Abstract
Background and objectives
Patients who develop post-operative acute kidney injury (AKI) have a poor prognosis, especially when undergoing high-risk surgery. Therefore, the objective of this study was to evaluate the outcome of patients with AKI acquired after non-cardiac surgery and the possible risk factors for this complication.
Methods
A multicenter, prospective cohort study with patients admitted to intensive care units (ICUs) after non-cardiac surgery was conducted to assess whether they developed AKI. The patients who developed AKI were then compared to non-AKI patients.
Results
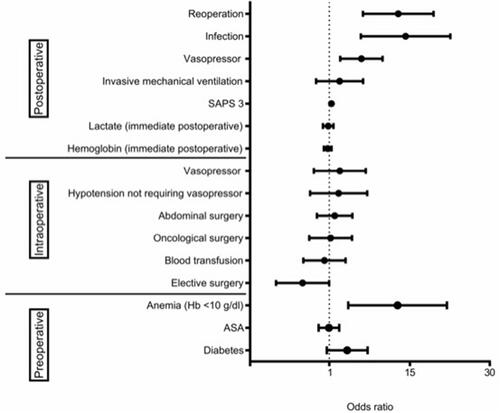
A total of 29 ICUs participated, of which 904 high-risk surgical patients were involved in the study. The occurrence of AKI in the post-operative period was 15.8%, and the mortality rate of post-operative AKI patients at 28 days was 27.6%. AKI was strongly associated with 28-day mortality (OR = 2.91; 95% CI 1.51–5.62; p = 0.001), and a higher length of ICU and hospital stay (p < 0.001). Independent factors for the risk of developing AKI were pre-operative anemia (OR = 7.01; 95% CI 1.69–29.07), elective surgery (OR = 0.45; 95% CI 0.21–0.97), SAPS 3 (OR = 1.04; 95% CI 1.02–1.06), post-operative vasopressor use (OR = 2.47; 95% CI 1.34–4.55), post-operative infection (OR = 8.82; 95% CI 2.43–32.05) and the need for reoperation (OR= 7.15; 95% CI 2.58–19.79).
Conclusion
AKI was associated with the risk of death in surgical patients and those with anemia before surgery, who had a higher SAPS 3, needed a post-operative vasopressor, or had a post-operative infection or needed reoperation were more likely to develop AKI post-operatively.
Background
Acute kidney injury (AKI) is characterized by a rapid and significant decrease in the glomerular filtration rate (GFR) and is usually of multifactorial origin [Citation1]. Studies that have evaluated perioperative AKI were performed mostly after cardiac and vascular surgery, and there are still major deficiencies in the literature regarding the development of AKI in patients undergoing non-cardiac surgery because most recent studies involve retrospective analyses [Citation2,Citation3].
The development of AKI is commonly associated with sepsis, low cardiac output, and the post-operative period of major surgeries [Citation4]. One out of every three cases of AKI occurs during the perioperative period [Citation5]; such cases represent ∼18–47% of the cases of hospital-acquired AKI [Citation6].
The incidence of AKI in surgical patients varies according to the type and severity of the surgery, with reported rates of 19% after cardiac surgery and ∼12–13% after general and thoracic surgery [Citation7]. In a large epidemiological study of patients undergoing major non-cardiac surgery in intensive care units (ICUs) in Brazil, 30% of patients had post-operative complications, and AKI was the post-operative complication with the second-highest occurrence rate. Post-operative complications are common in high-risk patients after major surgery [Citation8].
Therefore, we hypothesize that perioperative AKI is common and is associated with a worse prognosis. Since there are few prospective data on post-operative surgical patients in intensive care, assessing the incidence and characteristics of patients undergoing major non-cardiac surgery to develop post-operative AKI is relevant to adequately improve the therapeutic management of these patients.
The main objective of this study was to evaluate the incidence, impact on outcomes and main risk factors for developing AKI in patients who underwent non-cardiac surgery who developed AKI after admission to the ICU. Because of the importance of fluid balance in surgical patients, we also evaluated the association between this variable and the development of AKI.
