Abstract
Purpose
There are conflicting results as to the effect of inhaled nitric oxide (iNO) therapy on the risk of acute kidney injury (AKI). The aim of this study was to perform a meta-analysis to assess the updated data.
Methods
We systematically searched Web of Science, the Cochrane Library, Wanfang, and PubMed for relevant randomized control trials between database inception and 9/07/2020. Relative risks (RRs) with 95% confidence intervals (CIs) predicting the risk of AKI were extracted to obtain summary estimates using fixed-effects models. The Trim and Fill method was used to evaluate the sensitivity of the results and adjust for publication bias in meta-analysis.
Results
15 randomized controlled studies from 14 articles involving 1853 patients were included in the study. Analyzing the eligible studies we found: (1) iNO therapy significantly increased the risk of AKI in acute respiratory distress syndrome patients (RR 1.55, 95% CI 1.15–2.10, p = 0.004; I2 for heterogeneity 0%; Phet = 0.649). (2) The use of iNO was associated with reduced AKI risk in patients undergoing cardiac surgery (RR 0.80, 95% CI 0.64–0.99, p = 0.037; I2 for heterogeneity 0%; Phet = 0.528). (3) For organ transplantation recipients, there was no effect of iNO administration on the risk of AKI (RR 0.50, 95% CI 0.16–1.56, p = 0.233; I2 for heterogeneity 0%; Phet = 0.842). The Trim and Fill analysis showed that the overall effect of this meta-analysis was stable.
Conclusions
The effect of iNO on AKI risk might be disease-specific. Future RCTs with larger patient populations should aim to validate our findings.
Introduction
Nitric oxide (NO) is an important signaling substance and vasodilator. It can relax vascular smooth muscle cells as well as pericytes by binding to the heme moiety of cytosolic guanylate cyclase and ultimately causing a fall in intracellular Ca2+ [Citation1]. Inhalation of NO (iNO) leads to selective pulmonary vasodilatation and reduces pulmonary vascular resistance, increases arterial oxygenation, and improves pulmonary angiogenesis and lung alveolarization [Citation2]. Given these beneficial effects, iNO is used widely as therapy in the field of critical care and medicine in general, including acute respiratory distress syndrome (ARDS), neonatal pulmonary hypertension, and cardiac surgery.
Although iNO has excellent effectiveness and safety profile, its potential adverse effects should not be neglected. Two previous meta-analyses raised concerns about the relationship between the use of iNO and the risk for acute kidney injury (AKI) [Citation3,Citation4]. In 2007, Adhikari and colleagues included four randomized control trials (RCTs) involving 895 participants in a pooled analysis and suggested that iNO increased the risk for renal dysfunction (risk ratio 1.50, 95% confidence interval 1.11–2.02) [Citation3]. Another meta-analysis published in 2015 by Ruan et al. [Citation4] found that the use of iNO was associated with higher renal dysfunction risk especially with prolonged use in ARDS patients. Despite these interesting findings, there were some limitations to the meta-analyses. First, a sensitivity analysis was not performed and the stability of the results were not evaluated. Second, most included studies in these meta-analyses focused on ARDS and there was less evidence on other diseases. Third, the number of relevant researches has increased rapidly since these meta-analyses were published. The accumulating evidence needs to be reevaluated. Therefore, we aimed to perform an up-dated quantitative assessment of the relationship between iNO and AKI.
Methods
This meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [Citation5]. There was no registered protocol for this meta-analysis.
Search methods
Applying a predetermined search strategy, two independent investigators (JW and XC) searched Web of Science, the Cochrane Library, Wanfang, and PubMed in order to identify potentially relevant articles between database inception and 9 July 2020. The following search terms were used: ‘inhaled nitric oxide’ and ‘randomized controlled trial’. Secondary searching included a manual search of reference lists in previous meta-analyses, reviews, and all included studies. There were no language restrictions. The search details are shown in Supplementary Table S1.
