Abstract
Background
Patients with chronic kidney disease, especially those receiving hemodialysis (HD), are at risk of hyperkalemia (HK). This systematic review aimed to evaluate the prevalence of HK in patients with renal disease receiving HD and collate evidence on the effect of HK and differing HD patterns (i.e., long vs. short inter-dialytic intervals [LIDI and SIDI, respectively] in a thrice weekly schedule) on mortality.
Methods
Comprehensive searches were conducted across six databases and selected conference proceedings by two independent reviewers up to September 2020. A hundred and two studies reporting frequency of HK, mortality, or cardiovascular (CV) outcomes in adult patients with acute, chronic or end-stage renal disease in receipt of HD were included. Narrative synthesis of results was undertaken with key findings presented in tables and figures.
Results
Median prevalence of HK in patients with renal disease receiving HD was 21.6% and increased in patients receiving concomitant medications – mainly renin–angiotensin–aldosterone system inhibitors and potassium-sparing diuretics. Associations between elevated potassium levels and increased risk of both all-cause and CV mortality in the HD population were consistent across the included studies. In addition, there was a rise in all-cause and CV mortality on the day following LIDI compared with the day after the two SIDIs in patients on HD.
Conclusions
Evidence identified in this systematic review indicates a relationship between HK and LIDI with mortality in patients with renal disease receiving HD, emphasizing the need for effective monitoring and management to control potassium levels both in emergency and chronic HD settings.
Introduction
Hyperkalemia (HK) is a clinically important electrolyte abnormality characterized by an elevated serum potassium (S-K+) concentration above the normal range of 3.5–5.0 mmol/L [Citation1]. While there is no universally agreed definition for HK, the most widely used thresholds for the categorization of mild, moderate and severe HK are 5.5–5.9, 6.0–6.4 and ≥6.5 mmol/L, respectively [Citation2,Citation3]. Mild HK may be associated with symptoms such as nausea, fatigue or muscle weakness [Citation2]; however, more severe HK can cause alterations in cardiac physiology resulting in chest pain, cardiac dysrhythmia, shortness of breath, and – in very severe cases – cardiac arrest and death [Citation4,Citation5].
Renal excretion is the main route of K+ elimination; therefore, renally impaired patients are at increased risk of HK [Citation6]. Indeed, HK is reported in approximately 50% of patients with chronic kidney disease (CKD) [Citation7,Citation8], with prevalence increasing alongside disease severity. Patients with CKD that progress to end-stage renal disease (ESRD) or patients with acute renal failure are initiated and often maintained on hemodialysis (HD). HK is common in the dialysis setting [Citation9]; however, its true prevalence in patients receiving dialysis is unknown.
HD is normally prescribed thrice weekly with schedules of Monday-Wednesday-Friday (MWF) or Tuesday-Thursday-Saturday (TTS). The two shorter (48 h) breaks between HD sessions are referred to as the short inter-dialytic interval (SIDI) and the extended (72 h) break is known as the long inter-dialytic interval (LIDI). Given the impaired ability of patients receiving HD to excrete K+ and other electrolytes and their high prevalence of cardiovascular (CV) disease, patients are at increased risk of hospitalizations, cardiac arrhythmias and mortality following LIDI [Citation10,Citation11]. However, the interplay between S-K+, LIDI and clinical outcomes remains uncertain.
The aim of this study was to undertake a systematic literature review (SLR) to evaluate the effect of HK and LIDI on mortality in patients with renal disease or acute renal failure receiving HD. The findings will have the potential to provide further information on the frequency and management of HK to support healthcare professionals working in the dialysis setting.
Methods
This systematic review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation12] with the protocol developed a priori.
Search strategy
Comprehensive searches of electronic databases (MEDLINE, MEDLINE In-Process, Embase, Cochrane Library, University of York Center for Reviews and Dissemination) were conducted from database inception to 1st September 2020. A sample search strategy is provided in Supplementary Table S1. In addition, conference proceedings (American Society of Nephrology, European Renal Association-European Dialysis and Transplant Association, and International Society of Nephrology) were reviewed for the past four years for any studies that may not have been captured from database searches, and bibliographies of relevant studies and systematic reviews were visually scanned to identify relevant primary studies.
Study selection
EndNote X9 was used to facilitate the removal of duplicate records, study selection and referencing of literature search results. Two reviewers independently screened articles for eligibility using pre-determined inclusion/exclusion criteria according to a population- intervention-comparator-outcome-study design (PICOS) framework (). Any discrepancies between reviewers were resolved by consensus or involvement of a third reviewer.
Table 1. Eligibility criteria for the identification of studies describing HK and LIDI in HD patients.
Data extraction and quality assessment
Relevant information on study characteristics, cohort details and study outcomes were extracted from all included studies by a single reviewer and quality-checked by a second reviewer for accuracy and completeness. All data were extracted in a consistent manner using a standardized data extraction form.
Quality assessment of observational studies was performed using a checklist modified from the Downs and Black instrument [Citation13]. The Cochrane risk of bias tool was used to assess methodological quality and reporting in randomized controlled trials (RCTs) [Citation14].
Data synthesis
Narrative synthesis of included studies was undertaken. Between study heterogeneity and limited reference to the timepoint, where mortality was reported, precluded meta-analysis. Simple analysis was performed in the statistical package R [Citation15] with summary statistics presented as mean (± standard deviation) for demographic data, median (interquartile range) for prevalence and median (range) for mortality data.
Studies reporting a specific threshold for defining HK were included in the review and the HK definition was recorded as such. When studies did not provide a HK definition then they were included in the review if they reported the proportion of patients with pre-dialysis S-K+ concentrations ≥5.0 mmol/L. Prevalence was defined as the proportion of patients with HK in the total HD population included in a study in a given period.
A standardized definition for the different inter-dialytic intervals was used; for studies where results were presented based on days of the week (i.e., MWF or TTS HD schedule), data were aligned to SIDI and LIDI periods to enable comparison. Thus, Mondays (MWF schedule) and Tuesdays (TTS schedule) were categorized as ‘day after LIDI’, Wednesdays (MWF schedule) and Thursdays (TTS schedule) as ‘day after SIDI1’, and Fridays (MWF schedule) and Saturdays (TTS schedule) as ‘day after SIDI2’. An ‘end of LIDI’ group was used to refer to Sundays (MWF schedule) or Mondays (TTS schedule).