Methods
This is a pre-planned secondary analysis of a multicenter study recently published [Citation8]. Then, a prospective, multicenter cohort study was conducted between 1 May and 1 November 2018, with a 28-day follow-up period. This study was approved by the Research Ethics Committee of the study coordinating center, Hospital Israelita Albert Einstein (CAAE: 55828016.1.1001.0071), and of all participating centers. A signed written informed consent form was obtained from all patients or their respective legal representatives. Two of the participating centers were exempted from providing the form due to the observational nature of the study.
All patients in the BRASIS study [Citation8] for whom did not have AKI before surgery were selected for this secondary analysis. Patients aged 18 years or older who were undergoing non-cardiac surgery requiring post-operative ICU care were included. Because the criteria for determining the need for post-operative intensive care were not standardized among the centers, all patients with this indication were considered high-risk.
Patients with AKI before surgery, chronic kidney disease, terminal cancer, those receiving palliative care, and those with severe liver failure (Child C) were excluded due to lower, or no prospect of cure, thus their inclusion could lead to inaccurate results. Pregnant women were also excluded. Furthermore, we excluded patients with a length of hospital stay of <12 h because it was not possible to determine whether they were not considered high-risk and for not being able to have follow-up information for at least two days in the ICU. Patients who were readmitted to the ICU during the same hospitalization were excluded to avoid repeating them in the study more than once.
The following variables were evaluated: age; type of surgery; American Society of Anesthesiologists (ASA) classification; Simplified Acute Physiology Score III score (SAPS 3); Sequential Organ Failure Assessment score (SOFA); previous comorbidities; the presence of infection, sepsis or septic shock during ICU stay; creatinine level (mg/dl) at admission and during ICU stay; daily fluid balance; mean arterial blood pressure at ICU admission; mechanical ventilation; vasoactive drugs; length of hospital stay and complications. Day 1 was considered the day of surgery plus admission to the ICU before 11:59 pm. Baseline serum creatinine was the pre-operative value.
Then, AKI was identified by the presence of at least one of the following parameters, Kidney Disease Improving Global Outcomes (KDIGO) criteria 2012 diagnostic score: increase in SCr by ≥0.3 mg/dl (≥26.5 µmol/l) within 48 h; increase in SCr to ≥1.5 times baseline pre-operative, which is known or presumed to have occurred within the prior 7 days; urine volume <0.5 mL/kg/h for 6 h or need for renal replacement therapy during ICU stay in patients with no history of chronic kidney failure [Citation9,Citation10]. Creatinine was measured at the entrance and reassessed in the first 5 days of ICU stay. Oliguria was assessed for 24 h to classify AKI severity.
The fluid balance was calculated as the difference between the infused fluids (crystalloids, colloids, fluid-diluting drugs, blood derivatives, and fluid via nasogastric tube) and eliminated fluids (via diuresis, bleeding, dialysis, and drains). The need for transfusion was appointed when hemoglobin values were below 8 g/dl intraoperatively. Arterial hypotension was defined when mean blood pressure values intra- and post-operative presented a 30% drop from baseline preoperative values for at least 30 min.
The other post-operative complications were defined as follows: cardiovascular, based on the need for vasopressors for more than 1 h despite adequate volume resuscitation; respiratory, in the presence of a partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) <200 in patients without previous lung disease, the need for reintubation or the failure to wean from mechanical ventilation during the post-operative period; neurological, based on a sharply fluctuating and non-zero Richmond Agitation-Sedation Scale (RASS) score [Citation11], within 24 h and agitation (determined by a RASS score ≥ +2); and gastrointestinal, in the presence of acute abdominal distension, uncontrolled nausea or vomiting, or moderate- to high-output fistulas.
The main outcome was 28-day mortality after surgery, which was evaluated face-to-face or by telephone. A 28-day follow-up period was chosen to standardize the follow-up time specifically related to surgery.