Inclusion and exclusion criteria
Studies were included in this meta-analysis if they met the following inclusion criteria: (1) the eligible studies were RCTs; (2) they compared iNO with placebo or usual treatment; and (3) the number of patients with renal dysfunction was reported in iNO and control groups. The exclusion criteria were: (1) retrospective studies, cohort studies, and non-randomized controlled studies; (2) conference abstracts; and (3) data for renal dysfunction were not reported.
Data extraction
From all RCTs found eligible, the following information was extracted: the family name of the first author, country/region of research, year of publication, study designs and methods, participant number and details, intervention details, and the number of patients with renal dysfunction in iNO and control groups. Two investigators (JW and XC) performed data extraction. Any disagreements were resolved by discussion between the two investigators and JZ where necessary.
Assessment for risk of bias
Two investigators (JW and XC) assessed the risk of bias for each trial by using criteria according to the Cochrane Risk of Bias Tool, which allows for evaluating six domains: method of random sequencing, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other factors that may affect bias [Citation6]. Discrepancies were resolved by discussion or by consultation with the third investigator, JZ.
Meta-analyses
For dichotomous data, we used risk ratios (RRs) as the effect measure with 95% confidence intervals (CIs) calculated using the fixed-effects model, where a RR > 1 indicates an increased likelihood of renal dysfunction when treated with iNO compared with placebo. We generated summary forest plots to show the RRs and 95% CIs. Statistical heterogeneity between trial results was assessed using the I2 statistic and the Chi-square test. It was classified as large (75%), moderate (50%), and low (25%) [Citation7]. We considered it to be substantial heterogeneity when the I2 was greater than 50% and was accompanied by a statistically significant Chi2 statistic. The cause of heterogeneity was explored using subgroup analyses. Finally, publication bias was evaluated by graphical analysis of the funnel plot. Egger’s regression test was used to test the funnel plot [Citation8]. All analyses were performed in Stata software, version 12.0 (College Station, TX). The significance level for all statistical tests was p < 0.05 (two-tailed).
Results
The literature search yielded 741 articles. After the removal of duplicates, they were reduced to 596. At the screening of study titles and abstracts, an additional 572 articles were excluded as they did not meet our inclusion criteria. shows how we picked the studies. Ultimately, a total of 15 studies from 14 articles were analyzed in our final meta-analysis [Citation9–22]. All studies were performed in North America, Europe, China, Brazil, and Uganda. Among the included studies, 4 studies reported the results for ARDS [Citation9,Citation11–13], 4 studies reporting cardiac surgery [Citation15,Citation16,Citation21,Citation22], 3 studies reporting organ transplantation [Citation14,Citation17], and 4 studies reporting other diseases [Citation10,Citation18–20]. The number of participants in each trial varied with 29 subjects in the smallest study and 385 subjects in the largest study. The majority were men, except in one study. All the studies provided data for AKI. shows these studies selected for the final meta-analysis. Detailed risk of bias assessments are presented in .
Table 1. Characteristics of included studies.
Table 2. Assessment of risk of bias in individual studies.
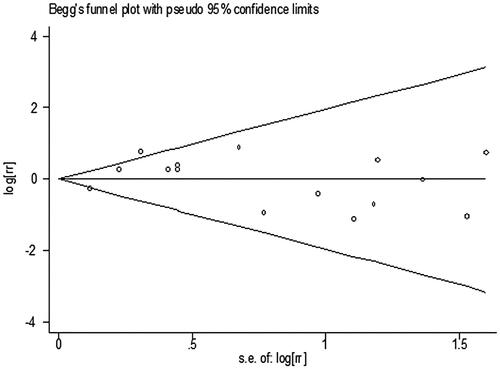
presents the RRs for AKI from each study. The pooled meta-analysis showed that iNO treatment was not associated with AKI risk, with a pooled RR of 1.00 (95% CI 0.84–1.18; p = 0.977; I2 for heterogeneity 31.6%; Phet = 0.116) ( and ). Egger’s test (p = 0.461), Begg’s test (p = 0.198), and visual evaluation of the funnel plot () indicated no publication bias. In subgroup analysis based on the fixed-effects model, the use of iNO was associated with increased risk of AKI in ARDS patients (RR 1.55, 95% CI 1.15–2.10, p = 0.004; I2 for heterogeneity 0%; Phet = 0.649) ( and ), but iNO treatment decreased AKI risk for individuals who received cardiac surgery (RR 0.80, 95% CI 0.64–0.99, p = 0.037; I2 for heterogeneity 0%; Phet = 0.528) ( and ). In addition, there was no effect of iNO therapy on the risk of AKI in patients undergoing organ transplantation ( and ). The robustness of the results was evaluated by a sensitivity analysis using the trim and fill method [Citation23]. As shown in , the results of the trim and fill test showed that sensitivity analyses for ARDS and cardiac surgery remained significant.