Results
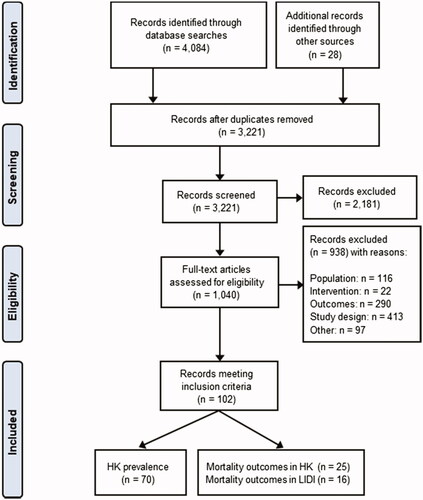
Systematic searches yielded 3221 records after the removal of duplicates, with 102 studies included in the final synthesis. The study selection process is illustrated in a PRISMA flow diagram [Citation12] ().
Figure 1. PRISMA flow diagram illustrating the study selection process. HK: hyperkalemia; LIDI: long inter-dialytic interval.
HK in patients with CKD or acute renal failure receiving HD
Seventy studies reported on the frequency of HK [Citation16–85], 25 studies reported on the associated mortality [Citation21,Citation22,Citation34,Citation41,Citation44,Citation45,Citation48,Citation68,Citation70,Citation86–101] in HD patients with CKD or acute renal failure, and nine studies [Citation21,Citation22,Citation34,Citation41,Citation44,Citation45,Citation48,Citation68,Citation70] reported on both frequency of HK and associated mortality.
Prevalence of HK in patients receiving HD
Of the 70 studies reporting on the frequency of HK (Supplementary Table S2), 45 were observational studies, 15 were RCTs, four were nonrandomized trials and six did not explicitly report a study design. The population size varied significantly between studies ranging from 5–74,219 patients. The mean age of patients was 58.3 ± 6.4 years and 59% were male. Of the identified studies, 40 examined patients with ESRD, 21 included CKD patients (not explicitly categorized as ESRD patients) in receipt of HD, four included patients with acute kidney injury (AKI) and five focused on emergency HD for the treatment of renal failure emergencies ().
Table 2. Summary of patient characteristics and outcomes in studies reporting frequency of HK in HD patients stratified by population.
Forty-eight studies did not consider any medication when reporting outcomes of interest, whereas 22 studies stratified results by the co-administered drug, including blood pressure medication (renin-angiotensin-aldosterone system inhibitors [RAASi]), K+-sparing diuretics (spironolactone [SPL], eplerenone), K+-binding agents (sodium polystyrene sulfonate [SPS], patiromer [PAT], sodium zirconium cyclosilicate [SZC]) and drugs for the treatment of hyperparathyroidism and anemia among others. Diabetes (n = 32) and hypertension (n = 18) were the most frequently reported comorbidities in the included HD populations (42% with diabetes and 76.2% with hypertension on average), followed by heart failure (31%) and coronary artery disease (35.7%).
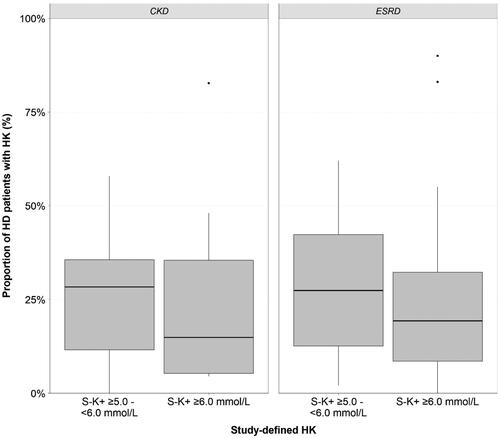
The definition of HK varied between studies. Where reported (n = 46), the majority of studies defined HK as pre-dialysis S-K+ concentration of ≥5.5 mmol/L (54%) and to a lesser extent ≥5.0 mmol/L (15%) and ≥6.0 mmol/L (22%); the rest of the studies reported moderate to severe HK without providing a HK threshold. Overall, 67 studies [Citation16–21,Citation23–45,Citation47–69,Citation71–85] reported the prevalence of HK as the proportion of patients with HK in the HD population studied and four reported HK incidence rates [Citation22,Citation46,Citation70,Citation82]. Median HK prevalence in HD patients with renal disease was 21.6% (7.3–39.3%) and was comparable between ESRD and CKD patients (). The prevalence of study-defined HK, when using different S-K+ thresholds, is presented in . Median prevalence of milder HK in patients with S-K+ 5.0–6.0 mmol/L (excluding all cases of serious HK, i.e., ≥6.0 mmol/L, where possible) was 27.4% (12.1–36.9%) and median prevalence of more serious HK (S-K+ ≥6.0 mmol/L) was 19.3% (7.3–35.4%). In patients with AKI, HK was the most common indication for initiation of HD in 27.3% of the cases, and HK was observed in 24% of patients requiring emergency HD ().
Figure 2. Median prevalence of HK (%) across the 70 included studies stratified by study-defined S-K+ thresholds. CKD: chronic kidney disease; ESRD: end-stage renal disease; HD: hemodialysis; HK: hyperkalemia; S-K+: serum potassium.
Thirteen studies [Citation19–21,Citation23,Citation35,Citation36,Citation42,Citation43,Citation54,Citation57,Citation64,Citation66,Citation67,Citation76] investigated the impact of drugs, mainly RAASi and K+-sparing diuretics, on the occurrence of HK. An increase in the proportion of HD patients presented with HK was observed in patients receiving medication on top of HD (24.4%; 7.3–46.1%) compared with those that received no medication or placebo (12.0%; 2.1–18.6%).
Mortality in HD patients with HK
Mortality outcomes were reported in 25 studies [Citation21,Citation22,Citation34,Citation41,Citation44,Citation45,Citation48,Citation68,Citation70,Citation86–88,Citation90–102] (Supplementary Table S3). Studies were mostly observational and therefore limitations such as confounding by indication should be considered when interpreting these results; only one study [Citation22] was an RCT. The size of the enrolled population varied significantly between studies (range: 8–51,297 patients), the mean age of patients was 60.1 ± 4.1 years and 54% were male. Sixteen studies enrolled patients with ESRD, eight with CKD (not explicitly categorized as ESRD patients) in receipt of HD and one study included patients receiving emergency HD due to renal failure.
