Abstract
Background
Gut dysbiosis may be implicated in the pathogenesis of IgA nephropathy (IgAN) through immune and/or metabolite pathways. Fecal microbiota transplantation (FMT) could reestablish the micro-ecological balance in IgAN, although this has never been attempted before. We explored whether FMT could be efficacious in treating IgAN in two patients with refractory IgAN.
Case presentation
Two Chinese female patients with IgAN failed to achieve clinical remission after receiving several rounds of immunosuppressive therapy and suffered from unbearable adverse effects due to immunosuppressants. Both patients received intensive fresh FMT conducted through transendoscopic enteral tubing (TET) regularly for 6–7 months, and were followed up for a further 6 months. Partial clinical remission was achieved in both patients, evidenced by a decrease in the 24-h urinary protein (24-hUP) to less than half of baseline during FMT treatment or follow-up, along with increased serum albumin (sAlb) and stable kidney function. The gut microbiota of both patients was distorted with lower biodiversity and altered composition, which was reversed following FMT. Phylum Proteobacteria decreased while genus Prevotella increased during and after FMT. The intensive fresh FMT was well-tolerated, and no severe adverse events occurred.
Conclusions
Preliminary evidence of the safety and efficacy of FMT for treating refractory IgAN may provide a new direction by which to decipher the pathogenesis of IgAN.
Introduction
IgA nephropathy (IgAN) is the most common primary glomerular disease with an annual worldwide incidence of 25 per 100,000 populations [Citation1]. The current dominant theory of IgAN pathogenesis is that immune complexes characterized bygalactose-deficient IgA1 (GdIgA1) are deposited in the glomerular mesangial area, causing kidney impairment [Citation2]. Increasing evidence indicates that gut dysbiosis plays a role in IgAN. Animal experiments showed that GdIgA1 production depended on the gut commensal microbiota [Citation3]. Genetic studies revealed that risk alleles associated with IgAN were also implicated in dysfunction of the gut barrier and regulation of the mucosal immune response to pathogens [Citation4]. Budesonide released in the distal ileum decreased proteinuria of IgAN patients, attributed to restoration of intestinal mucosal immunity [Citation5]. Lubiprostone also alleviated proteinuria and kidney dysfunction in two patients with IgAN [Citation6], attributed to manipulation of gut dysbiosis and reductions in uremic toxins [Citation7]. Fecal microbiota transplantation (FMT) has been explored in the management of some dysbiosis-related diseases with relatively high safety and efficacy [Citation8]. However, the application of FMT in treating IgAN has not been reported before. We administered intensive fresh FMT to two patients with refractory IgAN and gut dysbiosis. Informed consent was obtained from each patient. The treatment was approved by the ethics committee for clinical trials of the Xijing Hospital of the Fourth Military Medical University in China (KY20172080-1).
Case presentation
Case 1
Patient A was a 48-year-old woman who was referred to the Department of Nephrology of Xijing hospital due to renal dysfunction and persistent heavy proteinuria. Examination revealed: body height 165 cm, weight 60 kg, blood pressure 135/72 mmHg, 24-h urinary protein (24-hUP) 2605 mg, serum creatinine (sCr) 106 µmol/L, serum albumin (sAlb) 32.8 g/L, and estimated glomerular filtration ratio (eGFR) 51.0 mL/min/1.73 m2. Kidney biopsy revealed IgAN (Lee III, M1E1S1T0-C1). She initially in 1997 presented with gross hematuria, but did not receive any intervention. In July 2006, she was prescribed benazepril hydrochloride (gradually increased to maximum tolerated dose) when 24-hUP reached 2300 mg and urine occult blood (2+). However, edema occurred intermittently in both lower extremities and eyelids. She was not willing to administer glucocorticoid when she was awake to the potential adverse effect. In May 2017, she received tacrolimus (2 mg, b.i.d.) and valsartan amlodipine (one tablet, q.d.). Tacrolimus was reduced to 1 mg (b.i.d.) on 1 July 2017 and discontinued on 11 February 2018 due to severe hand tremor. Unfortunately, proteinuria was maintained around 2089 mg, and sCr increased to 128 µmol/L. She then received mycophenolate mofetil (MMF; 0.5 g, b.i.d.) from 26 April 2018. MMF was discontinued after 1.5 months due to intense gastro-intestinal discomfort. At that time, tests showed 24-hUP 2020 mg and urine occult blood (4+). Considering the poor response and intolerance to immunosuppressive drugs (Supplemental Digital Content, Table S1), we defined her condition as refractory, and treated her with intensive fresh FMT after a month of wash-out.