Figure 2. Meta-analysis of the effect of inhaled nitric oxide on acute kidney injury risk by pooling the 15 randomized controlled trials.
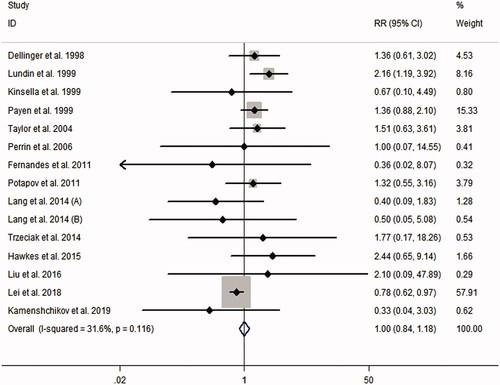
Figure 4. Meta-analysis of the effect of inhaled nitric oxide on acute kidney injury risk. (A) Acute respiratory distress syndrome. (B) Cardiac surgery. (C) Organ transplantation.
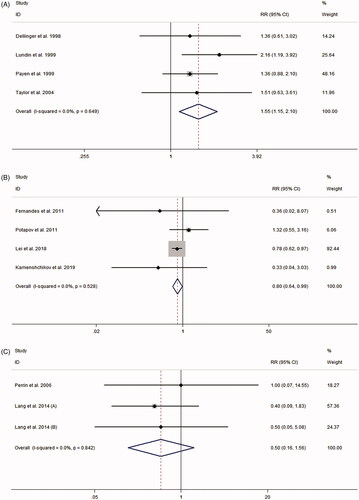
Table 3. Meta-analysis evaluation of the relationship between inhaled nitric oxide and acute kidney injury risk.
Table 4. Trim and Fill test results.
Heterogeneity
To quantify the between-study heterogeneity, we utilized the I2 statistic and the Chi-square test. There was no significant heterogeneity across the studies in both the overall meta-analysis and subgroup analyses. This enabled us to pool evidence from the included studies using the fixed-effects model.
Discussion
We carried out a meta-analysis by combining evidence from 15 randomized clinical studies to evaluate the effect of iNO therapy on the risk of AKI. The main findings of the meta-analysis are the following: (i) iNO therapy significantly increased the risk of AKI in ARDS patients; (ii) the use of iNO was associated with reduced AKI risk in patients undergoing cardiac surgery; (iii) for organ transplantation recipients, there was no effect of iNO administration on the risk of AKI.
