Abstract
Background
The role of indoxyl sulfate (IS), an important protein-bound uremic toxin, in arterial stiffness (AS) in patients with chronic kidney disease (CKD) is unclear.
Materials and methods
We investigated the association between serum IS levels and AS in a cross-sectional study of 155 patients with CKD. Patients in the AS group was defined as carotid-femoral pulse wave velocity (cfPWV) value >10 m/s measured by a validated tonometry system (SphygmoCor), while values ≤10 m/s were regarded as without AS group Serum IS was measured by liquid chromatography–mass spectrometry analysis.
Results
Of these CKD patients, AS was present in 51 (32.9%) patients, who were older, had a higher rate of diabetes, higher systolic blood pressure (SBP), and higher IS levels compared to those without AS. By multivariable logistic regression analysis, IS (adjusted odds ratio [aOR] 1.436, 95% confidence interval [CI] 1.085–1.901, p = 0.011), age (aOR 1.058, 95% CI 1.021–1.097, p = 0.002), and SBP (aOR 1.019, 95%CI 1.000–1.038, p = 0.049) were independent predictors of AS. By multivariable stepwise linear regression analysis, logarithmically transformed IS, age, DM, and SBP were significantly correlated with cfPWV. The area under the receiver-operating characteristic curve for serum log-IS was 0.677 (95%CI 0.598–0.750, p = 0.0001) to predict the development of AS in patients with CKD.
Conclusion
These finding demonstrate that in addition to older and higher SBP, a high serum IS level is a significant biomarker associated with AS in patients with CKD.
Introduction
The incidence of cardiovascular disease (CVD) is significantly higher in patients with chronic kidney disease (CKD) than in those without CKD. CVD in patients with CKD is associated with poor morbidity and mortality due to traditional risk factors including age, hypertension, and diabetes mellitus (DM) as well as CKD-specific factors such as arterial stiffness (AS) [Citation1,Citation2]. AS is caused by irreversible changes to the vascular structure, including dysregulation of elastin and collagen due to oxidative stress and inflammation, which result in impaired perfusion of vital organs [Citation3,Citation4]. Several studies have identified age, hypertension, DM, and abnormal renal function as risk factors for AS [Citation3,Citation5–10]. Importantly, indoxyl sulfate (IS), originally identified as a gut-derived, protein-bound uremic toxin, accumulates with declining renal function and contributes to the deterioration of kidney function through several mechanisms. For example, several lines of evidence from in vitro and in vivo studies have demonstrated that IS induces renal tubular damage and tubulointerstitial fibrosis through oxidative stress, inflammation, and trans-differentiation of renal tubular cells via the upregulation of intra-renal renin-angiotensin-aldosterone or mitogen-activated protein kinase pathway, which culminate in renal fibrosis [Citation11–13]. In addition, IS can augment oxidative stress, increase endothelial microparticle generation, impair endothelial cell repair, and induce vascular smooth muscle cell proliferation, ultimately resulting in aortic calcification and AS, which can lead to increased overall and cardiovascular mortality in patients with CKD [Citation14].
While IS may play a role in vascular dysfunction and can induce further cardiovascular events in patients with CKD, there are no reliable circulating biomarkers for vascular dysfunction. Therefore, we aimed to identify the predictors for the development of AS in patients with CKD and specifically examined the potential association between serum IS levels and AS in these patients.
Materials and methods
Participants
In this cross-sectional study conducted at a single hospital. After the approval from The Protection of the Human Subjects Institutional Review Board of Tzu-Chi University and Hospital (IRB108-96-B) and receiving the informed consents from all patients from January and December 2016, there were 155 out of 180 CKD patients enrolled because we excluded 25 patients due to missing data (15), acute infection (5), acute myocardial infarction or heart failure (4) and malignancy (1). Hypertension and DM were diagnosed according to SBP or DBP ≥ 140/90 mmHg or fasting plasma glucose level of ≥126 mg/dL as well as usage of relevant medications. Body mass index was measured as (BW)(kg)/(BH) [Citation2](m2).
Biochemical analysis
After obtaining blood samples, serum biochemical analysis was examined by an auto-analyzer (Siemens Advia 1800, Siemens Healthcare, Henkestr, Germany). The estimated GFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation [Citation1]: 141 X min(Scr/κ, 1) α X max(Scr/κ, 1)−1.209 × 0.993Age X 1.018 [if female] X 1.159 [if African American]; where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1. Patients were staged according to 2012 Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline [Citation1].
