Abstract
Background
Peritoneal dialysis (PD) patients experience accelerated arterial aging, which is characterized by elastin degradation. Elastin-derived peptides (EDPs) are direct products of elastin fragmentation. This study tried to explore the association between serum EDPs and abdominal aortic calcification (AAC) in PD patients.
Methods
Serum levels of EDPs were analyzed in 126 eligible PD patients and 30 controls. PD patients were grouped according to the annularity of AAC evaluated by an abdominal computed tomography (CT) scan. Serum EDPs were analyzed in relation to the presence of AAC or severe AAC in PD patients by logistic regression analysis.
Results
Serum EDPs in PD patients were significantly higher than age-matched controls. In 126 PD patients, higher EDPs was associated with greater risk of present AAC (OR = 1.056, 95%CI 1.010–1.103) and severe AAC (OR = 1.062, 95%CI 1.004–1.123). A combination of EDPs substantially improved the accuracy of diagnostic performance for AAC and severe AAC.
Conclusions
EDPs can predict the presence and extent of AAC in PD patients, indicating its possible role to recognize PD patients at risk for AAC and severe AAC.
Introduction
Cardiovascular disease (CVD) remains one of the major causes of death in patients starting peritoneal dialysis (PD) [Citation1], and those with vascular calcification are thought to have the highest risk of future CVD [Citation2]. The prevalence of abdominal aortic calcification (AAC) among dialysis patients in Asia is up to 46.8 − 85.71% [Citation3–5]. Potential biomarkers to improve AAC risk stratification may help understand its pathogenesis and even become targets.
Vascular calcification is thought to be a process of ageing accompanied by obvious extra-cellular matrix remodeling featured by elastin degradation [Citation6]. Concurrently with aging, the increased sympathetic activity, local inflammation, and oxidative stress could activate proteases including cysteine proteinases and matrix metalloproteinases (MMPs) [Citation7,Citation8], and irreversibly degrades elastin into elastin-derived peptides (EDPs) [Citation9].
In uremic patients with accelerated arterial aging, elastodystrophy of aorta occurred early and constructed a context of altered mineral metabolism for the pathogenesis of arterial medial calcification [Citation10]. In dialysis patients with more metabolic, hemodynamic, inflammatory, and hormonal changes [Citation11], elastic fiber fragmentation in epigastric artery was more obvious when compared with healthy kidney donors [Citation12]. These findings indicated PD patients underwent accelerated elastin degradation. In aneurysms featured by elastin breakdown, structural products of EDPs and desmosine in plasma increased and correlated with aneurysm rupture and aneurysm-related death [Citation13,Citation14]. Thus, we hypothesized EDPs in PD patients might also be elevated and correlate with AAC. In this study, we measured serum EDPs and evaluated its association with AAC in PD patients detected by a multi-slice spiral CT scan (MSCT).
Materials and methods
The study included adult PD patients and healthy volunteers from August 2018 to January 2020 in the First Affiliated Hospital of Soochow University. All participants signed an informed consent, and the study was approved by the ethics committee in accordance with the Declaration of Helsinki.
Study population
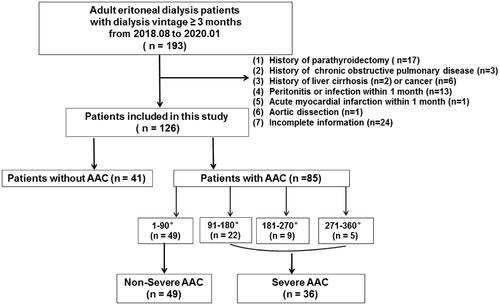
PD patients receiving continuous ambulatory peritoneal dialysis with low calcium (1.25 mmol/L) peritoneal dialysis solution (Baxter Healthcare CO.LTD, Guangzhou, China) for at least three months were included. Exclusion criteria were (1) history of parathyroidectomy, (2) chronic obstructive pulmonary disease (COPD), (3) liver cirrhosis, (4) cancer, (5) peritonitis or infection within 1 month, (6) acute myocardial infarction or aortic dissection within 1 month, and (7) incomplete information on AAC evaluation. The flowchart was displayed in .