Studies varied in the outcome measure used to report mortality. Twelve studies [Citation22,Citation87,Citation88,Citation91–99] reported the proportion of patients that died due to HK in the overall HD population ranging from 0–12.5% (follow-up range: 3–66 months, where reported) ().
Table 3. Studies reporting mortality due to HK in patients receiving HD (n = 12).
Eight studies [Citation41,Citation45,Citation48,Citation68,Citation86,Citation100–102] reported percentage all-cause mortality in different groups of patients. Alagusundaramoorthy et al. [Citation86] undertook a chart review of 346 adult ESRD patients admitted to hospital, who had HD for severe HK, and reported that in-hospital mortality for these patients was 6.9%. Chatoth et al. [Citation102] examined outcomes of patients with moderate to severe HK undergoing HD in a large ESRD provider network in the USA. For S-K+ >6.0 mmol/L, all-cause mortality over a 6-month period was 0.0%, 1.8% and 2.5% for patients receiving K+-binding agents PAT and SPS, and no K+-binder, respectively, compared to 2.8%, 2.5%, and 2.3% for S-K+ >5.5 mmol/L. Hwang et al. [Citation41] presented data from a retrospective single center study of ESRD patients receiving HD. Patients were divided into three groups according to the last mid-week pre-dialysis S-K+ concentrations: hypokalemia (<3.5 mmol/L), normokalemia (3.5–5.5 mmol/L), and HK (>5.5 mmol/L). The maximum duration of the follow-up period was 4.5 years. All-cause mortality was lowest for patients with normokalemia at 18.7%, compared to 21.6% for patients with HK. Kim et al. [Citation45] examined the differences in the relationship between S-K+ and all-cause mortality across non-Hispanic whites, African-Americans and Hispanics in a contemporary cohort of over 100,000 incident HD patients, who had a median follow-up of 1.3 years. The data demonstrated that while mortality was increased for whites and African–Americans with higher S-K+, this was not observed in Hispanic HD patients; all-cause mortality was 35.4%, 27.5% and 15% for white, African-American and Hispanic HD patients respectively with S-K+ >5.5 mmol/L, compared with 32.6%, 20.6% and 16.2% for patients with S-K+ 5.0–5.5 mmol/L. Kovesdy et al. [Citation48] reported results from a multicenter cohort of 81,013 CKD patients on maintenance HD (mean follow-up of 36 months). The study findings suggested that S-K+ between 4.0 and 5.3 mmol/L was associated with the lowest death rate (28–30%), whereas S-K+ >5.6 mmol/L was associated with increased all-cause mortality (≥32%). Yalin et al. [Citation68] reported data from 177 adult patients who underwent emergency HD between 2000 and 2010 in which all-cause mortality rate was 41.8%. Zulham et al. [Citation101] observed that early death in diabetic ESRD patients on HD was 49% in patients with HK compared with 25.2% in these without HK. Finally, Trajceska et al. [Citation100] reported that 19.5% of ESRD patients with HK died during the 36 months follow-up period.
Hazard ratios (HRs) for mortality were reported in five studies [Citation21,Citation34,Citation44,Citation70,Citation90]. For most studies, HR for all-cause mortality was increased with higher S-K+ levels, although the differences were not significant (Supplementary Table S3).
Four studies [Citation34,Citation41,Citation45,Citation48] reported CV mortality. CV mortality was generally increased in the HK population (>5.5 mmol/L) compared to patients with normokalemia (3.5–5.5 mmol/L); 9.8% compared to 5.5% in Hwang et al. [Citation41], 13.0–14.0% compared to 10.0–11.0% in Kovesdy et al. [Citation48], and 6.1–13.0% compared to 7.0–11.3% in Kim et al. [Citation45]. Genovesi et al. [Citation34] reported 3-year cumulative incidence of deaths in a cohort of 476 patients; for patients without HK (S-K+ <6.0 mmol/L) the 3-year cumulative incidence of deaths was 12.6% compared to 18.2% for patients with HK (≥6.0 mmol/L). An increase in cardiac arrest, coronary artery disease (CAD) and cardiac arrhythmia was also observed in patients with HK compared with normal S-K+ populations, with risk increasing with HK severity [Citation41,Citation44].
Mortality associated with the inter-dialytic period
Sixteen studies [Citation10,Citation34,Citation103–117] were identified reporting mortality outcomes at different days of the week based on the HD schedule (Supplementary Table S4). Using a standardized definition for the different inter-dialytic intervals, the following groups were recorded: day after LIDI; day after SIDI1; day after SIDI2; end of LIDI; during LIDI; during SIDI.
The majority of studies were observational, with the cohort size ranging from 28–61,152 patients. The mean age of included patients, where reported, was 61.9 ± 4.8 years and 59% were male. Nine studies recruited patients with ESRD and seven with CKD (not explicitly categorized as ESRD patients) receiving HD. All included studies, apart from one [Citation109], reported a thrice weekly HD schedule.
Five studies [Citation10,Citation105,Citation106,Citation111,Citation116] reported all-cause mortality. Three of the studies [Citation10,Citation105,Citation106] reported all-cause mortality rates with all observing an increased mortality on the day after LIDI compared with the day after the short intervals (22.1 vs. 18.9, 8.4 vs. 5.2, and 17 vs. 14 deaths per 100 person-years, respectively); follow-up ranging from 18.5–60.0 months. Two of the studies [Citation111,Citation116] reported mortality as proportion of deaths in the overall study population stratified by MWF or TTS HD schedule. The data from these studies also demonstrated a higher all-cause mortality on the day after LIDI (median [range]: 19.7% [17.9–23.7%] for MWF, 19.2% [16.9–22.3%] for TTS) compared with the day after SIDI (median [range]: 14.8% [13.3–19.1%] for MWF, 15.7% [11.9–17.9%] for TTS) and end of LIDI (median [range]: 12.4% [12.1–15.7%] for MWF, 14.4% [10.7–15.7%] for TTS).