FMT was performed 40 times consecutively (200 mL daily, 5 d/week) according to the protocol reported by Sudarshan Paramsothy et al. [Citation9], and then a further 57 times (200 mL daily, 10–15 d/month) over the next 5 months. Valsartan amlodipine was administered during FMT treatment and follow-up. Two healthy young men were selected to donate fresh feces; the findings of medical history, physical examination, and laboratory test of the two donors are described in Supplemental Digital Content Table S2. The procedure of FMT is shown in Supplemental Digital Content File 1. The patient was followed for six months after ceasing treatment (). The 24-hUP showed a slow downward trend during and after FMT, decreasing by 37.0% after 97 FMTs and to less than half of baseline three months after FMT, achieving partial clinical remission. The sAlb increased by 21.9 and 32.6% after treatment and follow-up compared to the baseline, respectively, supporting the reduction of protein loss. The sCr and eGFR fluctuated around the baseline, suggesting stable kidney function during and after the treatment process (, ).
Figure 1. Treatment timeline, Alpha diversity, Beta diversity, and 24-h urinary protein of patient A before, during, and post FMT. (A) Treatment timeline of patient A. The timeline shows the major clinical events during the treatment process of patient A. M: month; FK506: tacrolimus; MMF: mycophenolate mofetil; FMT: fecal microbiota transplantation. (B) Alpha diversity (Chao index), which refers to the diversity within a particular region or ecosystem and is a comprehensive indicator of richness and evenness of gut microbiota in patient A. Black dots represent different samples collected from patient A before (A_Pre), during (A_FMT), and post FMT (A_Post). (C) Beta diversity (PCOA index), which reflects the significant microbial community difference between gut microbiota of samples from patient A. Red dots represent samples from donors; Samples collected from patient A before (A_Pre, blue square), during (A_FMT, green triangle), and post FMT (A_Post, yellow diamond). (D) 24-h urinary protein of patient A before, during, and post FMT. Red solid line indicates the trend.
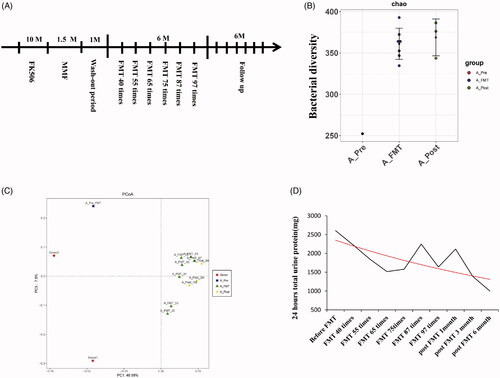
Table 1. Clinical parameters of patient A and B before, during, and post FMT.
The intestinal biodiversity of patient A was distorted, as evidenced by low alpha diversity [; Supplemental Digital Content (Tables S3 and S6 and Figure S1)] and distinct beta diversity (), which were reversed during and after FMT. Additionally, Phylum Proteobacteria was found with decreasing abundance, while genus Prevotella with increasing abundance, during FMT (Supplemental Digital Content, and SCitation3). The patient developed red papules on the limbs and body (skin biopsy showed spongioticdermatitis), and was infected with influenza A virus during hospitalization for FMT, and recovered after appropriate treatment.
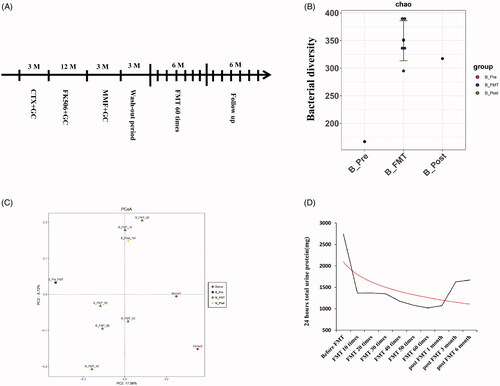
Figure 2. Treatment timeline, Alpha diversity, Beta diversity, and 24-h urinary protein of patient B before, during, and post FMT. (A) Treatment timeline of patient B. The timeline shows the major clinical events during the treatment process of patient B. M: month; CTX: cyclophosphamide; FK506: tacrolimus; MMF: mycophenolatemofetil; FMT: fecal microbiota transplantation. (B) Alpha diversity (Chao index) of gut microbiota in patient B. Black dots represent different samples collected from patient B before (B_Pre), during (B_FMT), and post FMT (B_Post). (C) Beta diversity (PCOA index) of gut microbiota in patient B. Red dots represent samples from donors; Samples collected from patient B before (B_Pre, blue square), during (B_FMT, green triangle), and post FMT (B_Post, yellow diamond). (D) 24-h urinary protein of patient B before, during, and post FMT. A Red solid line indicates the trend.