Determination of serum total is levels
Like our previous study, a Waters e2695 high-performance liquid chromatography system comprising a single quadrupole mass spectrometer with proper performance as well as adequate relative standard deviation of intra- and inter-day precision (within ±15%) was used to measure serum total IS levels (ACQUITY QDa, Waters Corporation, Milford, MA, USA) [Citation15,Citation16].
Measurement of carotid-femoral pulse wave velocity
AS previously studies [Citation17–19], we used an applanation tonometer (SphygmoCor, AtCor Medical, Australia) to calculate carotid-femoral pulse wave velocity (cfPWV) by recording the pulse waves of carotid and femoral arteries in series synchronously with an electrocardiogram as a timing reference. Calculation of cfPWV was as distance divided by transit time of propagation wave between sternal notch (N) and femoral artery (F) and sternal notch and carotid artery (C): ΔD/Δt = Distance (N – F) – Distance (N – C) /time interval (N-F) – time interval (N-C). Patients with or without AS were defined according to cfPWV >10 m/s or ≤10 m/s, individually [Citation20].
Statistical analysis
Continuous variables were analyzed by Student’s independent t test or the Mann-Whitney U test (two-tailed) and expressed as means ± standard deviation or medians with IQR according to the Kolmogorov–Smirnov test. Categorical variables were analyzed by chi-square test and expressed as numbers and percentage. Values of IS in different stage of CKD was analyzed by one-way analysis of variance. Log-transformed those skewed continuous variable were applied to achieve normal distribution and were used for linear regression analysis. To explore the correlation between variables and cfPWV and possible probabilities for the diagnosis of AS of CKD patients, multivariate linear and logistic regression analyses were applied. Additionally, the relationship between eGFR and IS values was analyzed by linear correlation analysis. To determine the optimal serum IS value to predict AS in CKD patient, a receiver-operating characteristic curve (ROC) analysis was used to calculate the area under the curve (AUC). Data were analyzed using the SPSS for Windows software (version 19.0; SPSS, Chicago, IL, USA).
Results
shows the clinical characteristics of the study cohort of 155 patients with CKD. The cohort comprised 66 (42.6%) and 122 (78.7%) patients with DM and hypertension, respectively. There were 51 (32.9%) patients who were diagnosed to have AS. Compared to patients without AS, patients with AS were older and had more DM (p < 0.001 and = 0.030, respectively). Additionally, the patients in the AS group had higher systolic blood pressure (p = 0.004), higher IS level (1.53 μg/mL [interquartile range (IQR) 0.70–3.68 μg/mL] vs. 0.88 μg/mL [IQR 0.43–1.76 μg/mL], p < 0.001), and lower estimated glomerular filtration rate (p = 0.014) compared to those without AS. There were no significant differences in sex, rate of hypertension, and use of medications between the two patient groups.
Table 1. Baseline characteristics of CKD patients with or without AS.
Multivariable logistic regression analysis performed after adjusting for factors including age, DM, SBP, eGFR, overweight, obesity, fasting glucose and IS, IS (adjusted odds ratio [aOR] 1.436, 95% confidence interval [CI] 1.085–1.901, p = 0.011), SBP (aOR 1.019, 95% CI 1.000–1.038, p = 0.049), and age (OR 1.058, 95% CI 1.021–1.097, p = 0.002) were significant and independent predictive factors for AS ().
Table 2. Factors predicted the development of AS among CKD patients.
By simple linear regression analysis, carotid-femoral pulse wave velocity (cfPWV) was significantly and positively correlated with age, DM, SBP, logarithmically transformed (log)-IS (log-IS), log-creatinine, and log-blood urea nitrogen (BUN) and was negatively correlated with eGFR. The multivariable stepwise linear regression analysis performed after adjusting for factors including DM, age, SBP, eGFR, and log-IS, cfPWV was significantly correlated with age (β = 0.267, adjusted R2 change = 0.052, p < 0.001), SBP (β = 0.283, adjusted R2 change = 0.094, p < 0.001), DM (β = 0.177, adjusted R2 change = 0.026, p = 0.011), and log-IS level (β = 0.217, adjusted R2 change = 0.133, p = 0.003) in patients with CKD ().
Table 3. Correlation between cfPWV and clinical variables among the CKD patients.