Statistical analysis
Considering data from the literature, we assumed a renal complication rate of 15% in high-risk surgical patients [Citation4,Citation7,Citation12]. Therefore, we estimated that at least 1000 patients would be required to conduct the study with the inclusion of fifteen explanatory variables in a robust logistic regression model, with 28-day mortality as the dependent variable. Fortunately, this result was the same found in the first study [Citation8], the current study just did not consider AKI patients before surgery.
Categorical variables are presented as absolute and relative frequencies. Quantitative variables are expressed as the mean and standard deviation (SD) or as the median and interquartile range (IQR), when appropriate. We used the Kolmogorov–Smirnov test to evaluate the distribution pattern of continuous numerical variables.
Proportions were compared using the chi-square test or Fisher’s exact test, as appropriate. Quantitative variables with multiple measurements were compared using general linear model (GLM) analysis, the consistency of the model was tested using Mauchly's sphericity test, and a post-hoc Bonferroni correction was performed in these analyses. The Bonferroni correction set the significance cutoff p-value; it was used to detect the time points at which the differences were significant in multiple comparisons.
The associations between explanatory and response variables were evaluated using fixed logistic regression models. Variables that were statistically significant in the univariate analyses (p < 0.05) were selected for inclusion in the multiple logistic regression models. Collinearity was first evaluated by examining the dispersion matrix and the Pearson correlation coefficient for continuous variables or the cross-tabulation for categorical variables. We also evaluated collinearity using the variance inflation factor (VIF). Variables with substantial collinearity (VIF ≥ 10) were excluded from the final model. The results of the logistic regression analyses are expressed as the odds ratio (OR) and respective 95% confidence interval (95% CI).
All probabilities of significance (p-values) were two-tailed. p-Values were considered statistically significant when p < 0.05. The Statistical Package for Social Sciences v. 26.0 (SPSS Inc.®; Chicago, IL, USA) and R v. 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) were used to perform the analyses.
Results
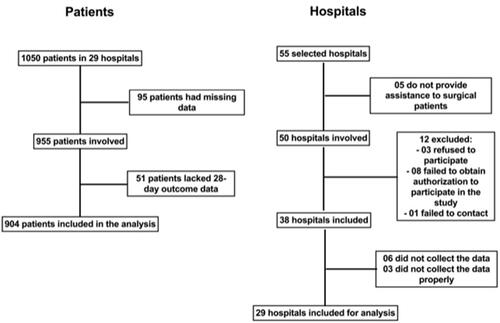
A total of 55 ICUs from 55 hospitals were selected for participation in the study (chosen for having accepted to participate in the screening). There were no significant differences in operational characteristics among the ICUs when the regions of the country were compared. Twelve of these ICUs (21.8%) were not eligible for participation for different reasons, i.e., 5 ICUs (9.1%) refused to participate because they did not treat the anticipated number of surgical patients, and 9 ICUs (16.4%) returned questionnaires with incomplete study data. Therefore, 29 ICUs participated in the study. During the study period, 25 500 patients underwent non-cardiac surgery. Of these, 904 (3.5%; 95% CI 3.3–3.8%) were admitted to the ICUs and involved in analyze of the study. The patients who entered the study were specifically high-risk surgical patients admitted to the ICU and did not have exclusion criteria ().
Figure 2. Risk factors related to AKI during the post-operative period (multivariate analysis). ASA: American Society of Anesthesiologists; Hb: hemoglobin; SAPS 3: Simplified Acute Physiology Score III. Area under the curve: 0.844; 95% confidence interval 0.814–0.871 .
The median (IQR) patient age was 62 (50–72) years, and 53.8% of the patients were male. The median (IQR) SAPS 3 was 42 (32–53) points. Approximately 80.4% of the patients had at least one comorbidity, and hypertension, cancer, and smoking were the most frequently occurring.
The median (IQR) length of ICU stay was 2 days (1–4). The median (IQR) length of hospital stay was 9.5 days (5.4–18.6).
The post-operative 28-day mortality rate for the entire cohort was 9.6%, and half of these patients (5.2%) presented with AKI. The total incidence of post-operative complications was 29.9%, with 15.8% of renal complications.
