Abstract
Objective
The aim of this study was to determine the efficacy and safety of lanthanum carbonate (LC) versus calcium salts, non-LC phosphate binders (PBs), sevelamer, or placebo in patients with chronic kidney disease (CKD).
Materials and methods
A literature search on PubMed, Embase, and Cochrane Library databases was conducted up to 18 June 2021. Data acquisition and quality assessment were performed by two reviewers. Meta-analysis was performed to evaluate the serum biochemical parameters, adverse events, and patient-level outcomes of LC, non-LC PBs, and sevelamer for hyperphosphatemia in patients with CKD. Heterogeneity across studies was assessed utilizing the I2 statistic and Q-test, and a random effect model was selected to calculate the pooled effect size.
Results
A total of 26 randomized, controlled trials and 3 observational studies were included. Compared to the other groups, better control effect of serum phosphorus (RR = 2.68, p < 0.001), reduction in serum phosphorus (95%CI = −1.93, −0.99; p < 0.001), Ca × P (95%CI = −13.89, −2.99; p = 0.002), serum intact parathyroid hormone levels (95%CI = −181.17, −3.96, p = 0.041) were found in LC group. Besides, reduced risk of various adverse effects, such as hypotension, abdominal pain, diarrhea, dyspepsia, and a score of coronary artery calcification were identified with LC in comparison to calcium salt, non-LC PBs, or placebo group. Significantly lower risk in mortality with LC treatment vs. non-LC PBs was observed, while no significant difference was identified between LC and calcium salt groups.
Conclusion
LC might be an alternative treatment for hyperphosphatemia in patients with CKD considering its comprehensive curative effect.
1. Introduction
Chronic kidney disease (CKD) is a significant health problem worldwide [Citation1]. Hyperphosphatemia is very common and harmful in patients undergoing maintenance dialysis [Citation2,Citation3]. Previous studies suggested that hyperphosphatemia is an independent risk factor for surrogate clinical endpoints like morbidity and mortality for patients with CKD [Citation4], or the development of coronary artery calcification in peritoneal dialysis patients [Citation5]. Evolving effective agents is an essential part of CKD therapy.
Currently, the management of hyperphosphatemia in patients with CKD is depending on intestinal phosphate binders (PBs), including non-calcium-based binders or calcium-based agents [Citation6]. Although effective in reducing serum phosphorus levels, security issues need to be considered and explored when choosing which one to use. Gastrointestinal adverse events and cardiovascular disease are major problems for the complication of these PBs. The progression of vascular calcification of media is considered to be the main influencing factor [Citation7]. A prospective randomized study in hemodialysis patients reveals that excessive use of calcium carbonate is likely to cause hypercalcemia, which is continuously related to progressive arterial calcification and decreased trabecular bone density within 2 years of observation [Citation8]. In addition, a meta-analysis has found that sevelamer, a nonabsorbed, calcium- and metal-free dietary PB, does not produce sustained superior biochemical outcomes in comparison to calcium-based therapies [Citation9]. However, another meta-analysis of 11 randomized trials (4622 patients) suggests that non-calcium-based PBs are superior to calcium-based PBs in decrease the all-cause mortality risk in patients with CKD [Citation10]. Yet to date, the impact of these agents on patient-level outcomes is still a controversial issue.
Lanthanum carbonate (LC), as a new non-calcium-based PB, is used to treat hyperphosphatemia in patients with CKD through binding phosphate via its trivalent cation [Citation6,Citation11]. Reportedly, healthy individuals receiving a dose of 3000 mg/day of LC can reduce urinary phosphorus excretion [Citation12]. In addition, a multicenter, randomized, and double-blind study showed that LC is a well-tolerated and efficacious oral PB with mild adverse effects for hemodialysis and patients with CKD [Citation13]. To further analyze the efficacy and safety of LC, the present meta-analysis was performed via comparing the effects of LC on serum biochemical parameters, various adverse events, and patient-level outcomes versus calcium salts (calcium acetate and calcium carbonate), sevelamer hydrochloride (SH), non-LC PBs (PBs) or placebo.