Four studies [Citation34,Citation103,Citation104,Citation115] reported the proportion of patient deaths recorded as sudden death at the end or after the LIDI period. Two studies defined sudden death as cardiac death. Bleyer et al. (1999) reported that in a cohort of 6137 ESRD patients receiving HD 18.1–20.8% of all deaths were sudden deaths occurring on the day after LIDI compared with 12.7–16.7% on the day after SIDI or at the end of LIDI [Citation104], whereas Wong et al. reported that in a total of ten deaths in a cohort of 50 CKD patients followed-up for 18 months, eight were sudden deaths, all of which occurred during LIDI [Citation115]. Two studies defined sudden death as unexpected death without known etiology occurring within one hour of the onset of symptoms. Genovesi et al. reported that in a cohort of 476 CKD patients 40.6% of all deaths were sudden deaths occurring during LIDI compared with 25–34.4% on the day after the two SIDI periods [Citation34], whilst Bleyer et al. (2006) reported that from the 228 patient deaths reviewed 20.6% were sudden deaths occurring at the end of LIDI compared to 11.3% on the day after LIDI [Citation103].
Five studies [Citation10,Citation104,Citation108,Citation109,Citation116] reported CV mortality using different outcome measures. All showed increased CV mortality on the day after LIDI compared with the day after SIDI. In one study [Citation108] of 10,338 ESRD patients the odds ratio (OR) associated with CV mortality on the day after LIDI was 1.27 (MWF schedule) and 1.10 (TWS schedule), compared with a range of 0.84–1.02 for other days during the HD schedule. In another study [Citation116] conducted in the US population showed that the relative risk associated with CV mortality on the day after LIDI was 1.45 (MWF schedule) and 1.56 (TTS schedule), compared with the day after SIDI (range: 1.03–1.09) or end of LIDI (range: 0.94–1.16). Data for other countries included in this study followed a similar pattern, demonstrating that cardiac deaths were more likely to occur on the day after LIDI than other periods during the HD schedule. Three studies reported on the percentage of CV deaths in the overall study population; in a study of 6137 ESRD patients on HD CV mortality was 18.5–20.2% on the day after LIDI compared with 13.5–14.9% on the day after SIDI and 13.3–15.2% at the end of LIDI [Citation104], in a study of 10,338 ESRD patients on HD CV mortality was 8.8–9.7% on the day after LIDI compared to 7.6–8.0% on the day after SIDI and 6.7% at the end of LIDI [Citation108], and in a study of 240 ESRD patients on HD CV mortality was 12.9% on the day after LIDI compared to 6.3% on the day after SIDI [Citation109]. One study reported CV mortality rates per 100 person-years and reported significantly higher mortality rates on the day after LIDI than on other days of the week (10.2 vs. 7.7–8.1 per 100 person-years, respectively) [Citation10].
The most commonly encountered CV events in the LIDI period was cardiac arrhythmia [Citation112–115] and cardiac arrest [Citation10,Citation107]. MI [Citation10], atrial fibrillation [Citation112], ventricular tachycardia [Citation113] and cardiopulmonary resuscitation (CPR) [Citation110] were also reported.
Association between LIDI and HK
Three studies reported an association between LIDI and prevalence of HK (). Chevarria et al. [Citation118] monitored 20 ESRD dialysis patients for 12 months and reported an association between LIDI and S-K+ >6.0 mmol/L (OR: 2.8 [95% CI, 1.47–5.33]). Similarly, Yusuf et al. [Citation70] observed in four annual cohorts of HD patients ranging from 28,774 to 36,888 patients that prevalence of HK on the day after LIDI was 2.0 − 2.4 times higher than on the day after SIDI. Finally, Singh et al. [Citation63] observed in 240 ESRD patients on HD that were monitored for 12 days that HK was more common during LIDI with 36 − 50% of patients having pre-dialysis HK after LIDI.
Table 4. Studies reporting an association between HK and the inter-dialytic period.
Quality assessment
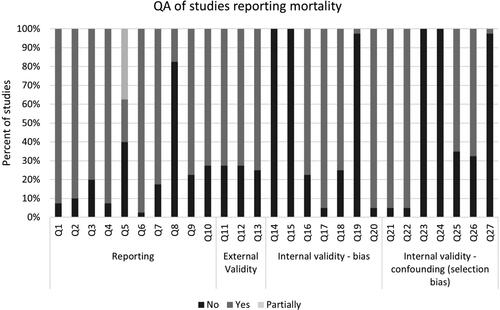
Quality assessment was performed for the 41 studies reporting mortality outcomes as this was the main objective of the review (). Overall, methodological quality and reporting were adequate in most studies, except reporting of all adverse events; however, this is of lesser importance as we specifically regarded mortality in this analysis. External validity was generally adequate. Regarding internal validity, due to the observational study design of the included studies, blinding and randomization were absent. Patient compliance with the intervention was not well assessed. The other tool items were met in 60% to 97.5% of the studies. The only included RCT was reported as a conference proceeding, and the quality assessment was not informative.
Discussion
In total, 102 studies met the eligibility criteria for inclusion in this systematic review. A median HK prevalence of 21.6% was observed in patients with renal disease receiving HD, based on study-defined criteria for HK, with more serious cases of HK occurring in an estimated 19.3% of the HD population. This is significantly higher than the prevalence in the general population which has been estimated to be 2–3% [Citation119,Citation120]. The incidence rate of HK in ESRD patients ranged from 15.1 to 26.0 events per 100 patient-months.
It is well established that HK is a major burden in terms of increased risk of CV outcomes (due to alterations in cardiac electrophysiology) and mortality. Death due to HK was observed in approximately 4% (range: 0–12.5%) of the overall HD population. Data identified in this review also indicated that both all-cause and CV mortality were increased in patients with HK receiving HD compared to those with normal S-K+ levels. These findings align with a recently conducted systematic review that also reported an increased risk of both all-cause and CV mortality with S-K+ levels outside the normokalemia range [Citation121]. Thus, the evidence collated in this SLR supports the existence of an association between elevated S-K+ levels and mortality across patient populations with renal disease receiving HD. However, the heterogeneity observed in the included studies relating to patient characteristics, the intervals of S-K+ used to define HK, and the outcome measures and timepoints used to report mortality may impact on the generalizability of the review findings. It should be noted that the prevalence of comorbidities in CKD patients such as diabetes, hypertension and CV disorders alongside HK may potentiate any CV risk and death rates in the HD population. In addition, there are indications that HK may not only have effects on cardiac excitability but may also contribute to peripheral neuropathy and cause renal tubular acidosis, thus increasing the risk of death [Citation122].