Case 2
Patient B was a 32-year-old woman who was referred to our center due to persistent heavy proteinuria. Examination revealed: body height 165 cm, weight 55 kg, blood pressure 110/65 mmHg, 24-hUP 1320 mg, sCr 100 µmol/L, sAlb 37.8 g/L, and eGFR 59.2 mL/min/1.73 m2. She initially noted foamy urine in April 2016, and underwent kidney biopsy with a diagnosis of IgAN (Lee III) in her local hospital, when tests showed 24-hUP 3614.7 mg, urine occult blood (+), sCr 61 µmol/L, and sAlb 33.6 g/L. She received prednisone (60 mg, q.d.), cyclophosphamide (intravenous, 1 g every 4 weeks), and candesartan (4 mg, q.d.) for 3 months. However, 24-hUP was not controlled and fluctuated between 4277.8 and 6320.8 mg. She then received tacrolimus (2 mg, b.i.d), prednisone, and irbesartan (150 mg, q.d; gradually increased to maximum tolerated dose) for 12 months. The 24-hUP decreased to 840 mg in August 2017, but never dropped further. Meanwhile, she developed severe tremors and alopecia. In March 2018, she was prescribed MMF (0.75 g, b.i.d) for 3 months. Unfortunately, the 24-hUP still did not decrease, sCr increased to 100 µmol/L, and sAlb deceased to 37.8 g/L. Immunosuppressive therapy failed to further improve her condition which was considered as refractory IgAN (Supplemental Digital Content, Table S4). The above therapy was discontinued for three months by which time 24-hUP increased to 2490 mg, and sAlb deceased to 32.6 g/L. We treated her with intensive fresh FMT, 60 treatments in 6 months (200 mL daily, 10–15 d/month), and followed up for 6 months (). Valsartan was prescribed during the FMT treatment and follow-up.
The 24-hUP presented a fast downward trend during FMT, decreasing to less than half of baseline after 10 FMTs and by 62.8% after 60 FMTs, achieving partial clinical remission which was maintained until the third month of follow-up. Meanwhile, sAlb increased by 19.6 and 29.1% after treatment and follow-up, and there was evidence of stable kidney function (; ).
Alteration of parameters of gut microbiota before and after FMT was similar to that of patient A, with the addition of decreasing Verrucomicrobia during FMT [; Supplemental Digital Content (Tables S5 and S6 and Figures S1–S3)]. The patient developed diarrhea once daily for three non-consecutive days within 4 h after receiving FMT, without further progression. She reported temporary but severe discomfort when deploying the transendoscopic enteral tube through colonoscopy each time, and alternatively chose retention enema on the sixth occasion.
Discussion and conclusions
To our knowledge, this is the first report of the management of IgAN with FMT. Two patients, who failed to achieve clinical remission after receiving several rounds of immunosuppressive therapy and experienced drug intolerance, reached partial clinical remission along with stable kidney function after receiving intensive fresh FMT for 6–7 months. The intensive fresh FMT was well tolerated and neither patient experienced severe adverse events. Despite the different patterns of remission, these findings may indicate encouraging efficacy of FMT compared to immunosuppressive strategies in patients with refractory IgAN. The gut dysbiosis present in both patients, possibly attributable to the disease or its drug treatment, was restored by intensive FMT with increased diversity and a more balanced composition compared to baseline. The patients received different FMT regimens with the aim of exploring the optimum dose, a long process of FMT may have a more stable efficacy, which may provide a basic notion for the later clinical trial and needs further verification. The longer treatment period was well-tolerated except for discomfort when deploying the transendoscopic enteral tube through colonoscopy each time, which raised the need for a more comfortable approach. Patient A developed pityriasisrosea during treatment, which has not been reported before and may be a negative response to FMT on systemic immunity.
FMT is effective and safe for restoring a diverse and balanced microbiome profile, and has been applied in the management of several dysbiosis-related diseases. Therefore, it is feasible that FMT could be safe and effective for treating IgAN in patients with evidence of gut dysbiosis. An abundance of phylum Proteobacteria and a reduced abundance of genus Prevotella observed prior to FMT in both patients is consistent with the characteristics of gut microbiota in patients with chronic kidney disease [Citation10]. Altered gut microbiota profiles in IgAN patients have been documented previously and Prevotella sp [Citation11], Prevotella copri [Citation11], and Prevotella_9 [Citation12] were significantly decreased, and the reversal of this imbalance during FMT to some extent supports our findings. Interestingly, Proteobacteria was also expanded in the blood microbiome of patients with chronic kidney disease [Citation13], providing evidence for bacterial translocation through a ‘leaking’ gut barrier. Sputum Proteobacteria was also associated with bronchiectasis severity [Citation14]. Gamma-Proteobacteria was enriched in newborn mice and depleted in normal adult microbiota, which was regulated by a gamma-Proteobacteria-specific IgA response. A higher abundance of gamma-Proteobacteria was associated with sustained intestinal inflammation [Citation15]. However, gut dysbiosis and IgAN may interact as both cause and effect, and the mechanism by which these altered bacterial taxa or micro-ecological imbalance play a role in the progression of IgAN warrants further investigation.