It is widely accepted that the effects of NO are limited to the lungs when delivered by inhalation. Many published RCTs suggest that iNO is associated with a very low incidence of adverse effects in the usual range of dosage. However, because iNO forms many metabolites that can act as endocrine carriers of NO in circulation, the safety aspects of iNO remain the focus of research. Lundin and coworkers focused on the adverse effects of iNO on ARDS in a European multicentre study [Citation11]. They found that although iNO administration was not associated with bleeding complications, marked methemoglobinemia, or increased frequency of pneumothorax, there was an association between acute renal failure and iNO in ARDS (RR 2.16, 95% CI 1.19–3.92) [Citation11]. This raised a concern about iNO-related renal dysfunction in ARDS patients. Several other studies from the USA and Europe also evaluated this possible relationship, including Dellinger et al. [Citation9], Payen et al. [Citation12], and Taylor et al. [Citation13]. Based on the findings, two meta-analyses reviewed the data and concluded that the use of iNO increased AKI risk on ARDS [Citation3,Citation4]. Our meta-analysis confirmed the association between iNO administration and renal dysfunction in ARDS patients (RR 1.55). In addition, using the trim and fill method (), the meta-analysis results were shown to be stable, and no evidence of publication bias was detected. Previous meta-analyses and ours highlighted the potential AKI risk associated with iNO therapy in ARDS. It remains unknown why iNO administration in ARDS is linked to increased AKI risk. However, it is commonly believed that prolonged treatment with iNO might cause detrimental cell damage by reacting with reactive oxygen species and the resulting formation of reactive nitrogen species [Citation24]. NO oxidative products can oxidize DNA bases and create DNA strand damage [Citation25]. They also disrupt the maintenance of oxidation-sensitive enzymes, inhibit mitochondrial respiration, induce nuclear factor-kappaB-mediated protein degradation, and enhance caspase activation [Citation26,Citation27]. Dellinger and coworkers found a positive link between iNO doses and circulating levels of NO oxidative products (such as NO2) in patients with ARDS [Citation9]. There is evidence that the generation of NO oxidative products contributed to glomerular cell apoptosis [Citation28]. In addition, animal studies showed that the prolonged administration of iNO could induce apoptosis of the cells of the collecting ducts and the distal convoluted tubular cells, leading to renal injury [Citation29]. In a rat model of diabetic kidney disease, endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS)-derived high NO production and oxidative stress contributed to apoptosis in the kidney [Citation30]. However, the precise mechanisms of iNO-induced AKI in ARDS patients remain largely unknown, deserving further research in the future.
Cardiac surgery is a procedure that is commonly carried out in the world. Despite promising technical advances, it is still a high-risk surgery. AKI is a common and serious complication post cardiac surgery. AKI has been found in approximately 40–70% of patients undergoing cardiac surgery and is associated with an unfavorable prognosis [Citation31]. The primary mechanisms of cardiac surgery-associated AKI include hemolysis, renal ischemia, and inflammation [Citation31]. Unlike patients with ARDS, clinical studies showed that patients undergoing cardiac surgery developed a NO deficient state due to hemolysis. NO depletion induces a proinflammatory cascade, causes oxidative stress, produces vasoconstriction, and impairs endothelial function and tissue perfusion [Citation32]. Preclinical and clinical data demonstrated that NO was a renal-protective agent during hemolysis and might thus prevent cardiac surgery-associated AKI. In a canine model of water-induced hemolysis, the use of iNO significantly reversed the vasoconstrictor effect of hemolysis, reduced serum creatinine, and attenuated renal impairment [Citation33]. Several RCTs evaluated the effect of iNO therapy on renal dysfunction in patients undergoing cardiac surgery. In a Chinese RCT of 244 patients undergoing multiple valve replacement, Lei and coworkers found that NO administration during cardiopulmonary bypass and for the first 24 h postoperatively was associated with a decreased incidence of postoperative AKI and major adverse kidney events [Citation21]. The trial by Lei et al. also indicated that the nephroprotective effect of iNO might be attributed to iNO’s impact on hemodynamics and right ventricular afterload. Their findings were supported by a recent European study in which Lomivorotov et al. demonstrated cardioprotective and nephroprotective effects of NO administration in cardiac surgery [Citation22]. Combining results from different RCTs, this meta-analysis indicated a 20% decreased risk of postoperative AKI in cardiac surgery patients who received iNO therapy. Our results supported a previous meta-analysis by Hu et al. [Citation34]. It is worth mentioning that several RCTs (such as NCT02836899 and NCT03527381) are underway to further assess the nephroprotective effect of NO in cardiac surgery. These trials may help to elucidate the underlying mechanisms of NO therapy for protecting AKI post-cardiac surgery.