Current evidence indicates that the risk of mortality in patients receiving HD peaks the day after LIDI potentially due to the greater accumulation of uremic toxins, acids and electrolytes, especially K+, in this time interval [Citation123–125]. Based on this SLR, an increased risk of all-cause mortality was observed on the day after LIDI when compared with the day after the two short intervals. Similarly, a rise in CV mortality was evident on the day after LIDI when compared with the day after SIDI or any other day of the week. Some – but not all – studies also reported an elevated mortality on the last day of LIDI (usually 60–72 h after the last HD session). The findings from this SLR indicate that LIDI is a time of increased risk among many patients receiving HD and highlights the need for further research into dialysis patterns (twice vs thrice weekly), to determine the frequency of HD with the lowest risk of adverse outcomes.
This review was limited by the high heterogeneity of studies identified which may affect the generalizability of the findings. Included studies were heterogeneous regarding the study population and cohort size (5–74,219 patients) by selecting specific patient populations alongside CKD (including HCV or HIV-infected, the presence of pruritus, obese, with hyperparathyroidism or leprosy) and by estimating the frequency of HK associated with medications administered to treat comorbidities or interventions to control K+ levels. In addition, the S-K+ threshold used to define HK varied significantly between studies (ranging from 5.0–6.0 mmol/L with many studies not providing a definition) making difficult to stratify outcomes based on HK severity. Furthermore, some of the studies included in the analysis reported HK as an adverse event of interventions assessed in clinical trials (this may underestimate its frequency and it is difficult to interpret due to the lack of information concerning the definition of HK). Information regarding follow-up, HD vintage and weekly schedule were sparse throughout the studies. Specifically, for studies reporting on mortality, in addition to population heterogeneity, the wide range of outcome measures used and the limited reference to the timepoint those outcomes were assessed at precluded pooling of data and meta-analysis was not considered appropriate.
In conclusion, evidence identified in this systematic review indicated a relationship between HK and LIDI with mortality in patients receiving HD for renal disease or acute renal failure. The majority of the evidence of this association is from observational studies of a retrospective nature, therefore further research that includes prospective studies and RCTs is required to determine if HK plays a causal role in the observed associations. In the meantime, improved monitoring and management of S-K+ levels in patients with renal disease may have the potential to improve outcomes in a chronic and an emergency HD setting.
Author contributions
DB led the design and implementation of the research and the writing of the manuscript and undertook the systematic literature review. BW and IZ undertook the systematic literature review. KB and DB contributed to the analysis of the data. DS and PM provided senior review and technical expertise and contributed to the design of the research and writing of the manuscript. ET provided senior review, contributed to the design and implementation of the research and supported manuscript development. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. As guarantor and corresponding author, DB takes full responsibility for the work as a whole and the decision to submit and publish the manuscript.
Supplemental Material
Download PDF (814.2 KB)Acknowledgements
Medical writing and editorial support were provided by Dr Angharad R. Morgan and Carissa Dickerson of Health Economics and Outcomes Research Ltd. Support for this assistance was provided by AstraZeneca.
Disclosure statement
DB, DS, BW, IZ, KB, DB and PM are employees of Health Economics and Outcomes Research Ltd and received funding from AstraZeneca to undertake the research outlined in this study. ET is an employee of AstraZeneca. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Additional information
Funding
References
- Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92:487–495.
- Soar J, Perkins G, Abbas G, al. e. European Resuscitation Council Guidelines for Resuscitation 2010 Section 8. Cardiac arrest in special circumstances: electrolyte abnormalities, poisoning, drowning, accidental hypothermia, hyperthermia, asthma, anaphylaxis, cardiac surgery, trauma, pregnancy, electrocution. Resuscitation. 2010;81:1400–1433.
- Tran HA. Extreme hyperkalemia. South Med J. 2005;98:729–732.
- Lehnhardt A, Kemper M. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26:377–384.
- Spodick DH. Effects of severe hyperkalemia. Am Heart Hosp J. 2008;6:68.
- Jain N, Kotla S, Little BB, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–1513.
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162.
- Sarafidis PA, Blacklock R, Wood E, et al. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. CJASN. 2012;7:1234–1241.
- Ahmed J, Weisberg LS. Hyperkalemia in dialysis patients. Semin Dial. 2001;14:348–356.
- Foley RN, Gilbertson DT, Murray T, et al. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365:1099–1107.
- Fotheringham J, Fogarty DG, El Nahas M, et al. The mortality and hospitalization rates associated with the long interdialytic gap in thrice-weekly hemodialysis patients. Kidney Int. 2015;88:569–575.
- Moher D, Liberati A, Tetzlaff J, PRISMA Group, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. w64.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384.
- Viswanathan M, Ansari MT, Berkman ND, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Methods guide for effectiveness and comparative effectiveness reviews. Rockville (MD): AHRQ Methods for Effective Health Care; 2008.
- The R Foundation. The R Project for Statistical Computing 2018 [updated 2018 Sep 14]. Available from: https://www.r-project.org/
- Ahmad Z. Hyperkalemia as a medical emergency in patients with ESRD in hemodialysis. Pak J Med Sci. 2010;26:117–122.
- Balamuthusamy S, Reddi A, et.al. Prevalence of hyperkalaemia in patients with ESRD undergoing haemodialysis. J Am Soc Nephrol. 2017;28:388.
- Belmar Vega L, Galabia ER, Bada da Silva J, et al. Epidemiology of hyperkalemia in chronic kidney disease. Nefrologia: Publicacion oficial de la Sociedad Espanola Nefrologia; 2019.
- Block GA, Chertow GM, Sullivan JT, et al. An integrated analysis of safety and tolerability of etelcalcetide in patients receiving hemodialysis with secondary hyperparathyroidism. PLoS One. 2019;14:e0213774.
- Block GA, Rosenbaum DP, Leonsson-Zachrisson M, et al. Effect of tenapanor on interdialytic weight gain in patients on hemodialysis. CJASN. 2016;11:1597–1605.
- Chan KE, Ikizler TA, Gamboa JL, et al. Combined angiotensin-converting enzyme inhibition and receptor blockade associate with increased risk of cardiovascular death in hemodialysis patients. Kidney Int. 2011;80:978–985.
- Charytan D, Himmelfarb J, et.al. Safety and cardiovascular efficacy of spironolactone (SPL) in dialysis-dependent ESRD (Spin-D): S pilot trial of the NIDDK haemodialysis novel therapies consortium. J Am Soc Nephrol. 2017;28:B9.
- Charytan DM, Himmelfarb J, Ikizler TA, et al., Hemodialysis Novel Therapies Consortium. Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney Int. 2019;95:973–982.