In the logistic regression model, the independent factors associated with 28-day mortality were age (OR = 1.034; 95% CI 1.013–1.054), SAPS 3 (OR = 1.036; 95% CI 1.012–1.061), SOFA at ICU admission (OR = 1.159; 95% CI 1.053–1.275), emergency surgery (OR = 2.7441; 95% CI, 1.197–6.273) and post-operative development of AKI (OR = 2.911; 95% CI 1.507–5.623) ().
Table 1. Factors related to post-operative 28-day mortality (univariate and multivariable analysis).
As shown in there was a negative impact of AKI post-operatively. Therefore, this specific population was explored. So, when comparing patients with and without AKI, it was identified that Male sex, higher SAPS 3 and SOFA scores at admission, physical status other than ASA 2, diabetes and anemia, i.e., hemoglobin level before surgery ˂10 g/dl, had a significant association with AKI ().
Table 2. Characteristics of patients presenting with and without acute kidney injury (AKI).
During the intraoperative period, the variables that were significantly associated with AKI were the type of surgery, intraoperative hypotension without the need for reversal with vasopressors (reversal of hypotension with volume), need for vasopressors and blood transfusion, and abdominal and oncological surgery ().
In addition, during the post-operative period, AKI was more strongly correlated with a lower hemoglobin level, higher arterial lactate level, and mechanical ventilation at ICU admission, in addition to the need for vasopressors during this period, the presence of post-operative infection, and the need for unplanned reoperation (). It can be noted that from the values of diuresis, creatinine, and need for dialysis that of the AKI patients 13.4% were KIDGO 3, and 86.6% developed KIDGO 1 and 2.
However, when logistic regression was applied to the perioperative variables that were identified as statistically significant in the univariate analysis comparing patients with and without AKI during the post-operative period (except for the male gender and SOFA score variables, because they present collinearity with SAPS 3 score, therefore, these variables did not participate in the logistic regression model), only preoperative anemia (OR = 7.01; 95% CI 1.69–29.07), elective surgery (OR = 0.45; 95% CI 0.21–0.97), SAPS 3 (OR = 1.04; 95% CI 1.02–1.06), post-operative vasopressor use (OR = 2.47; 95% CI 1.34–4.55), post-operative infection (OR = 8.82; 95% CI 2.43–32.05) and the need for reoperation (OR = 7.15; 95% CI 2.58–19.79) were independent factors associated with AKI ().
Specifically, regarding daily fluid adjustment during the perioperative period, AKI exhibited an association in the univariate analysis with the total volume and 0.9% saline volume received every 24 h. Nevertheless, when these variables were adjusted for the identified risk factors for developing AKI, there was no association with AKI ().
Table 3. Comparison of patients with or without acute kidney injury (AKI) according to fluids received during the perioperative period, univariate and multivariate analysis (adjusted for SAPS 3, preoperative anemia, elective surgery, post-operative vasopressor use, post-operative infection, and reoperation).
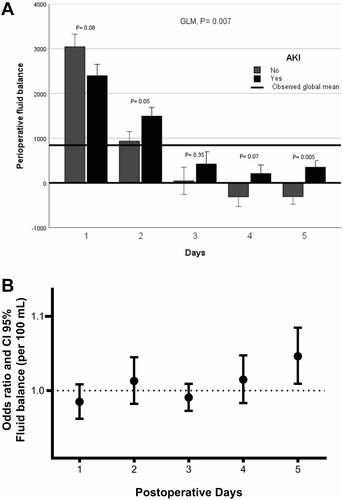
Regarding the evolution of the daily fluid balance in patients with AKI, the GLM showed significant differences (p = 0.007) in fluid balance from the day of surgery to the fifth day between patients with and without AKI. The difference was most evident on the second (p = 0.05 Bonferroni correction) and fifth (p = 0.005 Bonferroni correction) post-operative days. Furthermore, when the daily fluid balance was adjusted for the identified risk factors for developing AKI, only a high fluid balance on the fifth post-operative day was stronger correlated with AKI (OR per 100 mL= 1.051; 95% CI 1.010–1.094) ().