Figure 1. Flowchart of study participants for the cross-sectional study. AAC indicates abdominal aortic calcification.
At the same time, 30 healthy subjects were randomly selected among age- and sex-matched people undergoing medical examination in the Medical Examination Center. Every of them had a normal glomerular filtration rate and underwent a CT scan to rule out the presence of AAC. They also had no history of proteinuria, hematuria, hypertension, or diabetes.
Evaluation of AAC
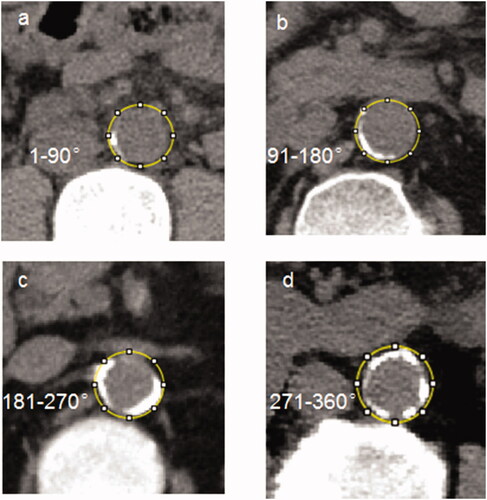
All patients received noncontrast abdominal MSCT during hospitalization, and the detailed CT protocol was presented in Online Resource 1. AAC was defined as calcified plaques with area ≥1mm3 and density ≥130 Hounsfield unit [Citation15] along the abdominal aorta walking path from diaphragm to the common iliac artery. Calcification of abdominal aorta was measured on cross-sectional images by one author (Xiao-chun Yang, with 20 years of radiology research experience and blinded to our study). Annularity was scored into five categories as absent, 1–90, 91–180, 181–270, and 271–360 degree, which was a simple method highly correlated with the Agatston score quantification [Citation16]. Examples of the semi-quantitative patterns were shown in .
Blood sampling and analysis
Venous samples were collected for at least 10-h fast. After clotting for 30 min at room temperature, the samples were centrifuged at 4000 g for 15 min at 4 °C. The upper serum was separated and stored at −80 °C. Until the investigation, serum samples were thawed at room temperature. Specific competitive enzyme-linked immunosorbent assay kits (Aviscera bioscience, USA) were used to measure serum EDPs. The principle and assay procedures were summarized in Online Resource 1.
Data collection
Age, gender, body mass index (BMI), dialysis vintage (PD vintage), weekly urea clearance (total Kt/V), and blood pressure were recorded on purpose. At the same time, baseline comorbid conditions of hypertension, type-2 diabetes mellitus (T2DM), and CVD consisting of coronary artery disease, cerebral artery disease, and aortic aneurysm were abstracted from previous medical records, as well as the related medications.
Hemoglobin (HB), neutrophil cells (NE), platelets (PLT), lipid profile, creatinine (Cr), β2-microglobulin (β2-MG), uric acid (UA), albumin (Alb), and high sensitivity-C reactive protein (hs-CRP), mineral metabolism parameters including calcium (Ca), phosphate, intact parathyroid hormone (iPTH) and alkaline phosphatase (ALP) [Citation17] were obtained with routine laboratory methods at the beginning of study. Serum albumin in our hospital was measured by Bromcresol green method, and when albumin was lower than 34 g/L, serum calcium was corrected with the formula: corrected Ca = uncorrected Ca + (34 – [Alb (g/L)]) * 0.0176 [Citation18,Citation19].
Statistical analysis
The continuous variables were expressed as mean ± standard deviation (SD) or median (Q1 – Q3). One-way analysis of variance (ANOVA) or Kruskal–Wallis H test, and independent sample T-test or Mann–Whitney test were used appropriately. Categorical variables were presented as frequencies (percentages) and compared by the Chi-square test or Fisher’s exact test when necessary. Spearman correlation analysis was used to find association of EDPs with other parameters.