- Checherita IA, David C, Diaconu V, et al. Potassium level changes–arrhythmia contributing factor in chronic kidney disease patients. Romanian J Morphol Embryol. 2011;52:1047–1050.
- Cho JH, Hwang JY, Lee SE, et al. Nutritional status and the role of diabetes mellitus in hemodialysis patients. Nutr Res Pract. 2008;2:301–307.
- Cobo Sanchez JL, Alconero Camarero AR, Casaus Perez M, et al. Hyperkalaemia and haemodialysis patients: eletrocardiographic changes. J Ren Care. 2007;33:124–129.
- de Almeida L, Sette L, Fonseca F, et al. Metabolic and volume status evaluation of hemodialysis patients with and without residual renal function in the long interdialytic interval. J Bras Nefrol. 2019;41:481–491.
- Douvris A, Zeid K, Hiremath S, et al. Safety lapses prior to initiation of hemodialysis for acute kidney injury in hospitalized patients: a patient safety initiative. JCM. 2018;7:317.
- Khedr E, Abdelwhab S, El-Sharkawy M, et al. Prevalence of hyperkalemia among hemodialysis patients in Egypt. Ren Fail. 2009;31:891–898.
- Eron JJ Jr, Lelievre JD, Kalayjian R, et al. Safety of elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide in HIV-1-infected adults with end-stage renal disease on chronic haemodialysis: an open-label, single-arm, multicentre, phase 3b trial. The Lancet HIV. 2019;6:e15–e24.
- Farese S, Kruse A, Pasch A, et al. Glycyrrhetinic acid food supplementation lowers serum potassium concentration in chronic hemodialysis patients. Kidney Int. 2009;76:877–884.
- Frazão CM, de Sá JD, Medeiros AB, et al. The adaptation problems of patients undergoing hemodialysis: socio-economic and clinical aspects. Rev Lat Am Enfermagem. 2014;22:966–972.
- Garagarza CA, Valente AT, Oliveira TS, et al. Effect of personalized nutritional counseling in maintenance hemodialysis patients. Hemodial Int. 2015;19:412–418.
- Genovesi S, Valsecchi MG, Rossi E, et al. Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24:2529–2536.
- Hammer F, Malzahn U, Donhauser J, et al., MiREnDa Study Group. A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney Int. 2019;95:983–991.
- Han SW, Won YW, Yi JH, et al. No impact of hyperkalaemia with renin-angiotensin system blockades in maintenance haemodialysis patients. Nephrol Dial Transplant. 2007;22:1150–1155.
- He YL, Yang SJ, Hu CH, et al. Safety and efficacy of sofosbuvir-based treatment of acute hepatitis C in end-stage renal disease patients undergoing haemodialysis. Aliment Pharmacol Ther. 2018;47:526–532.
- Hooker R, Young M, Hendey GW. Hyperkalemia frequently presents as symptomatic bradycardia in the prehospital setting. Acad Emerg Med. 2015;22:S86.
- Huang CW, Lee MJ, Lee PT, et al. Low potassium dialysate as a protective factor of sudden cardiac death in Hemodialysis patients with Hyperkalemia. PLoS One. 2015;10:e0139886.
- Hussain S, Dreyfus DE, Marcus RJ, et al. Is spironolactone safe for dialysis patients? Nephrol Dialy Transplant. 2003;18:2364–2368.
- Hwang JC, Wang CT, Chen CA, et al. Hypokalemia is associated with increased mortality rate in chronic hemodialysis patients. Blood Purif. 2011;32:254–261.
- Iseki K, Arima H, Kohagura K, et al., Olmesartan Clinical Trial in Okinawan Patients Under OKIDS (OCTOPUS) Group. Effects of angiotensin receptor blockade (ARB) on mortality and cardiovascular outcomes in patients with long-term haemodialysis: a randomized controlled trial. Nephrol Dial Transplant. 2013;28:1579–1589.
- Karaboyas A, Xu H, Morgenstern H, et al. DOPPS data suggest a possible survival benefit of renin angiotensin-aldosterone system inhibitors and other antihypertensive medications for hemodialysis patients. Kidney Int. 2018;94:589–598.
- Karaboyas A, Zee J, Brunelli SM, et al. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2017;69:266–277.
- Kim T, Rhee CM, Streja E, et al. Racial and ethnic differences in mortality associated with serum potassium in a large hemodialysis cohort. Am J Nephrol. 2017;45:509–521.
- Knoll GA, Sahgal A, Nair RC, et al. Renin-angiotensin system blockade and the risk of hyperkalemia in chronic hemodialysis patients. Am J Med. 2002;112:110–114.
- Kourtellidou S, Ashby D, et.al. Oral sodium bicarbonate reduces inter-dialytic potassium gain – the BicHD trial. J Am Soc Nephrol. 2016;27:595A.
- Kovesdy CP, Regidor DL, Mehrotra R, et.al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. CJASN. 2007;2:999–1007.
- Kovesdy CP, Rowan CG, Conrad A, et al. Real-World evaluation of patiromer for the treatment of hyperkalemia in hemodialysis patients. Kidney Int Rep. 2019;4:301–309.
- Lee MJ, Kim S, Park I, et al. Comparison of estimated glomerular filtration rate equations at the time of hemodialysis initiation. Kidney Res Clin Pract. 2015;34:207–211.
- Lin HH, Yang YF, Chang JK, et al. Renin-angiotensin system blockade is not associated with hyperkalemia in chronic hemodialysis patients. Ren Fail. 2009;31:942–945.
- Maoujoud O, Zajjari Y, Asseraji M, et al. Commentary: the practice of dialysis in the intensive care unit in a developing country. Ethnicity Disease. 2014;24:226–228.
- Mattos A, Sanches L, Bibian M, et al. Clinical characteristics of patients treated by hemodialysis. Kidney Res Clin Pract. 2012;31:A57– A7.
- Movilli E, Camerini C, Gaggia P, et al. Use of renin-angiotensin system blockers increases serum potassium in anuric hemodialysis patients. Am J Nephrol. 2018;48:79–86.
- Nemati E, Taheri S. Electrocardiographic manifestations of hyperkalemia in hemodialysis patients. Saudi J Kidney Dis Transplant. 2010;21:471–477.