Figure 3. (A) Multiple analysis model (GLM) for variables repeated over time in relation to fluid balance in patients with and without AKI. p-Values were corrected by the Bonferroni method and (B) multivariate analysis for daily post-operative fluid balance (per 100 mL) adjusted for SAPS 3, preoperative anemia, elective surgery, post-operative vasopressor use, post-operative infection, and reoperation.
Discussion
The main findings of this study were a high incidence of AKI (15.8%) with high mortality in non-cardiac surgery patients admitted to the ICU and the association of AKI with non-elective surgeries, infection, reoperation, circulatory shock, preoperative anemia, and inappropriate positive fluid balance.
Major surgeries account can reach 40% of cases of hospital-acquired AKI [Citation13]. Patients with AKI admitted to the ICU during the post-operative period in cardiovascular surgery accounted for 7.7–40% of cases [Citation14], and gastrointestinal tract surgery patients accounted for almost 22% [Citation15]. Specific data on surgical patients requiring intensive care are scarce.
It is important to comment that although this study does not include surgical cardiac patients, the findings of our study can be extrapolated in several aspects for these patients, because cardiac surgical patients suffer some complications and have some similar characteristics to high-risk non-cardiac surgery, thus, the risk factors for AKI can be the same [Citation16].
The presence of AKI during the perioperative period in this subgroup of patients undergoing major surgery was associated with unfavorable outcomes, with a hospital mortality rate of 27.6%. In addition, the length of hospital stay of patients with AKI was higher than that of patients non-AKI; patients with AKI spent approximately one extra week in the hospital. Similarly, a large multicenter observational study in European countries reported that mortality among patients with AKI was more than twice that observed in other patients [Citation17]. In a recent study, the occurrence and severity of AKI were strongly associated with the risk of death after surgery. However, the relationship between preoperative renal function, as assessed by serum creatinine-based estimated GFR, and the risk of death depended on patient age and whether AKI developed post-operatively [Citation18].
In the current study, patients who developed AKI were more severe (higher SAPS 3), non-elective operations, had a greater need for advanced support with catecholamines, and frequently had infections.
Non-elective operations make it difficult to prepare and assess the risk for AKI, which prevents the proper management of these patients, making them more vulnerable to this problem [Citation19,Citation20]. In addition, the intensity of the inflammatory response to surgical trauma determines both tissue hypoperfusion and the ischemia-reperfusion response, which intensifies the inflammatory response. Prospective studies have reported a relationship between AKI and sepsis and the need for mechanical ventilation [Citation6,Citation21,Citation22].
There is a close correlation between hypotension and the development of AKI. A recent meta-analysis showed that goal-directed hemodynamic adjustments reduce the likelihood of AKI in orthopedic and abdominal surgery patients [Citation23]. We observed the most frequent use of vasopressors in this group of patients. The duration of intraoperative hypotension (particularly relative to the patient’s normal arterial blood pressure), including brief episodes of mean arterial pressure lower than 55 mmHg, has been associated with kidney injury [Citation5]. Careful maintenance of cardiovascular stability, including fluid infusion, throughout this period, is vital for protecting renal perfusion while avoiding volume overload. A study of high-risk surgical patients showed that the customized maintenance of blood pressure according to the patient's previous levels during surgery was able to reduce organ dysfunction [Citation24]. Moreover, the association between the use of vasoactive drugs and mortality is consistent with the results of other studies [Citation25–27].
Interestingly, a correlation was noted between preoperative anemia (such as hemoglobin concentration <10 g/dl) and impaired renal function. This pathophysiology is multifactorial, but mainly involves a decrease in the oxygen transport capacity and subsequent tissue hypoxia [Citation28]. Patients in the AKI group had more transfusions, which may have induced more systemic inflammation, in addition to having more patients with anemia (Hb < 10 g/dl) at entry, a condition that may have facilitated tissue hypoxia.
Besides, patients who developed AKI had a more positive daily fluid balance for a long time. In it is quite clear that AKI patients maintain a positive balance until the fifth day and non-AKI patients more rapidly decrease the fluid balance.