In addition to ARDS and cardiac surgery, our meta-analysis also evaluated the effect of iNO on AKI incidence in other conditions, including organ transplantation. Some animal studies and RCTs reported that administrating iNO during the intraoperative period had protective effects on ischemia-reperfusion induced injury post-transplantation [Citation14,Citation17,Citation35,Citation36]. However, the results of our meta-analysis did not show any difference in the incidence of AKI post-transplantation between the iNO group and the control group.
Our meta-analysis has several limitations. Firstly, it remains unclear if the effect of iNO therapy on AKI risk in different diseases is dose-related. Although there was no significant heterogeneity in our meta-analysis, the included studies did vary in study design and iNO administration, making it difficult to assess dose-related effects. Secondly, most of the included studies were performed in adults and the number of pediatric studies was small. There were only three pediatric studies on the topic. It requires more research to investigate the effect of iNO therapy on renal function and AKI in pediatric patients. Thirdly, the incidence of AKI was not constantly reported by RCTs evaluating the effect of iNO therapy. Although Egger’s test did not suggest the presence of publication bias, we further performed the Trim and Fill test to assess the sensitivity of the results and adjust for publication bias in meta-analysis. As shown in , the results indicated that the overall effect of this meta-analysis was stable.
In conclusion, this meta-analysis suggested that iNO therapy increased the risk of AKI in ARDS patients, but the use of iNO was associated with a decreased risk of AKI in patients undergoing cardiac surgery. For organ transplantation recipients, iNO therapy had no effects on AKI incidence post-transplantation. Future RCTs are necessary to evaluate if the effects of iNO on AKI incidence is disease-specific. In addition, future RCTs should focus on the relation of iNO duration and dose with renal function.
Author contributions
JZ contributed to the conception of research. JW and XC performed the search, extracted data, and conducted statistical analyses. All authors contributed to the writing and revision of the manuscript.
Supplemental Material
Download PDF (303.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bhatraju P, Crawford J, Hall M, et al. Inhaled nitric oxide: current clinical concepts. Nitric Oxide. 2015;50:114–128.
- Barnes M, Brisbois EJ. Clinical use of inhaled nitric oxide: local and systemic applications. Free Radic Biol Med. 2020;152:422–431.
- Adhikari NK, Burns KE, Friedrich JO, et al. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334(7597):779.
- Ruan SY, Huang TM, Wu HY, et al. Inhaled nitric oxide therapy and risk of renal dysfunction: a systematic review and meta-analysis of randomized trials. Crit Care. 2015;19(1):137.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. Version 2. BMJ. 2011;343:d5928.
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998;26(1):15–23.
- Kinsella JP, Walsh WF, Bose CL, et al. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial. Lancet. 1999;354(9184):1061–1065.
- Lundin S, Mang H, Smithies M, et al. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. Intensive Care Med. 1999;25(9):911–919.
- Payen D, Vallet B. Results of the French prospective multicentric randomized double-blind placebo-controlled trial on inhaled nitric oxide in ARDS. Intensive Care Medicine. 1999;25S:166.
- Taylor RW, Zimmerman JL, Dellinger RP, et al. Inhaled Nitric Oxide in ARDS Study Group. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291(13):1603–1609.
- Perrin G, Roch A, Michelet P, et al. Inhaled nitric oxide does not prevent pulmonary edema after lung transplantation measured by lung water content: a randomized clinical study. Chest. 2006;129(4):1024–1030.
- Fernandes JL, Sampaio RO, Brandão CM, et al. Comparison of inhaled nitric oxide versus oxygen on hemodynamics in patients with mitral stenosis and severe pulmonary hypertension after mitral valve surgery. Am J Cardiol. 2011;107(7):1040–1045.
- Potapov E, Meyer D, Swaminathan M, et al. Inhaled nitric oxide after left ventricular assist device implantation: a prospective, randomized, double-blind, multicenter, placebo-controlled trial. J Heart Lung Transplant. 2011;30(8):870–878.