PD patients were firstly assigned into AAC versus non-AAC group, and a logistic regression analysis was conducted among PD patients to examine the relationship between EDPs with AAC. We also conducted a subgroup analysis in patients with AAC. Considering the imbalanced sample size, patients were further grouped into severe versus non-severe group with the cutoff annular of 90 degree, as shown in . Receiver operation characteristic (ROC) curves were used to determine optimal cutoff values of EDPs by maximizing the Youden index (sensitivity + specificity − 1). Additionally, for sensitivity analysis, EDPs were transferred to a dichotomous variable according to the respective cutoff point of two ROC curves, and then again fully adjusted. A probability of < 0.05 was considered statistically significant for all analyses. All of the analyses were conducted with the IBM SPSS (version 22.0, IBM Inc., NY, USA).
Results
Participants
Finally, our study enrolled 126 patients on PD and 30 healthy controls. Patients and controls were comparable in age and sex ratio; however, serum EDPs in PD patients were significantly higher than that in controls. Hyperlipidemia was absent in PD patients, while disorders of mineral parameters were more prevalent (higher serum P and ALP). Detailed demographic and clinical characteristics of all PD patients are summarized in .
Table 1. Comparisons of demographic data and laboratory parameters among PD patients with or without AAC and the controls.
Comparisons of characteristics among PD patients with or without AAC
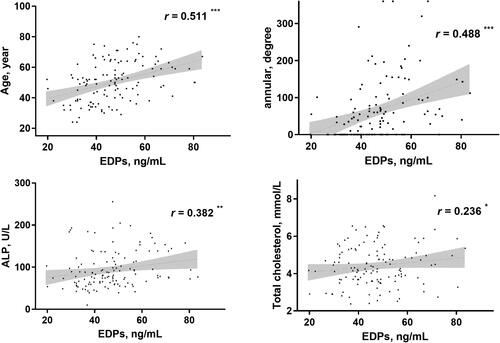
The mean age of all PD patients was 50 ± 13 years and the median dialysis vintage was 22.5 (7.0 − 51.0) months. Correlation analysis showed positive association of EDPs with age (r = 0.511), annularity of AAC (r = 0.488), ALP (r = 0.382) and TC (r = 0.236) as shown in .
Figure 3. Correlation analyses of EDPs with other parameters in PD patients. *p < 0.05, **p < 0.01, ***p < 0.001
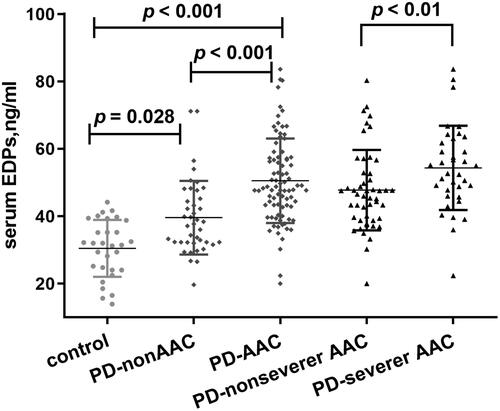
We divided all patients into two groups according to the presence or absence of AAC. 85 (67.5%) patients were in the AAC group, and 41 (32.5%) were within the non-AAC group. As shown in , patients with AAC presented higher serum EDPs than those without AAC. The AAC group also exhibited higher age, longer PD vintage and lower DBP, along with higher UA and lower albumin than non-AAC group. Prevalence of T2DM tended to be higher in AAC group, but was not significant. We found no obvious differences in mineral metabolism parameters with the exception for ALP ().
Figure 4. Comparisons of EDPs among different groups presented as a dot-plot. EDPs of PD patients with or without AAC were greatly higher than healthy controls. In all PD patients, EDPs of AAC were significantly higher than those without AAC. In patients with severer AAC, EDPs were also higher than those without severer AAC. AAC indicates abdominal aortic calcification; PD: peritoneal dialysis; EDPs, elastin-derived peptides.
Prediction values of EDPs and mineral metabolism parameters for AAC
ROC curves of EDPs and mineral bone disorder parameters showed that EDPs had the most excellent power to discriminate between PD patients with or without AAC. The area under the curve (AUC) of EDPs was 0.767, which was the largest (Online Resource 1, Figure S2).