- Patil VC, Kulkarni C, Rajput A, et al. Incidence, etiology and clinical profile of newly detected Chronic kidney disease (CKD) at teaching hospital. Res J Pharmaceut Biol Chem Sci. 2015;6:1092–1110.
- Poulikakos D, Shah A, Persson M, et al. Safety and efficacy of beta blockers in haemodialysis. Nephrol Dial Transplant. 2012;27:ii248.
- Pourfarziani V, Ghanbarpour F, Nemati E, et al. Laboratory variables and treatment adequacy in hemodialysis patients in Iran. Saudi J Kidney Dis Transplant. 2008;19:842–846.
- Raza H, Courts A, Quadri K, et al. The effect of active nutritional counseling in improving biochemical nutritional parameters and fluid overload problems in maintenance hemodialysis patients. Saudi J Kidney Dis Transplant. 2004;15:140–143.
- Robertson J, Benner D, Levine R. Analysis of potassium profiles among hemodialysis (HD) patients. Am J Kidney Dis. 2009;53:A65.
- Ross J, DeatherageHand D. Evaluation of potassium levels before hemodialysis access procedures. Semin Dial. 2015;28:90–93.
- Sacchetti A, Stuccio N, Panebianco P, et al. ED hemodialysis for treatment of renal failure emergencies. Am J Emerg Med. 1999;17:305–307.
- Singh B, Block G, Lerma EV, et al. Hyperkalemia and serum potassium variability in patients on hemodialysis. Am J Kidney Dis. 2017;69:A3.
- Taheri S, Mortazavi M, Shahidi S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi J Kidney Dis Transplant. 2009;20:392–397.
- Tzamaloukas AH, Avasthi PS. Temporal profile of serum potassium concentration in nondiabetic and diabetic outpatients on chronic dialysis. Am J Nephrol. 1987;7:101–109.
- Vukusich A, Kunstmann S, Varela C, et al. A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1380–1387.
- Walsh M, Manns B, Garg AX, et al. The safety of eplerenone in hemodialysis patients: a noninferiority randomized controlled trial. Clin J Am Soc Nephrol. 2015;10:1602–1608.
- Yalin SF, Trabulus S, Yalin AS, et al. Factors associated with mortality in patients underwent emergency hemodialysis. Nephrol Dial Transplant. 2012;27:ii211–ii2.
- Yang G, Wang J, Sun J, et al. Perioperative hyperkalemia in hemodialysis patients undergoing parathyroidectomy for renal hyperparathyroidism. Intern Emerg Med. 2019;14:1065–1071.
- Yusuf AA, Hu Y, Singh B, et al. Serum potassium levels and mortality in hemodialysis patients: a retrospective cohort study. Am J Nephrol. 2016;44:179–186.
- Acha K, Awasume E, Ama Moor V, et al. SAT-216 factors associated with disorders of serum potassium in patients on maintenance hemodialysis in a facility without a renal dietician. Kidney Int Rep. 2020;5:S92–S3.
- Borgia SM, Dearden J, Yoshida EM, et al. Sofosbuvir/velpatasvir for 12-weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J Hepatol. 2019;71:660–665.
- Brown P, Omar RL. S. The indications and timing of haemodialysis in critically ill patients with acute kidney injury admitted to ICU. Southern Afr J Crit Care. 2019;35:34–35.
- Chen N, Hao C, Liu BC, et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381:1011–1022.
- Fishbane S, Ford M, Fukagawa M, et al. A phase 3b, randomized, double-blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. JASN. 2019;30:1723–1733.
- Fishbane S, Jamal A, Munera C, et al. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382:222–232.
- Fishbane S, Spinowitz BS, Wisemandle WA, et al. Randomized controlled trial of subcutaneous epoetin alfa-epbx versus epoetin alfa in end-stage kidney disease. Kidney Int Rep. 2019;4:1235–1247.
- Jebali H, Ghabi H, Mami I, et al. Evaluation of electrocardiographic findings before and after hemodialysis session. Saudi J Kidney Dis Transplant. 2020;31:639–646.
- Megahed AF, Tawfik M, Holes G, et al. Enoxaparin use as an anticoagulant during hemodialysis is associated with therapeutic benefits. Nephrol Dial Transplant. 2019;34:a514.
- Nyandwi J, Ndirahisha E, Manirakiza S, et al. SUN-006 prognosis of acute kidney injury in the era of renal replacement therapy in Burundi. Kidney Int Rep. 2020;5:S206–S207.
- Rafique Z, Aceves J, Espina I, et al. Can physicians detect hyperkalemia based on the electrocardiogram? Am J Emerg Med. 2020;38:105–108.
- Thomas A, Silver SA, Perl J, et al. The frequency of routine blood sampling and patient outcomes among maintenance hemodialysis recipients. Am J Kidney Dis. 2020;75:471–479.
- Triqui C, Najjar M, Ben Amor S, et al. SAT-094 hemodialysis emergencies in a nephrology department: about 117 cases. Kidney Int Rep. 2020;5:S42.
- Vaz de Melo Ribeiro P, Miranda Hermsdorff HH, Balbino KP, et al. Effect of a nutritional intervention, based on transtheoretical model, on metabolic markers and food consumption of individuals undergoing hemodialysis. J Ren Nutr. 2020;30:430–439.
- Woods J, Polkinghorne K, Kerr P, et al. SUN-321 investigating the effectiveness and safety of a very low calorie diet (VLCD) as a method of weight loss in patients receiving haemodialysis therapy. Kidney Int Rep. 2019;4:S293.
- Alagusundaramoorthy S, Gregory A, et.al. Timing is everything: decreasing mortality in severe hyperkalemia. J Am Soc Nephrol. 2018;29:500.
- Balle C, Schollmeyer P. Morbidity of patients with analgesic-associated nephropathy on regular dialysis treatment and after renal transplantation. Klin Wochenschr. 1990;68:38–42.
- Chaaban A, Abouchacra S, Gebran N, et al. Potassium binders in hemodialysis patients: a friend or foe? Ren Fail. 2013;35:185–188.
- Chatoth D, Wahl P, Rakov V, et al. FP531 Real-world outcomes of hyperkalemia management with patiromer in end-stage renal disease patients undergoing hemodialysis in the United States. Nephrol Dial Transplant. 2018;33:i218.
- Ferrey A, You AS, Kovesdy CP, et.al. Dialysate potassium and mortality in a prospective hemodialysis cohort. Am J Nephrol. 2018;47:415–423.