- Lang JD Jr., Smith AB, Brandon A, et al. A randomized clinical trial testing the anti-inflammatory effects of preemptive inhaled nitric oxide in human liver transplantation. PLoS One. 2014;9(2):e86053.
- Trzeciak S, Glaspey LJ, Dellinger RP, et al. Randomized controlled trial of inhaled nitric oxide for the treatment of microcirculatory dysfunction in patients with sepsis*. Crit Care Med. 2014;42(12):2482–2492.
- Hawkes MT, Conroy AL, Opoka RO, et al. Inhaled nitric oxide as adjunctive therapy for severe malaria: a randomized controlled trial. Malar J. 2015;14(1):421.
- Liu DH, Gao HX, Yi B, et al. Inhaled nitric oxide in neonates with hypoxic respiratory failure: a clinical randomized controlled study. Chinese J Neonatol. 2016;31(3):182–188.
- Lei C, Berra L, Rezoagli E, et al. Nitric oxide decreases acute kidney injury and stage 3 chronic kidney disease after cardiac surgery. Am J Respir Crit Care Med. 2018;198(10):1279–1287.
- Kamenshchikov NO, Mandel IA, Podoksenov YK, et al. Nitric oxide provides myocardial protection when added to the cardiopulmonary bypass circuit during cardiac surgery: randomized trial. J Thorac Cardiovasc Surg. 2019;157(6):2328–2336.
- Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98.
- Rehberg S, Maybauer MO, Maybauer DM, et al. The role of nitric oxide and reactive nitrogen species in experimental ARDS. Front Biosci. 2010;2:18–29.
- Tang CH, Wei W, Liu L. Regulation of DNA repair by S-nitrosylation. Biochim Biophys Acta. 2012;1820(6):730–735.
- Blaise GA, Gauvin D, Gangal M, et al. Nitric oxide, cell signaling and cell death. Toxicology. 2005;208(2):177–192.
- Creagh-Brown BC, Griffiths MJ, Evans TW. Bench-to-bedside review: inhaled nitric oxide therapy in adults. Crit Care. 2009;13(3):221.
- Hruby Z, Rosinski M, Tyran B. Parenchymal injury in remnant-kidney model may be linked to apoptosis of renal cells mediated by nitric oxide. J Nephrol. 2008;21(5):686–693.
- Goździk W, Albert J, Harbut P, et al. Prolonged exposure to inhaled nitric oxide transiently modifies tubular function in healthy piglets and promotes tubular apoptosis. Acta Physiol. 2009;195(4):495–502.
- Dincel G, Yildirim S. Increased expressions of eNOS and iNOS correlate with apoptosis of diabetic nephropathy in streptozotocin-induced type 1 diabetic rats. Kafkas Univ Vet Fak Derg. 2016;22:381–390.
- Mosa OF, Skitek M, Kalisnik JM, et al. Evaluation of serum cysteine-rich protein 61 and cystatin C levels for assessment of acute kidney injury after cardiac surgery. Ren Fail. 2016;38(5):699–705.
- Arellano DL. Acute kidney injury following cardiothoracic surgery. Crit Care Nurs Clin North Am. 2019;31(3):407–417.
- Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115(12):3409–3417.
- Hu J, Spina S, Zadek F, et al. Effect of nitric oxide on postoperative acute kidney injury in patients who underwent cardiopulmonary bypass: a systematic review and meta-analysis with trial sequential analysis. Ann Intensive Care. 2019;9(1):129.
- Murakami S, Bacha EA, Mazmanian GM, et al. Effects of various timings and concentrations of inhaled nitric oxide in lung ischemia-reperfusion. The Paris-Sud University Lung Transplantation Group. Am J Respir Crit Care Med. 1997;156(2):454–458.
- Katsumi H, Nishikawa M, Yamashita F, et al. Prevention of hepatic ischemia/reperfusion injury by prolonged delivery of nitric oxide to the circulating blood in mice. Transplantation. 2008;85(2):264–269.