subgroups. AKI was associated with a significantly higher mortality in COVID-19 and MERS. In the SARS subgroup, this did not reach statistical significance possibly due to the small number of studies included. The incidence of urgent-start KRT use in coronavirus infected patients with AKI was 8.9% with an associated mortality of 80.7%. This probably reflects the fact that AKI patients requiring urgent-start KRT are generally more critically ill, likely to need ventilatory support or extracorporeal membrane oxygenation (ECMO) [Citation87]. Many dialysis modalities [Citation88–90], including CRRT, high-volume hemofiltration, plasma exchange, plasma adsorption and acute peritoneal dialysis have been reported, mostly as case reports or small case series. A consensus recommendation regarding the optimal dialysis modality, timing, dosage and duration for management of AKI in coronavirus diseases is urgently needed.
Our analysis also showed that the presence of CKD or ESKD was significantly associated with increased mortality. The overall prevalence of ESKD was higher than that of preexisting CKD, possibly due to different number of studies being enrolled for each analysis. Patients with MERS had the highest mortality in prevalent patients with CKD or ESKD. Several studies have reported on virus prevalence and mortality in patients on prevalent hemodialysis [Citation30,Citation38,Citation42,Citation44,Citation46–51,Citation53,Citation54,Citation61,Citation74,Citation75]. The incidence of COVID-19 was 8.0% in routine hemodialysis patients, which was higher than SARS or MERS, but similar to the general population. The mortality rate for this subgroup was 25.7%, which was nearly the same as SARS but much less than with MERS (75%). Our analysis confirms that prevalent patients with CKD or on urgent-start KRT are at much higher risk of infection and of subsequent worse outcomes. Epidemic prevention measures must be strengthened especially in dialysis centers [Citation91]. Specific measures that could be introduced include the setting up of isolation areas for dialysis centers, wearing personal protective equipment, tracking and isolating contacts and environmental disinfection. For infected patients, continuous bedside dialysis has been successfully deployed [Citation92].
Our study had several limitations. First, we combined studies with a certain degree of heterogeneity, owing to the differences in the study design, sample size and population characteristics of the studies included. The specific reasons were as follows: (1) inclusion of case control study and case series can introduce bias to the result, which may lead to the high heterogeneity; (2) the number of infected patients enrolled in the included articles varied widely; (3) the inconsistent definitions of AKI or CKD or ESKD could have accounted for the variation of our results on AKI; (4) differences in the timing of outbreaks, geographical locations, ages, genders, habits, cares, and treatments may also contribute to the high heterogeneity; (5) the total sample size of SARS and MERS related studies was much smaller than COVID-19. Second, sampling bias may have contributed to part of our analysis when less than five cases were excluded. Third, renal function follow-up to assess renal recovery was not available. Fourth, several case series (about 8.62%) were included in our study, which could reduce the strength of the generated evidences. Then we tried to do the sensitivity analysis excluding all the case series, but found too few studies left. Also shown with the GRADE tool, the level of evidences generated in this study were low or very low; thus, more future high-quality researches are urged to confirm our results.
In conclusion, the kidney is commonly affectedly in patients with COVID-19, SARS and MERS. Renal events including AKI, preexisting CKD, and ESKD significantly increased the risk of mortality. Prevalent patients on urgent-start KRT also have an increased risk of infection and mortality. Routine hemodialysis patients were also at high risk of infection and mortality.
Author contributions
AO and ZM: act as guarantor for the validity of the study report. ZM and JX: study concept and design. JX and CX: acquisition of data. SZ and CX: extraction of data. JX and CX: checking of data. CX and BY: analysis and interpretation of data. XJ, CX, and SZ: drafting of the manuscript. AO and ZM: critical revision of the manuscript for important intellectual content.
Supplemental Material Table 1
Download MS Word (31.8 KB)Supplemental Material Table 2
Download MS Word (18.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization Coronavirus disease (COVID-19) pandemic. Available at: https://covid19.who.int/
- Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/types.html
- Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389–397.
- Chan JF, Lau SK, To KK, et al. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28(2):465–522.
- Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–424.
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061.
- Meena P, Bhargava V, Rana DS, et al. COVID-19 and the kidney: a matter of concern. Curr Med Res Pract. 2020;10(4):165–168.
- Rabb H. Kidney diseases in the time of COVID-19: major challenges to patient care. J Clin Invest. 2020;130(6):2749–2751.
- Zhang X, Li S, Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad Med J. 2020;96(1137):403–407.
- Michaud V, Deodhar M, Arwood M, et al. ACE2 as a therapeutic target for COVID-19; its role in infectious processes and regulation by modulators of the RAAS system. J Clin Med. 2020;9(7):2096.
- Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254.
- Nimkar A, Naaraayan A, Hasan A, et al. Incidence and risk factors for acute kidney injury and its effect on mortality in patients hospitalized from Covid-19. Mayo Clin Proc Innov Qual Outcomes. 2020.DOI:10.1016/j.mayocpiqo.2020.07.003. [online ahead of print].
- Panitchote A, Mehkri O, Hastings A, et al. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):74.
- Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013; 9(5):255–265.
- Cohen G. Immune dysfunction in Uremia 2020. Toxins (Basel). 2020;12(7):439.
- Syed-Ahmed M, Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26(1):8–15.
- Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63.
- Guyatt GH, Oxman AD, Vist GE, et al.; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926.
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice. Z Evid Fortbild Qual Gesundhwes. 2009;103(6):391–400.
- Murad MH, Mustafa RA, Schunemann HJ, et al. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med. 2017;22(3):85–87.
- Atkins D, Eccles M, Flottorp S, et al.; GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004;4(1):38.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
- IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802.
- Luo X, Xia H, Yang W, et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. 2020.DOI:10.1101/2020.03.19.20033175
- Chen M, Fan Y, Wu X, et al. Clinical characteristics and risk factors for fatal outcome in patients with 2019-coronavirus infected disease (COVID-19) in Wuhan, China (2/27/2020). Lancet 2020.
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091.
- Xiong F, Tang H, Liu L, et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31(7):1387–1397.
- Guan WJ, Ni ZY, Hu Y, China Medical Treatment Expert Group for Covid-19, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720.
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062.
- Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):748–755.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606.
- Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–348.
- Ma Y, Diao B, Lv X, et al. COVID-19 in hemodialysis (HD) patients: report from one HD center in Wuhan, China. medRxiv. 2020. DOI:10.1101/2020.02.24.20027201
- Lu H, Ai J, Shen Y, et al. A descriptive study of the impact of diseases control and prevention on the epidemics dynamics and clinical features of SARS-CoV-2 outbreak in Shanghai, lessons learned for metropolis epidemics prevention. medRxiv. 2020. DOI:10.1101/2020.02.19.20025031
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513.
- Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020. DOI:10.1101/2020.03.04.20031120
- Arslan H, Musabak U, Ayvazoglu Soy EH, et al. Incidence and immunologic analysis of coronavirus disease (COVID-19) in hemodialysis patients: a single-center experience. Exp Clin Transplant. 2020; 18(3):275–283.
- Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020; 97(5):829–838.
- Jung HY, Lim JH, Kang SH, et al. Outcomes of COVID-19 among patients on in-center hemodialysis: an experience from the epicenter in South Korea. J Clin Med. 2020; 9(6):1688.
- Pei G, Zhang Z, Peng J, et al. Renal Involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–1165.
- Goicoechea M, Sanchez Camara LA, Macias N, et al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020; 98(1):27–34.
- Dudreuilh C, Kumar N, Moxham V, et al. De-isolation of COVID-19-positive hemodialysis patients in the outpatient setting: a single-center experience. Kidney Int. 2020;98(1):236–237.
- Manganaro M, Baldovino S, Working group of the P, Aosta Valley Section of the SIN. First considerations on the SARS-CoV-2 epidemic in the Dialysis Units of Piedmont and Aosta Valley, Northern Italy. J Nephrol. 2020;33(3):393–395.
- Albalate M, Arribas P, Torres E, et al. High prevalence of asymptomatic COVID-19 in haemodialysis: learning day by day in the first month of the COVID-19 pandemic. Nefrologia. 2020;40(3):279–286.
- Alberici F, Delbarba E, Manenti C, et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020; 98(1):20–26.
- Trujillo H, Caravaca-Fontan F, Sevillano A, et al. SARS-CoV-2 infection in hospitalized patients with kidney disease. Kidney Int Rep. 2020;5(6):905–909.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612.
- Fisher M, Yunes M, Mokrzycki MH, et al. Chronic hemodialysis patients hospitalized with COVID-19 – short-term outcomes in Bronx, New York. Kidney360. 2020;1(8):755–762.
- Valeri AM, Robbins-Juarez SY, Stevens JS, Ahn W, et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020; 31(7):1409–1415.
- Richardson S, Hirsch JS, Narasimhan M, et al.; and the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052.
- Chu KH, Tsang WK, Tang CS, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698–705.
- Wong PN, Mak SK, Lo KY, et al. Clinical presentation and outcome of severe acute respiratory syndrome in dialysis patients. Am J Kidney Dis. 2003;42(5):1075–1081.
- Peiris JS, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772.
- Wu VC, Huang JW, Hsueh PR, et al.; Medicine SRGoNTUCo. National Taiwan University H: Renal hypouricemia is an ominous sign in patients with severe acute respiratory syndrome. Am J Kidney Dis. 2005;45(1):88–95.
- Chen LL, Hsu CW, Tian YC, et al. Rhabdomyolysis associated with acute renal failure in patients with severe acute respiratory syndrome. Int J Clin Pract. 2005;59(10):1162–1166.
- Kwan BC, Leung CB, Szeto CC, et al. Severe acute respiratory syndrome in dialysis patients. J Am Soc Nephrol. 2004;15(7):1883–1888.
- Farcas GA, Poutanen SM, Mazzulli T, et al. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J Infect Dis. 2005;191(2):193–197.