We did not examine alterations in the intestinal mucosal immune system and uremia profile before and after FMT, and at this stage cannot identify whether FMT improved the abnormal mucosal immune system or mitigated the accumulation of toxins. However, a pilot study to further explore the efficacy of FMT in IgAN is underway.
Ethical approval
The treatment was approved by the ethics committee for clinical trials of the Xijing Hospital of the Fourth Military Medical University in China, and conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The two patients have given their written informed consent to publish their cases.
Author contributions
Data curation: Jin Zhao and Ming Bai; Project administration: Shiren Sun; Methodology: Jin Zhao, Ming Bai, Xiaoxia Yang, Yang Wang, and Rong Li; Writing – original draft: Jin Zhao; Writing – review and editing: Jin Zhao, Ming Bai, and Shiren Sun.
| Abbreviations | ||
| IgAN | = | IgA nephropathy |
| 24-hUP | = | 24-hour urinary protein |
| FMT | = | Fecal microbiota transplantation |
| TET | = | transendoscopic enteral tubing |
| sCr | = | serum creatinine |
| sAlb | = | serum albumin |
| eGFR | = | estimated glomerular filtration ratio |
Supplemental Material
Download PDF (513 KB)Supplemental Material
Download PDF (328.2 KB)Supplemental Material
Download PDF (508.6 KB)Supplemental Material
Download PDF (187.8 KB)Supplemental Material
Download PDF (184.2 KB)Supplemental Material
Download PDF (185.8 KB)Supplemental Material
Download PDF (190.7 KB)Supplemental Material
Download PDF (187.6 KB)Supplemental Material
Download TIFF Image (10.9 MB)Supplemental Material
Download TIFF Image (13.7 MB)Supplemental Material
Download TIFF Image (18.8 MB)Acknowledgments
We thank the two patients and their families for participating in this study, the two donors for contributing fecal specimen, nursing team of nephrology of Xijing hospital for caring the patients during the procedure, and Shanghai Mobio Biomedical Technology Co., Ltd. for bioinformatics analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The sequencing data of our manuscript has been deposited in data repository, National Center of Biotechnology Information (NCBI) (NO.PRJNA630819).
Additional information
Funding
References
- McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414–430.
- Mestecky J, Novak J, Moldoveanu Z, et al. IgA nephropathy enigma. Clin Immunol. 2016;172:72–77.
- McCarthy DD, Kujawa J, Wilson C, et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest. 2011;121(10):3991–4002.
- Floege J, Feehally J. The mucosa-kidney axis in IgA nephropathy. Nat Rev Nephrol. 2016;12(3):147–156.
- Fellström BC, Barratt J, Cook H, et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet. 2017;389(10084):2117–2127.
- Takeshita M, Tanaka A, Nakamura T, et al. Effect of lubiprostone on urinary protein excretion: a report of two IgA nephropathy patients with chronic constipation. Intern Med. 2019;58(22):3255–3259.
- Mishima E, Fukuda S, Shima H, et al. Alteration of the intestinal environment by lubiprostone is associated with amelioration of adenine-induced CKD. J Am Soc Nephrol. 2015;26(8):1787–1794.
- Bafeta A, Yavchitz A, Riveros C, et al. Methods and reporting studies assessing decal microbiota transplantation: a systematic review. Ann Int Med. 2017;167(1):34–39.
- Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389(10075):1218–1228.
- Jiang S, Xie S, Lv D, et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci Rep. 2017;7(1):2870.
- De Angelis M, Montemurno E, Piccolo M, et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLOS One. 2014;9(6):e99006.
- Zhong Z, Tan J, Tan L, et al. Modifications of gut microbiota are associated with the severity of IgA nephropathy in the Chinese population. Int Immunopharmacol. 2020;89(Pt B):107085.
- Shah NB, Allegretti AS, Nigwekar SU, et al. Blood microbiome profile in CKD: a pilot study. Clin J Am Soc Nephrol. 2019;14(5):692–701.
- Guan WJ, Yuan JJ, Li HM, et al. Proteobacteria community compositions correlate with bronchiectasis severity. Int j Tuberc Lung Dis. 2018;22(9):1095–7920.
- Mirpuri J, Raetz M, Sturge CR, et al. Proteobacteria-specific IgA regulates maturation of the intestinal microbiota. Gut Microbes. 2014;5(1):28–39.