EDPs as a predictor of AAC
To examine the association between serum EDPs and AAC in PD patients, eligible covariates with p < 0.1 in univariate regression, along with mineral metabolism parameters, total cholesterol (TC), total triglyceride (TG), and history of T2DM were incorporated into the multivariate regression model. After fully adjusted, higher EDPs (OR = 1.056), along with higher age (OR = 1.097), and longer PD vintage (OR = 1.042) were associated with greater odds of AAC in PD patients. However, UA was also a significant determinant (OR = 0.987).
ROC curve analysis showed that the cutoff point of EDPs to predict AAC was 43.22 ng/mL, and the sensitivity and specificity values were 74.1% and 70.7%. When EDPs were transferred to a categorical variate according to the cutoff point, OR of EDPs increased to 2.810 (). A combined model of age, PD vintage, UA, and EDPs yielded a significant increase in AUC when compared with other factors alone ().
Figure 5. Receiver operating characteristic curves to predict AAC in PD patients. A combined model of age, PD vintage, UA, and EDPs yielded a significant increase in AUC when compared with other factors alone (Union factor 1, AUC = 0.903). The respective AUC of other parameters including EDPs, PD vintage, age, and UA was 0.766, 0.721), 0.813), and 0.645.
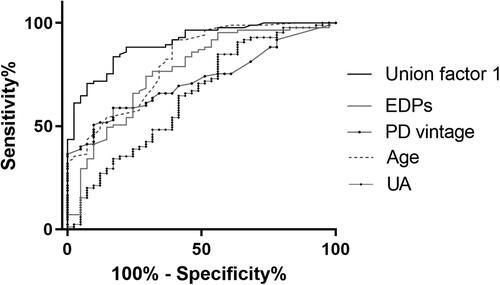
Table 2. Univariate and multivariate logistic regression analysis for effects of EDPs on AAC in PD patients.
Baseline characteristics of PD patients stratified by the severity of AAC
Of all 85 PD patients with AAC, 36 (42.35%) patients were categorized into the severe group with AAC annular > 90 degree, and 49 (57.65%) were in the nonsevere one. When compared with each other, patients with severe AAC were older with longer PD vintage and higher EDPs. Noticeably, classical risk factors of vascular calcification such as phosphate, iPTH and hs-CRP were significantly higher in severe AAC group. They also tended to have higher uremic toxicity biomarkers of β2-MG and Cr (). At the same time, the use of statin was more frequent among the severe group (Online Resource 1, Table S2).
Table 3. Comparisons of demographic data and laboratory parameters with or without severer AAC in PD patients.
EDPs as a predictor of severe AAC
As shown in , after fully adjusted, higher EDPs (OR = 1.062) was also associated with greater odds of severe AAC. The cutoff point of EDPs to predict severe AAC was 48.55 ng/mL, with a sensitivity of 72.2% and a specificity of 67.3%. When EDPs was again used in a binary fashion in the model, its association with severe AAC remained significant (OR = 6.521, ). After incorporation of age, PD vintage, phosphate, and EDPs, the AUC increased to 0.886 to predict severe AAC ().
Figure 6. Receiver operating characteristic curves to predict severe AAC in PD patients. A combined model of age, PD vintage, P, and EDPs yielded a significant increase in AUC when compared with other factors alone (Union factor 2, AUC = 0.886). The respective AUC of other parameters including EDPs, PD vintage, age, and P was 0.681, 0.856, 0.715, and 0.633.
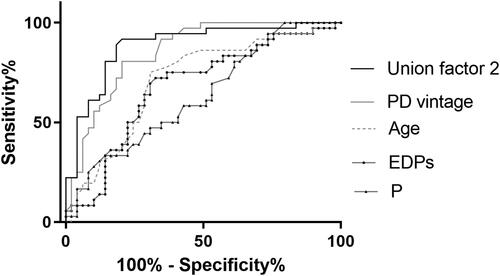
Table 4. Logistic regression analysis of EDPs on severer AAC in PD patients.