2. Materials and methods
2.1. Data sources
A systematic search strategy was performed and LC relevant clinical articles were obtained from PubMed (http://www.ncbi.nlm.nih.gov/PubMed), Embase (http://www.embase.com), and Cochrane Library (http://www.cochranelibrary.com/) electronic databases. Following terms including (Lanthanum carbonate) OR fosrenol OR (dilanthanumtricarbonate) OR (lanthanum carbonate hydrate) AND (CKD OR (chronic renal failure) OR (chronic kidney disease) OR (chronic nephropathy) OR hemodialysis OR (Peritoneal dialysis) OR (end-stage renal disease) OR ESRD) were used for searching. Based on titles and abstracts, the relevant studies were screened by two investigators separately (LJZ and AL), and reviewed by a third one (GSX). Any disagreement between the two researchers was resolved by a third person review (Kappa = 0.895, Se = 0.024, p < 0.001). The literature was published in English and the deadline for the literature search was 18 June 2021. The details of the retrieval strategy are shown in Supplementary Tables 1–3.
2.2. Inclusion and exclusion criteria
The inclusion criteria of the study were as follows: (a) the subjects of this study were CKD patients with hyperphosphatemia, including those who underwent hemodialysis, peritoneal dialysis, or did not undergo dialysis; (b) patients in the treatment group were treated with LC; (c) patients in the control group were treated with calcium-phosphorus binders (e.g., calcium acetate or calcium carbonate, etc.), non-calcium-phosphorus binders (e.g., sevelamer, iron, etc.), placebo or without using phosphorus reducing agents; (d) randomized controlled trial (RCT) or observational research; and (e) providing information regarding the effectiveness and safety outcomes, such as blood phosphorus control, blood phosphorus levels, alkaline phosphatase, death, as well as adverse reactions. The exclusion criteria of the study were as follows: (a) studies that could not be used for statistical analysis due to incomplete data; (b) non-original articles: including review, letter, and comments; and (c) duplicated publications.
2.3. Data extraction and quality assessment
Data extraction was conducted independently by two authors (LJZ and AL) and reviewed by a third one (GSX). The disagreements between two researchers were resolved by a third person review (Kappa = 0.638, Se = 0.041, p < 0.001). The following data were extracted and recorded: the first author of the literature, the year of publication, study type, age and gender of the subjects, sample size, therapy intervention, follow-up period, and the outcome data of patients. Differences were resolved by discussion.
Cochrane risk of bias assessment tool was used to evaluate the quality of the included RCT study [Citation14]. Evaluation items included sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases. Meanwhile, the methodological quality of the observational study was assessed by the quality evaluation criteria provided by the Newcastle–Ottawa Scale (NOS), including the evaluation of exposure selection, comparability, and outcome, with a full score of 9 points [Citation15]. Importantly, disagreements regarding the quality evaluation were solved after a group discussion with the third author.
2.4. Statistical analysis
All statistical analysis was performed by Stata 11.0 software. Relative ratio (RR) and 95% confidence interval (CI) were used for the categorical variables. However, weighted mean difference (WMD) and 95% CI were used as the combined index for the continuous variables. Random effect models were used to merge the outcomes of all studies. In addition, Cochran's Q statistics and I2 test were used for the heterogeneity test [Citation16]. Heterogeneity was significant among studies if p < 0.05 or/and I2>50%. While p ≥0.05 and I2≤50% indicated there was no significant heterogeneity. Furthermore, subgroup analysis was used to explore the effects of age (<60 years or ≥60 years), region (Asian or western), and sample size (<100 or ≥100) on the merger results. Publication bias was tested by Egger's test, and p <0.05 was considered significant.
3. Results
3.1. General characteristics of selected literature
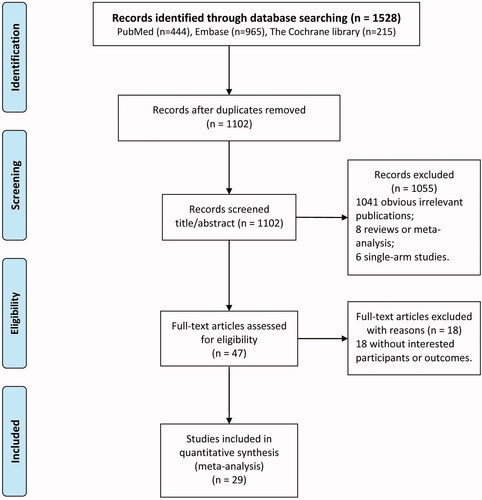
The flowchart of literature search and study selection is displayed in . A total of 1528 potential articles were relevant to the search terms (444 from PubMed, 965 from Embase, and 215 from Cochrane library). After eliminating 426 duplicate literature, 1041 obvious irrelevant publications, 8 reviews or meta-analysis, 6 single-arm studies, 18 articles without interested participants or outcomes, a total of 29 eligible studies [Citation13,Citation17–44]. were included for this meta-analysis.