It is expected that, in many cases, simply restoring the circulating volume does not improve the results and maybe counterproductive [Citation4,Citation5]. Organ edema distorts tissue architecture, impairs oxygen and metabolite diffusion, and obstructs the capillary flow and lymphatic drainage. These effects are particularly pronounced in encapsulated organs, such as the kidney, which cannot accommodate additional volume without significant increases in interstitial pressure and compromised blood flow. Elevated intratubular pressure decreases glomerular filtration and activation of tubuloglomerular feedback, with consequent preglomerular vasoconstriction, which leads to an additional reduction in glomerular filtration [Citation29–31]. Studies have shown that excess fluid is an independent factor for the development of AKI and that in patients with AKI, a more positive fluid balance was correlated with higher mortality [Citation32–35]. Regarding the type of solution used, epidemiologic data suggest that 0.9% saline solution, when compared with balanced salt solutions, such as balanced solutions, may increase the risk of AKI. In addition, there is evidence of harm (increased rates of AKI) with the use of hetastarch solutions, which should generally be avoided [Citation36]. In our study was not detect a negative influence of any type of colloid or crystalloid on the development of AKI.
Based on these, patients should be stratified according to their risk of developing AKI based on their exposure and susceptibility [Citation37,Citation38], and preemptive measures should be taken [Citation5].
Study limitations
The main strengths of our study are associated with its multicenter nature, as it included ICUs located in several regions of Brazil. However, there was a reasonable rate of refusal to participate in the study that affected external validity to some degree. In addition, the adjusted regression model could be performed with a priori defined AKI prediction variables, and no based on statistically important findings from the unadjusted analysis.
There were failures in capturing some relevant data that could have been included in the analyses, such as nephrotoxic agents or humoral factors, contrast exposure perioperatively, and the nature of oncological or vascular surgery. Additionally, the need to obtain informed consent in epidemiological studies, such as this tends to skew the sample due to the non-consenting of more critical patients, whose families may be psychologically fragile. Another aspect to be considered is the lack of standardization among the centers regarding indications for post-operative intensive care and treatments, but this fact does not invalidate the findings, because we tried to show what happens in real practice. Besides, our finding regarding inappropriate positive fluid balance and renal failure only generate a hypothesis, it may not necessarily be a cause-effect relationship. Furthermore, this study was not able to assess other possible causes of renal failures, such as intra-abdominal hypoperfusion and increased airway pressures [Citation39], also long-term complications and mortality, because some complications may have occurred after the study period.
Conclusion
AKI is a major complication in intensive care surgical patients and is associated with the risk of death, for this reason, it deserves attention in the perioperative. Patients with anemia before surgery, those with a higher SAPS 3, those requiring vasopressors during the post-operative period, and those with post-operative infection or the need for reoperation are the most likely to develop AKI, as are patients with an inappropriate higher perioperative fluid balance for a long time, therefore care should be considered in the perioperative period for these problems. The fluid type did not influence AKI development.
Ethical approval
This study was approved by the Research Ethics Committee of the study’s coordinating center, the Hospital Israelita Albert Einstein (CAAE: 55828016.1.1001.0071). Written informed consent was obtained from all patients or their legal representatives.
Author contributions
J.M.S.Jr. and H.T.K. conceived and designed the study. J.M.S.Jr., B.C.G., R.C.F.C., T.D.C., S.M.A.L., M.S.C.A., and L.M.S.M. performed the study. J.M.S.Jr., H.T.K., B.C.G., and A.S.N. analyzed the data. BRASIS study group contributed to data collection. J.M.S.Jr., H.T.K., B.C.G., S.M.A.L., and L.M.S.M. wrote the paper. All authors have read and approved the manuscript.