Discussion
The evaluation of cardiovascular calcification has been the focus in dialysis patients as it predicts a higher risk of CVD [Citation2]. PD patients with severe baseline AAC showed greater risk of cardiovascular events and death during follow-up compared with no or mild calcification [Citation20–22], which strengthened the importance of AAC risk stratification. In our study, AAC detected by nonenhanced MSCT is prevalent in 67.5% PD patients, higher than that evaluated by lateral lumber radiography [Citation5]. Most importantly, we highlighted the role of EDPs as a potential biomarker of AAC risk assessment in PD patients. EDPs had the greatest discriminatory power to differentiate the presence of AAC compared with mineral metabolism parameters. Its elevation was associated with higher risk of AAC and severe AAC in PD patients.
Elastin degrades irreparably with aging, and circulating concentration of EDPs increased steadily throughout the human life span [Citation23]. Patients with chronic kidney disease (CKD) experience early vascular ageing [Citation24], and higher EDPs in PD patients than in the age-matched controls were found in our study.
Elastin degradation participates in the pathogenesis of atherosclerosis and medial calcification [Citation25,Citation26], both of which coexist in CKD. EDPs in CKD patients were significantly higher than in controls and were associated with increased aortic stiffness and all-cause mortality [Citation27]. Desmosine was also found to correlate with coronary artery calcification rather than emphysema in patients with COPD [Citation28], indicating EDPs was more relevant to elastin of the vascular rather than the lungs. Apart from that, circulating EDPs were also considerably elevated in diabetes with microvascular complications such as albuminuria and retinopathy [Citation29]. Similar to those researches of strong correlation between EDPs and vascular disease, we found serum EDPs was significantly higher in PD patients with AAC, and it increased as the calcificaiton worsened. Further, elevated EDPs was a vital factor related to the risk of AAC and severe AAC in PD patients, prompting its important role in AAC risk stratification.
The strongest correlation of age with increased EDPs in PD patients of our study also suggested that elastin degradation might also be age-related, but further accelerated in those patients [Citation30–32]. Activation of elastases, such as MMPs and cathepsin, were observed in the abdominal aorta of uremic mice, along with obvious elastin loss [Citation30,Citation31]. While inhibition of the enzymes alleviated elastin loss and might attenuate vascular calcification [Citation31,Citation32]. Further activation of elastases might be a critical component in linking dialysis and the elevation of EDPs. On the other hand, immature elastogenesis might also play a role in the elevation [Citation33,Citation34]. Healthy arteries contain little tropoelastin, the dominant building block of elastic fibers. However, specific labeled molecular MRI targeting tropoelastin suggested a significant accumulation of tropoelastin in atherosclerosis progression [Citation33], which failed to transfer to mature elastin fibers [Citation6] and could be nonspecifically catalyzed by elastases [Citation34], leading to further accumulation of EDPs. However, the abnormal elastogenesis in PD patients needs further research.
Vascular calcification is such a complex process that other factors participating it should also be taken when evaluating the risk of AAC. We found that longer dialysis vintage played an important role in both AAC and severe AAC of PD patients. This was identical to several larger studies, in which duration of dialysis was related to premature AAC evaluated by lateral lumber radiography or conduit arteries medial calcification detected by ultrasound [Citation35–37]. Besides, we detected uric acid as a protective factor in AAC. Consistently, Lai et al also found higher SUA was associated with lower cardiovascular and all-cause mortality in 492 PD patients [Citation38]. While a U-shaped relationship between UA and all-cause mortality was found in a nationwide cohort study in Japan [Citation39]. The role of UA in vascular calcification in PD patients seemed to be controversial and needs further investigation.
At the same time, there exist several limitations in our study. First, this is a cross-sectional study with a small sample size. The association of EDPs and vascular calcification needs further validation in future multicenter researches. Second, we cannot figure out the causal link between EDPs and AAC progression because of a lack of following up. Finally, the model was based on limited clinical information. EDPs are biologically active and can interact with various cells and participate in physiological and pathological processes [Citation40], thus in vitro and in vivo studies are needed to elucidate its relationship with AAC in PD patients.