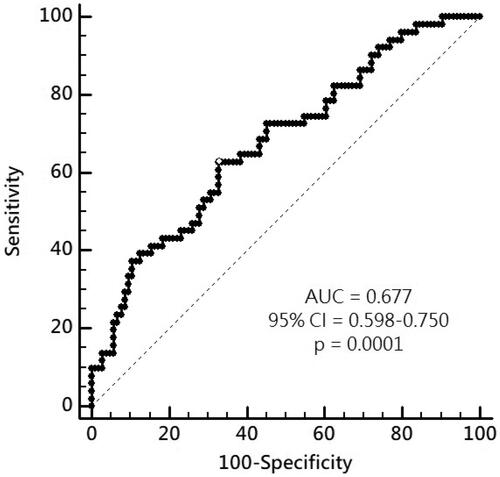
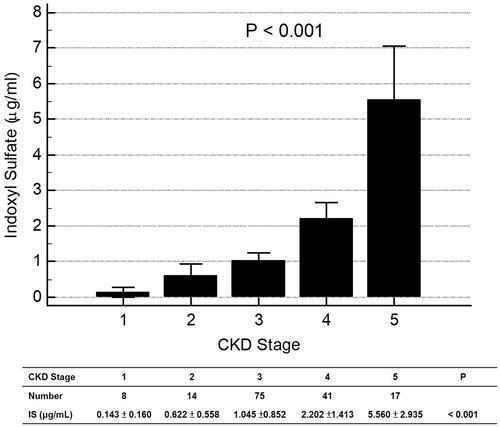
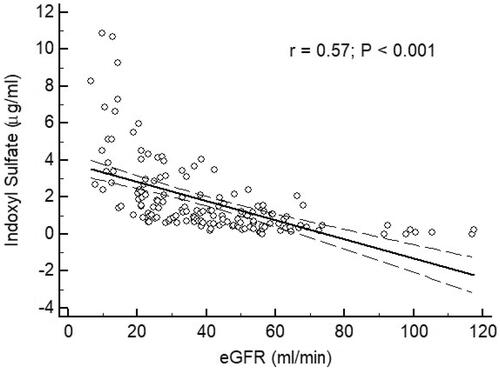
By analysis of ROC curve, the optimal value of IS to predict AS was 1.227 μg/mL (Youden index 0.3005) with AUC as 0.677 (95%CI 0.598–0.750, p = 0.0001), with a sensitivity of 62.75% and specificity of 67.31% (). showed progressively significant higher values of IS as renal function worsened. In addition, there was significant negative correlation between values of IS and eGFR of patients ().
Discussion
In this cross-sectional study including 155 patients with CKD, we found that, in addition to old age and hypertension, higher serum IS levels were also positively associated with cfPWV, suggesting its utility as a significant predictor of AS in patients with CKD.
AS is caused by irreversible anatomical changes in vascular wall structures, accompanied with functional abnormalities and increased mechanical strain to low-impedance circulation to vital organs, which subsequently result in renal dysfunction, CVD, and mortality [Citation3,Citation4]. A study of 16,867 European subjects revealed that cfPWV at any age was associated with BP elevation, which indicated that high BP mutually contributed to increased cfPWV [Citation5]. Aging can indeed lead to marked changes in the mechanical and geographic characteristics of the vasculature. However, aged-related changes in stiffness gradient is accelerated in patients with end-stage renal disease (ESRD) and studies have shown a significantly steeper correlation between age and aortic pulse wave velocity (PWV) in association with mechanisms leading to elastin fragmentation and calcification of the medial layer [Citation3,Citation6]. Moreover, studies demonstrated a negative association between renal function and PWV in patients with coronary arterial disease and stage 3 or 4 CKD [Citation7,Citation8]. On the other hand, a meta-analysis revealed that every PWV standard deviation increased was associated with 8% higher risk of incident CKD [Citation21], indicating that vascular stiffness might lead to renal dysfunction. The decreased vascular lumen diameter with the progression of vascular stiffness results in increased pulse pressure and SBP as well as decreased DBP, along with microvascular dysfunction and increased all-cause and cardiovascular mortality [Citation22]. In patients with hypertension with normal renal function, DM with normal renal function, or end stage renal disease patients, studies reported that both BP and age could positively predict cfPWV [Citation17,Citation23,Citation24]. Moreover, in a systematic review on risk factors associated with cfPWV, Cecelja et al. found that age and BP were associated with cfPWV in 91% and 90% of the analyzed studies, respectively; however, only 52% of the studies reported a positive association between DM and cfPWV [Citation9]. In addition, other studies showed that poor glucose tolerance, higher advanced glycation end products, and DM duration were independently associated with central AS, independent of age, sex, and BP [Citation25–27]. Consistent with these studies, we found that old age, higher SBP, impaired renal function, and DM were associated with higher cfPWV. Furthermore, after adjusting for these factors, old age and SBP remained significant predictors of AS in patients with CKD.