| Abbreviations | ||
| AKI | = | acute kidney injury |
| ASA | = | American Society of Anesthesiologists |
| GFR | = | glomerular filtration rate |
| ICU | = | intensive therapy unit |
| KDIGO criteria | = | Kidney Disease Improving Global Outcomes criteria |
| RASS | = | Richmond Agitation-Sedation Scale Score |
| SAPS 3 | = | Simplified Acute Physiology Score III |
| SOFA | = | Sequential Organ Failure Assessment |
Acknowledgments
The following have made substantial contributions to the study. We would like to thank the important work and support of the BRASIS research group, which carried out data collection and care for patients and their data for research: Cristina Prata Amendola, Neymar Elias de Oliveira, Viviane Cordeiro Veiga, Salomón Soriano Ordinola Rojas, Bruno Melo Nobrega de Lucena, Raphael Augusto Gomes de Oliveira, Luciana Coelho Sanches, Antonio Paulo Nassar Junior, Álvaro Réa-Neto, Alexandre Amaral, Flávio Geraldo Rezende de Freitas, Antônio Tonete Bafi, Fernando José Ramos, Maria Augusta Santos Rahe Pereira, Danielle Dourado Magalhães, Diogo Oliveira Toledo, Fernando Suparregui Dias, Marciano de Sousa Nobrega, Claudio Piras, Paulo Lisboa Bittencourt, Mirella Cristine de Oliveira, Jarbas da Silva Motta Júnior, Paula Geraldes David, Luana Alves Tannous, Lorena Macedo Araújo Miranda, José Arthur dos Santos Brasil, Rafael Alexandre de Oliveira Deucher, Marcos Henrique Borges Ferreira, Wagner Luis Nedel, Wilson de Oliveira Filho, Gilberto Friedman, Afonso José Celente Soares.
The present study was endorsed by the Brazilian Research in Intensive Care Network (AMIBnet).
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Acedillo RR, Wald R, McArthur E, et al. Characteristics and outcomes of patients discharged home from an emergency department with AKI. Clin J Am Soc Nephrol. 2017;12(8):1215–1225.
- Causey MW, Maykel JA, Hatch Q, et al. Identifying risk factors for renal failure and myocardial infarction following colorectal surgery. J Surg Res. 2011;170(1):32–37.
- Cho A, Lee JE, Kwon GY, et al. Post-operative acute kidney injury in patients with renal cell carcinoma is a potent risk factor for new-onset chronic kidney disease after radical nephrectomy. Nephrol Dial Transplant. 2011;26(11):3496–3501.
- Amendola CP, Silva JM, Jr Carvalho T, et al. Goal-directed therapy in patients with early acute kidney injury: a multicenter randomized controlled trial. Clinics. 2018;73:e327.
- Golden D, Corbett J, Forni LG. Peri-operative renal dysfunction: prevention and management. Anaesthesia. 2016;71 Suppl 1:51–57.
- Romagnoli S, Ricci Z. Postoperative acute kidney injury. Minerva Anestesiol. 2015;81(6):684–696.
- Gameiro J, Fonseca JA, Neves M, et al. Acute kidney injury in major abdominal surgery: incidence, risk factors, pathogenesis and outcomes. Ann Intensive Care. 2018;8(1):22.
- Silva Junior JM, Chaves RCF, Correa TD, et al. Epidemiology and outcome of high-surgical-risk patients admitted to an intensive care unit in Brazil. Rev Bras Ter Intensiva. 2020;32(1):17–27.
- Lameire N, Vanmassenhove J, Lewington A. Did KDIGO guidelines on acute kidney injury improve patient outcome? Intensive Care Med. 2017;43(6):921–923.
- Zarbock A, John S, Jörres A, et al. [New KDIGO guidelines on acute kidney injury. Practical recommendations]. Anaesthesist. 2014;63(7):578–588.
- Chanques G, Ely EW, Garnier O. The 2014 updated version of the confusion assessment method for the intensive care unit compared to the 5th version of the diagnostic and statistical manual of mental disorders and other current methods used by intensivists. Ann Intensive Care. 2018;8(1):33.
- Gumbert SD, Kork F, Jackson ML, et al. Perioperative acute kidney injury. Anesthesiology. 2020;132(1):180–204.
- Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67–75.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19–32.