In conclusion, EDPs improved the risk stratification of AAC and severe AAC among PD patients and provided novel insights into EDPs on AAC as a potential indicator.
Supplemental Material
Download PDF (634.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in figshare at http://doi.org/10.6084/m9.figshare.13172660.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302(16):1782–1789.
- Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters. Kidney Int. 2017;92(1):26–36.
- Avramovski P, Avramovska M, Sotiroski K, Sikole A. Acute-phase proteins as promoters of abdominal aortic calcification in chronic dialysis patients. Saudi J Kidney Dis Transpl. 2019;30(2):376–386.
- Yao Z, Wang C, Zhang Q, Ma S, Gui B, Duan C. Prevalence of abdominal artery calcification in dialysis patients with end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. 2017;49(11):2061–2069.
- Liu ZH, for the China Dialysis Calcification Study Group, Yu XQ, Yang JW, et al. Prevalence and risk factors for vascular calcification in Chinese patients receiving dialysis: baseline results from a prospective cohort study. Curr Med Res Opin. 2018;34(8):1491–1500.
- Wahart A, Hocine T, Albrecht C, et al. Role of elastin peptides and elastin receptor complex in metabolic and cardiovascular diseases. FEBS J. 2019;286(15):2980–2993.
- Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65(2):252–256.
- Toba H, Lindsey ML. Extracellular matrix roles in cardiorenal fibrosis: potential therapeutic targets for CVD and CKD in the elderly. Pharmacol Ther. 2019;193:99–120.
- Cocciolone AJ, Hawes JZ, Staiculescu MC, Johnson EO, Murshed M, Wagenseil JE. Elastin, arterial mechanics, and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2018;315(2):H189–H205.
- Pai A, Leaf EM, El-Abbadi M, Giachelli CM. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol. 2011;178(2):764–773.
- Jegatheesan D, Cho Y, Johnson DW. Clinical studies of interventions to mitigate cardiovascular risk in peritoneal dialysis patients. Semin Nephrol. 2018;38(3):277–290.
- Chung AW, Yang HH, Kim JM, et al. Upregulation of matrix metalloproteinase-2 in the arterial vasculature contributes to stiffening and vasomotor dysfunction in patients with chronic kidney disease. Circulation. 2009;120(9):792–801.
- Nakagawa D, Zanaty M, Hudson J, et al. Plasma soluble human elastin fragments as an intra-aneurysmal localized biomarker for ruptured intracranial aneurysm. J Am Heart Assoc. 2018;7(17):e010051.
- Mordi IR, Forsythe RO, Gellatly C, et al. Plasma desmosine and abdominal aortic aneurysm disease. J Am Heart Assoc. 2019;8(20):e013743.
- Harada K, Suzuki S, Ishii H, et al. Nutrition status predicts severity of vascular calcification in non-dialyzed chronic kidney disease. Circ J. 2017;81(3):316–321.
- Hendriks EJE, de Jong PA, Beulens JWJ, et al. Annularity of aorto-iliac arterial calcification and risk of all-cause and cardiovascular mortality. JACC Cardiovasc Imaging. 2018;11(11):1718–1719.
- Wang M, Obi Y, Streja E, et al. Association of parameters of mineral bone disorder with mortality in patients on hemodialysis according to level of residual kidney function. CJASN. 2017;12(7):1118–1127.
- Labriola L, Wallemacq P, Gulbis B, Jadoul M. The impact of the assay for measuring albumin on corrected ('adjusted') calcium concentrations. Nephrol Dial Transplant. 2009;24(6):1834–1838.
- de Roij van Zuijdewijn CLM, de Haseth DE, van Dam B, et al. Role of albumin assay on calcium levels and prescription of phosphate binders in chronic hemodialysis patients. Nephron. 2018;140(3):211–217.
- Ma DH, Yan H, Yang XX, Yu ZZ, Ni ZH, Fang W. Abdominal aortic calcification score as a predictor of clinical outcome in peritoneal dialysis patients: a prospective cohort study. BMC Nephrol. 2020;21(1):151.