IS, which is metabolized in the liver from indole, a product of tryptophan that is produced by intestinal bacterial flora, accumulates with the deterioration of renal function and contributes to the progression of CKD [Citation28,Citation29]; IS is also a marker of poor long-term outcomes in patients with CKD and ESRD [Citation29,Citation30]. Moreover, eGFR correlated negatively with PWV in CAD and CKD patients [Citation7,Citation8], IS was as well to induce dysfunction of vascular endothelial and smooth muscle cells [Citation31–33]. In vitro studies demonstrated that endothelial cells incubated with IS exhibited impaired proliferation, wound repair, nitric oxide production, and increased cell senescence and oxidative stress, indicating the adverse effects of IS on endothelial function [Citation32,Citation34]. In addition, IS increased the production of reactive oxygen species, as evidenced by increased expression levels of superoxide and peroxynitrite, and reduced the production of nitric oxide through the induction of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the inhibition of glutathione, ultimately leading to endothelial cell dysfunction [Citation31,Citation34]. Furthermore, in vitro and in vivo studies demonstrated that IS-treated human aortic smooth muscle cells could promote aortic calcification and aortic wall thickening and enhance the expression of osteoblast-specific proteins, senescence, and calcification due to oxidative stress resulting from NADPH oxidase upregulation [Citation35,Citation36]. In studies of patients with CKD, baseline IS levels were negatively correlated with vascular reactivity index [Citation15] and positively correlated with cfPWV and aortic calcification; these studies also found that IS was a predictor for overall and cardiovascular mortality [Citation30]. Importantly, an oral sorbent was reported to reduce serum IS levels and oxidative stress in vitro [Citation36]. The same oral sorbent, AST-120, was also demonstrated to reduce PWV and increase flow-mediated endothelium-dependent dilatation in patients with CKD not undergoing dialysis; the underlying mechanism included the inhibition of NADPH oxidase and/or mitochondrial respiration, leading to reduced production of reactive oxygen species [Citation33,Citation37,Citation38]. Collectively, these studies suggest that IS-induced oxidative stress is detrimental to vascular structures through the altered functions of endothelial and smooth muscle cells. In the present study, we found that serum IS levels were positively correlated with cfPWV and that serum IS was a significant predictor for the development of AS; these findings highlight the potentially crucial role of IS in the development of AS by mechanisms including the derangement of pro- and anti-oxidative systems in vascular structures. Further investigation is warranted to elucidate the detailed mechanisms underlying these effects.
The present study has several limitations. First, this was a cross-sectional, single-center study including a small number of patients with CKD. Second, inflammatory markers such as C-reactive protein did not measured. Third, differences in dietary habits as well as dietary supplements can impact the colonic microbiome, which can result in fluctuations of serum IS levels; Fourth, the detailed mechanisms of IS to influence the process of AS could not be clearly deduced without in vitro studies. Therefore, the causal relationship between serum IS levels and AS should be evaluated with controlled dietary and nutritional supplement intake in prospective studies of larger patient cohorts and in vitro studies to clarify the harmful effects of IS on the vasculature.
In the present study, we demonstrated that, in addition to old age and elevated SBP, serum IS might also be a potential biomarker associated with AS in patients with CKD, indicating that IS might mediate the progression of AS; however, the underlying mechanisms require further investigation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830.
- Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87(7):E10–E17.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327.
- Karras A, Haymann JP, Bozec E, et al. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60(6):1451–1457.
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338–2350.
- London GM, Safar ME, Pannier B. Aortic aging in ESRD: structural, hemodynamic, and mortality implications. JASN. 2016;27(6):1837–1846.
- Ilyas B, Dhaun N, Markie D, et al. Renal function is associated with arterial stiffness and predicts outcome in patients with coronary artery disease. QJM. 2008;102(3):183–191.
- Ford ML, Tomlinson LA, Chapman TP, et al. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010;55(5):1110–1115.
- Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54(6):1328–1336.