- Jadoul M, Thumma J, Fuller DS, et al. Modifiable practices associated with sudden death among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. CJASN. 2012;7:765–774.
- Li H, Wang SX. Analysis of death causes and its related factors in young patients on maintenance hemodialysis. Blood Purification. 2012;33:247–248.
- Lomonte C, Chiarulli G, Cazzato F, et al. End-stage renal disease in leprosy. J Nephrol. 2004;17:302–305.
- Morduchowicz G, Winkler J, Derazne E, et al. Causes of death in patients with end-stage renal disease treated by dialysis in a center in Israel. Israel J Med Sci. 1992;28:776–779.
- Onuigbo M, Onuigbo N, Bellasi A, et al. Penultimate pulse wave velocity, better than baseline pulse wave velocity, predicted mortality in Italian ESRD cohort study – a case for daily hemodialysis for ESRD patients with accelerated pulse wave velocity changes. G Ital Nefrol. 2013;30:gin/30.2.22.
- Poulikakos D, Malik M, Banerjee D. Risk profiling based on cardiac autonomic modulation and repolarisation indices from intradialytic computerized electrocardiography. Nephrol Dial Transplant. 2015;30:iii560.
- Pun PH, Herzog CA, Middleton JP. Improving ascertainment of sudden cardiac death in patients with end stage renal disease. CJASN. 2012;7:116–122.
- Shibata M, Kishi T, Iwata H. Clinical study of complications in dialyzed diabetics. Tohoku J Exp Med. 1983;141 Suppl :417–425.
- Li M, Ye ZC, Li CM, et al. Low serum uric acid levels increase the risk of all-cause death and cardiovascular death in hemodialysis patients. Ren Fail. 2020;42:315–322.
- Trajceska L, Selim G, Sikole A. Potassium level, malnutrition and mortality in dialysis patients. Int J Artif Organs. 2019;42:426.
- Zulham RM, Martini S, Widati S. Risk factor of early death in diabetic terminal renal failure patients receiving hemodialysis. Ind J Forensic Med Toxicol. 2019;13:1585–1590.
- Chatoth D, Wahl P, Rakov V, et al. Real-world outcomes of hyperkalemia management with patiromer in end-stage renal disease patients undergoing hemodialysis in the United States. Nephrol Dial Transplant. 2017;32:iii657.
- Bleyer AJ, Hartman J, Brannon PC, et.al. Characteristics of sudden death in hemodialysis patients. Kidney Int. 2006;69:2268–2273.
- Bleyer AJ, Russell GB, Satko SG, et.al. Sudden and cardiac death rates in hemodialysis patients. Kidney Int. 1999;55:1553–1559.
- Fotheringham J, Sajjad A, Stel VS, et al. The association between longer haemodialysis treatment times and hospitalization and mortality after the two-day break in individuals receiving three times a week haemodialysis. Nephrol Dial Transplant. 2019;34:1577–1584.
- Fotheringham J, Smith M, Froissart M, et.al. The variation in hospitalisation and mortality following non-attendance for haemodialysis according to dialysis day of the week in a European cohort. Nephrol Dial Transplant. 2018;33:i344.
- Karnik JA, Young BS, Lew NL, et.al. Cardiac arrest and sudden death in dialysis units. Kidney Int. 2001;60:350–357.
- Krishnasamy R, Badve SV, Hawley CM, et al. Daily variation in death in patients treated by long-term dialysis: comparison of in-center hemodialysis to peritoneal and home hemodialysis. Am J Kidney Dis. 2013;61:96–103.
- Kumar P, Prabhu AR, Bairy M. Difference in interdialytic intervals leading to hospital admission and mortality in hemodialysis patients. Value Health. 2013;16:A636.
- Lafrance JP, Nolin L, Senécal L, et al. Predictors and outcome of cardiopulmonary resuscitation (CPR) calls in a large haemodialysis unit over a seven-year period. Nephrol Dial Transplant. 2006;21:1006–1012.
- Rhee C, You A, Streja E, et al. Dialysis schedule and day-of-week mortality in a national dialysis cohort. Nephrol Dial Transplant. 2017;32:iii355–iii357.
- Roy-Chaudhury P, Tumlin JA, Koplan BA, et al., MiD investigators and committees. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int. 2018;93:941–951.
- Tangweerapong K. Association between pre-dialysis electrolytes and incidence of arrhythmia in long inter-dialytic interval among chronic haemodialysis patients. J Am Soc Nephrol. 2017;1043.
- Tumlin J, Roy-Chaudhury P, Koplan B, et al., MiD investigators and Committees. Relationship between dialytic parameters and reviewer confirmed arrhythmias in hemodialysis patients in the monitoring in dialysis study. BMC Nephrol. 2019;20:80.
- Wong MCG, Kalman JM, Pedagogos E, et al. Temporal distribution of arrhythmic events in chronic kidney disease: highest incidence in the long interdialytic period. Heart Rhythm. 2015;12:2047–2055.
- Zhang H, Schaubel D, Kalbfleisch J, al. e. Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule affects day-of-week mortality. Kidney Int. 2012;81:1108–1115.
- Fotheringham J, Smith MT, Froissart M, et al. Hospitalization and mortality following non-attendance for hemodialysis according to dialysis day of the week: a European cohort study. BMC Nephrol. 2020;21:218.
- Chevarría J, Sánchez M, Pérez K, et al. Hyperkalemia in hemodialysis patients not all is said. NDT Plus. 2010;3:iii312.
- Kovesdy C. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128:1281–1287.
- Kovesdy CP. Epidemiology of hyperkalemia: an update. Kidney Int Suppl (2011). 2016;6:3–6.
- Palaka E, Grandy S, Darlington O, et al. Associations between serum potassium and adverse clinical outcomes: a systematic literature review. Int J Clin Pract. 2020;74:e13421.
- Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant. 2019;34:iii2–iii11.
- Rhee CM. Serum potassium and the long interdialytic interval: minding the gap. Am J Kidney Dis. 2017;70:4–7.
- Brunelli SM, Du Mond C, Oestreicher N, et al. Serum potassium and short-term clinical outcomes among hemodialysis patients: impact of the long interdialytic interval. Am J Kidney Dis. 2017;70:21–29.
- Marano M, Marano S, Gennari FJ. Beyond bicarbonate: complete acid-base assessment in patients receiving intermittent hemodialysis. Nephrol Dial Transplant. 2017;32:528–533.