- Makela S, Asola M, Hadimeri H, et al. Abdominal aortic calcifications predict survival in peritoneal dialysis patients. Perit Dial Int. 2018;38(5):366–373.
- Verbeke F, CORD Study Investigators, Van Biesen W, Honkanen E, et al. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the Calcification Outcome in Renal Disease (CORD) Study. Clin J Am Soc Nephrol. 2011;6(1):153–159.
- Baydanoff S, Nicoloff G, Alexiev C. Age-related changes in the level of circulating elastin-derived peptides in serum from normal and atherosclerotic subjects. Atherosclerosis. 1987;66(1-2):163–168.
- Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82(4):388–400.
- Van den Bergh G, Opdebeeck B, D'Haese PC, Verhulst A. The vicious cycle of arterial stiffness and arterial media calcification. Trends Mol Med. 2019;25(12):1133–1146.
- Mohindra R, Agrawal DK, Thankam FG. Altered vascular extracellular matrix in the pathogenesis of atherosclerosis. J Cardiovasc Transl. 2021. DOI:10.1007/s12265-020-10091-8.
- Smith ER, Tomlinson LA, Ford ML, et al. Elastin degradation is associated with progressive aortic stiffening and all-cause mortality in predialysis chronic kidney disease. Hypertension. 2012;59(5):973–978.
- Rabinovich RA, Miller BE, Wrobel K, et al. Circulating desmosine levels do not predict emphysema progression but are associated with cardiovascular risk and mortality in COPD. Eur Respir J. 2016;47(5):1365–1373.
- Nikolov A, Tsinlikov I, Nicoloff G, et al. Abnormal levels of age-elastin derived peptides in sera of diabetic patients with arterial hypertension. CEJOI. 2014;39 (3):345–351.
- Zhou YB, Zhou H, Li L, et al. Hydrogen sulfide prevents elastin loss and attenuates calcification induced by high glucose in smooth muscle cells through suppression of Stat3/Cathepsin S signaling pathway. Int J Mol Sci. 2019;20(17):4202.
- Hecht E, Freise C, Websky KV, et al. The matrix metalloproteinases 2 and 9 initiate uraemic vascular calcifications. Nephrol Dial Transplant. 2016;31(5):789–797.
- Figueiredo JL, Aikawa M, Zheng C, et al. Selective cathepsin S inhibition attenuates atherosclerosis in apolipoprotein E-deficient mice with chronic renal disease. Am J Pathol. 2015;185(4):1156–1166.
- Phinikaridou A, Lacerda S, Lavin B, et al. Tropoelastin: A novel marker for plaque progression and instability. Circ Cardiovasc Imaging. 2018;11(8):e007303.
- Miekus N, Luise C, Sippl W, Baczek T, Schmelzer CEH, Heinz A. MMP-14 degrades tropoelastin and elastin. Biochimie. 2019;165:32–39.
- Honkanen E, Kauppila L, Wikstrom B, et al. Abdominal aortic calcification in dialysis patients: results of the CORD study. Nephrol Dial Transpl. 2008;23(12):4009–4015.
- Chen HC, Chou CY, Lin HJ, Huang CC, Chang CT. Abdominal aortic calcification score predicts the occurrence of coronary artery disease in middle-aged peritoneal dialysis patients. Nephrology (Carlton). 2019;24(3):336–340.
- Coll B, Betriu A, Martinez-Alonso M, et al. Large artery calcification on dialysis patients is located in the intima and related to atherosclerosis. Clin J Am Soc Nephrol. 2011;6(2):303–310.
- Lai KJ, Kor CT, Hsieh YP. An inverse relationship between hyperuricemia and mortality in patients undergoing continuous ambulatory peritoneal dialysis. J Clin Med. 2018;7(11):416.
- Sugano N, Maruyama Y, Ohno I, et al. Effect of uric acid levels on mortality in Japanese peritoneal dialysis patients. Perit Dial Int. 2021;41(3):320–327. .
- Le Page A, Khalil A, Vermette P, et al. The role of elastin-derived peptides in human physiology and diseases. Matrix Biol. 2019;84:81–96.