- Yapei Y, Xiaoyan R, Sha Z, et al. Clinical significance of arterial stiffness and thickness biomarkers in type 2 diabetes mellitus: an Up-To-date meta-analysis. Med Sci Monit. 2015;21:2467–2475.
- Milanesi S, Garibaldi S, Saio M, et al. Indoxyl sulfate induces renal fibroblast activation through a targetable heat shock protein 90-dependent pathway. Oxid Med Cell Longevity. 2019;2019:1–11.
- Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One. 2012;7(3):e34026.
- Kim SH, Yu MA, Ryu ES, et al. Indoxyl sulfate-induced epithelial-to-mesenchymal transition and apoptosis of renal tubular cells as novel mechanisms of progression of renal disease. Lab Invest. 2012;92(4):488–498.
- Vanholder R, Schepers E, Pletinck A, et al. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. JASN. 2014;25(9):1897–1907.
- Wang CH, Lai YH, Kuo CH, et al. Association between serum indoxyl sulfate levels and endothelial function in non-dialysis chronic kidney disease. Toxins. 2019;11(10):589.
- Shu C, Chen X, Xia T, et al. LC-MS/MS method for simultaneous determination of serum p-cresyl sulfate and indoxyl sulfate in patients undergoing peritoneal dialysis. Biomed Chromatogr. 2016;30(11):1782–1788.
- Hou JS, Wang CH, Lai YH, et al. Serum malondialdehyde-modified low-density lipoprotein is a risk factor for central arterial stiffness in maintenance hemodialysis patients. Nutrients. 2020;12(7):2160.
- Lai YH, Wang CH, Kuo CH, et al. Serum P-cresyl sulfate is a predictor of central arterial stiffness in patients on maintenance hemodialysis. Toxins. 2019;12(1):10.
- Hsieh YJ, Hsu BG, Lai YH, et al. Association of low serum L-carnitine levels with aortic stiffness in patients with non-dialysis chronic kidney disease. Nutrients. 2020;12(10):2918.
- Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448.
- Sedaghat S, Mattace-Raso FU, Hoorn EJ, et al. Arterial stiffness and decline in kidney function. CJASN. 2015;10(12):2190–2197.
- Laurent S, Boutouyrie P. Arterial stiffness: a new surrogate end point for cardiovascular disease? J Nephrol. 2007;20 (Suppl 12):S45–S50.
- Ramirez AJ, Christen AI, Sanchez RA. Serum uric acid elevation is associated to arterial stiffness in hypertensive patients with metabolic disturbances. CHYR. 2018;14(2):154–160.
- Levisianou D, Melidonis A, Adamopoulou E, et al. Impact of the metabolic syndrome and its components combinations on arterial stiffness in Type 2 diabetic men. Int Angiol. 2009;28(6):490–495.
- Schram MT, Henry RM, van Dijk RA, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43(2):176–181.
- Semba RD, Najjar SS, Sun K, Lakatta EG, et al. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am J Hypertens. 2009;22(1):74–79.
- Agnoletti D, Mansour AS, Zhang Y, et al. Clinical interaction between diabetes duration and aortic stiffness in type 2 diabetes mellitus. J Hum Hypertens. 2017;31(3):189–194.
- Wu IW, Hsu KH, Lee CC, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26(3):938–947.
- Lin CJ, Liu HL, Pan CF, et al. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch Med Res. 2012;43(6):451–456.
- Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. CJASN. 2009;4(10):1551–1558.
- Tumur Z, Niwa T. Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells. Am J Nephrol. 2009;29(6):551–557.
- Dou L, Bertrand E, Cerini C, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65(2):442–451.
- Yu M, Kim YJ, Kang DH. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. CJASN. 2011;6(1):30–39.
- Dou L, Jourde-Chiche N, Faure V, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007;5(6):1302–1308.
- Muteliefu G, Enomoto A, Jiang P, et al. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol Dial Transplant. 2009;24(7):2051–2058.
- Muteliefu G, Shimizu H, Enomoto A, et al. Indoxyl sulfate promotes vascular smooth muscle cell senescence with upregulation of p53, p21, and prelamin A through oxidative stress. Am J Physiol. 2012;303(2):C126–C134.
- Niwa T, Nomura T, Sugiyama S, et al. The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kid Int Suppl. 1997;62:S23–S28.
- Nakamura T, Kawagoe Y, Matsuda T, et al. Oral ADSORBENT AST-120 decreases carotid intima-media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press Res. 2004;27(2):121–126.