- Kim M, Brady JE, Li G. Variations in the risk of acute kidney injury across intraabdominal surgery procedures. Anesth Analg. 2014;119(5):1121–1132.
- Canet E, Bellomo R. Perioperative renal protection. Curr Opin Crit Care. 2018;24(6):568–574.
- Payen D, de Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74.
- Chaudery H, MacDonald N, Ahmad T, et al. Acute kidney injury and risk of death after elective surgery: prospective analysis of data from an international cohort study. Anesth Analg. 2019;128(5):1022–1029.
- Bihorac A, Hobson CE. Acute kidney injury: precision perioperative care protects the kidneys. Nat Rev Nephrol. 2017;14(1):8–10.
- Hobson C, Ruchi R, Bihorac A. Perioperative acute kidney injury: risk factors and predictive strategies. Crit Care Clin. 2017;33(2):379–396.
- Deng Y, Yuan J, Chi R, et al. The incidence, risk factors and outcomes of postoperative acute kidney injury in neurosurgical critically ill patients. Sci Rep. 2017;7(1):4245.
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818.
- Giglio M, Dalfino L, Puntillo F, et al. Hemodynamic goal-directed therapy and postoperative kidney injury: an updated meta-analysis with trial sequential analysis. Crit Care. 2019;23(1):232.
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–1357.
- Herrera-Anazco P, Taype-Rondan A, Pacheco-Mendoza J, et al. Factors associated with mortality in a population with acute kidney injury undergoing hemodialysis in Peru. J Brasil Nefrol. 2017;39(2):119–125.
- Depret F, Boutin L, Jarkovsky J, et al. Prediction of major adverse kidney events in critically ill burn patients. Burns. 2018;44(8):1887–1894.
- Palevsky PM. Dialysis modality and dosing strategy in acute renal failure. Semin Dial. 2006;19(2):165–170.
- Fowler AJ, Ahmad T, Phull MK, et al. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg. 2015;102(11):1314–1324.
- Butcher BW, Liu KD. Fluid overload in AKI: epiphenomenon or putative effect on mortality? Curr Opin Crit Care. 2012;18(6):593–598.
- Patil VP, Salunke BG. Fluid overload and acute kidney Injury. Indian J Crit Care Med. 2020;24(Suppl 3):S94–S97.
- Prowle JR, Echeverri JE, Ligabo EV, et al. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6(2):107–115.
- Silva JM, Jr., de Oliveira AM, Nogueira FA, et al. The effect of excess fluid balance on the mortality rate of surgical patients: a multicenter prospective study. Crit Care. 2013;17(6):R288.
- Kahan BC, Koulenti D, Arvaniti K, et al. Critical care admission following elective surgery was not associated with survival benefit: prospective analysis of data from 27 countries. Intensive Care Med. 2017;43(7):971–979.
- Messina A, Robba C, Calabrò L, et al. Association between perioperative fluid administration and postoperative outcomes: a 20-year systematic review and a meta-analysis of randomized goal-directed trials in major visceral/noncardiac surgery. Crit Care. 2021;25(1):43.
- Myles PS, Andrews S, Nicholson J, et al. Contemporary approaches to perioperative IV fluid therapy. World J Surg. 2017;41(10):2457–2463.
- Griffin BR, Liu KD, Teixeira JP. Critical care nephrology: core curriculum 2020. Am J Kidney Dis. 2020;75(3):435–452.
- Blitz JD, Shoham MH, Fang Y, et al. Preoperative renal insufficiency: underreporting and association with readmission and major postoperative morbidity in an academic medical center. Anesth Anal. 2016;123(6):1500–1515.
- Kendale SM, Lapis PN, Melhem SM, et al. The association between pre-operative variables, including blood pressure, and postoperative kidney function. Anaesthesia. 2016;71(12):1417–1423.
- Kopitko C, Medve L, Gondos T. The value of combined hemodynamic, respiratory and intra-abdominal pressure monitoring in predicting acute kidney injury after major intraabdominal surgeries. Ren Fail. 2019;41(1):150–158.