- Khalid I, Alraddadi BM, Dairi Y, Khalid TJ, et al. Acute management and long-term survival among subjects with severe Middle East respiratory syndrome coronavirus pneumonia and ARDS. Respir Care. 2016;61(3):340–348.
- Alfaraj SH, Al-Tawfiq JA, Assiri AY, et al. Clinical predictors of mortality of Middle East respiratory syndrome coronavirus (MERS-CoV) infection: a cohort study. Travel Med Infect Dis. 2019;29:48–50.
- Arabi YM, Al-Omari A, Mandourah Y, et al. Critically Ill patients with the Middle East respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med. 2017;45(10):1683–1695.
- Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761.
- Al-Jasser FS, Nouh RM, Youssef RM. Epidemiology and predictors of survival of MERS-CoV infections in Riyadh region, 2014-2015. J Infect Public Health. 2019;12(2):171–177.
- Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015; 70(7):2129–2132.
- Sherbini N, Iskandrani A, Kharaba A, et al. Middle East respiratory syndrome coronavirus in Al-Madinah City, Saudi Arabia: demographic, clinical and survival data. J Epidemiol Glob Health. 2017;7(1):29–36.
- Alfaraj SH, Al-Tawfiq JA, Altuwaijri TA, et al. Middle East respiratory syndrome coronavirus in pediatrics: a report of seven cases from Saudi Arabia. Front. Med. 2019;13(1):126–130.
- Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014;59(2):160–165.
- Khalid M, Khan B, Al Rabiah F, et al. Middle Eastern respiratory syndrome corona virus (MERS CoV): case reports from a tertiary care hospital in Saudi Arabia. Ann Saudi Med. 2014;34(5):396–400.
- Garout MA, Jokhdar HAA, Aljahdali IA, et al. Mortality rate of ICU patients with the Middle east respiratory syndrome – coronavirus infection at King Fahad Hospital, Jeddah, Saudi Arabia. Cent Eur J Public Health. 2018;26(2):87–91.
- Assiri A, Abedi GR, Bin Saeed AA, et al. Multifacility outbreak of Middle East Respiratory syndrome in Taif, Saudi Arabia. Emerg Infect Dis. 2016;22(1):32–40.
- Hastings DL, Tokars JI, Abdel Aziz IZ, et al. Outbreak of Middle East respiratory syndrome at tertiary care hospital, Jeddah, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22(5):794–801.
- Alqahtani FY, Aleanizy FS, Ali El Hadi Mohamed R, et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect. 2018;147:1–5.
- Omrani AS, Saad MM, Baig K, et al. Albarrak AM: Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14(11):1090–1095.
- Alanazi KH, Killerby ME, Biggs HM, et al. Scope and extent of healthcare-associated Middle East respiratory syndrome coronavirus transmission during two contemporaneous outbreaks in Riyadh, Saudi Arabia, 2017. Infect Control Hosp Epidemiol. 2019;40(1):79–88.
- Al Ghamdi M, Alghamdi KM, Ghandoora Y, et al. Treatment outcomes for patients with Middle Eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect Dis. 2016;16:174.
- Cha RH, Joh JS, Jeong I, Critical Care Team of National Medical Center, et al. Critical care team of national medical C: renal complications and their prognosis in Korean patients with Middle East respiratory syndrome-coronavirus from the central MERS-CoV designated hospital. J Korean Med Sci. 2015;30(12):1807–1814.
- Ng JJ, Luo Y, Phua K, et al. Acute kidney injury in hospitalized patients with coronavirus disease 2019 (COVID-19): a meta-analysis. J Infect. 2020;81(4):647–679.
- Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020; 581(7807):221–224.
- Hui KPY, Cheung MC, Perera R, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med. 2020;8(7):687–695.
- Behzad S, Aghaghazvini L, Radmard AR, et al. Extrapulmonary manifestations of COVID-19: Radiologic and clinical overview. Clin Imaging. 2020;66:35–41.
- Chen YT, Shao SC, Lai EC, et al. Mortality rate of acute kidney injury in SARS, MERS, and COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24(1):439.
- Yang X, Jin Y, Li R, et al. Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24(1):356.
- Liu S, Ding X, Cao J, et al. [Nationwide survey on clinical treatment of coronavirus disease 2019 in 9 provinces and municipalities]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32(4):397–400.
- El Shamy O, Patel N, Abdelbaset MH, Chenet L, et al. Acute start peritoneal dialysis during the COVID-19 pandemic: outcomes and experiences. J Am Soc Nephrol. 2020;31(8):1680–1682.
- Swol J, Lorusso R. Additive treatment considerations in COVID-19-The clinician's perspective on extracorporeal adjunctive purification techniques. Artif Organs. 2020;44(9):918–925.
- Chua HR, MacLaren G, Choong LH, et al. Ensuring sustainability of continuous kidney replacement therapy in the face of extraordinary demand: lessons from the COVID-19 pandemic. Am J Kidney Dis. 2020;76(3):392–400.
- Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994.
- Fu D, Yang B, Xu J, et al. COVID-19 infection in a patient with end-stage kidney disease. Nephron. 2020;144(5):245–247.