The characteristics of included studies are listed in . In total, 29 studies including 26 RCT and 3 retrospective cohort studies were included. The publication year of included studies ranged from 2003 to 2021, and studies were conducted in various countries, including China, Japan, Korea, USA, UK, India, Macedonia, and Australia. The duration of follow-up ranged from 4 weeks to 5 years. Notably, there were four control groups according to the type of binders used, including calcium salts, sevelamer, non-LC PBs, and placebo. For each study, no significant differences in baseline information between control and experimental groups were found.
Table 1. Characteristics of the studies included in the meta-analysis.
3.2. Quality assessment
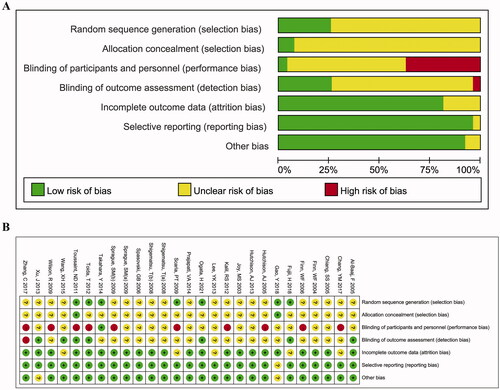
The results of the quality assessment are shown in and Supplementary Table 4. Due to most of the RCT did not elaborate the specific randomization, allocation concealment methods, and the method for the blind implementation, the degree of bias in the sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment were dominated by unclear outcomes. Besides, the bias in the incomplete outcome data, selective outcome reporting, and other biases was low risk. Overall, the RCT included in this study has a good methodological quality. The quality of the three retrospective cohort studies scored 6–7 and was also a moderate quality study.
3.3. Meta-analysis of pooled quantitative data
3.3.1. Serum biochemical parameters
3.3.1.1. Serum phosphorus control
Serum phosphorus control refers to the achievement of standard serum phosphorus levels in the literature. As shown in , there were 8, 2, and 1 studies that reported the serum phosphorus control comparison for LC vs. placebo, LC vs. Calcium, and LC vs. non-LC PBs. The combined results of LC vs. placebo were RR (95% CI) =2.68 (1.88, 3.82) and p < 0.001, without heterogeneity among studies (I2 = 48.7%, p = 0.058), indicating that LC had a better serum phosphorus control effect compared with placebo. However, no significant difference was observed among LC vs. calcium salts (RR = 1.03, 95% CI = 0.88 − 1.20; p = 0.750) or LC vs. non-LC PBs (RR = 0.94, 95% CI = 0.84 − 1.05; p = 0.269).
Figure 3. Pooled results for the serum biochemical parameters of LC treatment versus calcium salts, non-LC PBs, sevelamer, and placebo in patients with chronic kidney disease (CKD). (A) serum phosphorus control; (B) serum phosphorus; (C) Ca × P levels; (D) serum calcium; (E): serum intact parathyroid hormone; (F) serum alkaline phosphatase.

3.3.1.2. Serum phosphorus level
As shown in , there were 7, 7, 1, and 2 studies that reported the level of serum phosphate (mg/dL) comparison for LC vs. placebo, LC vs. calcium salts, LC vs. non-LC PBs, and LC vs. sevelamer. There was a significant decrease in serum phosphorus levels with LC in comparison with placebo (WMD= −1.46, 95%CI = −1.93, −0.99; p < 0.001), with considerable heterogeneity among studies (I2 = 82.9%, p < 0.001). Similarly, patients treated with LC had lower serum phosphorus levels compared with sevelamer (WMD= −0.25, 95%CI = −0.42, −0.08; p = 0.003), while no significant heterogeneity was found (I2 = 0.0%, p = 0.772).
3.3.1.3. Ca × P product
As shown in , there were 4, 6, and 1 studies that reported the Ca × P product comparison for LC vs. placebo, LC vs. calcium salts, and LC vs. sevelamer. There was a significantly lower Ca × P product in patients treated with LC in comparison with placebo (WMD= −8.44, 95%CI = −13.89, −2.99; p = 0.002), while a relatively higher Ca × P levels with LC in comparison to sevelamer (WMD= 2.27, 95%CI = 0.81, 3.73; p = 0.002) was detected, without significant heterogeneity among studies (p ≥ 0.05 or I2 ≤ 50%).
3.3.1.4. Serum calcium
As shown in , there were 4, 7, 1, and 2 studies reported in serum calcium (mg/dL) comparison for LC vs. placebo, LC vs. calcium salts, LC vs. non-LC PBs, and LC vs. sevelamer. Patients treated with LC had a relatively lower serum calcium level compared with calcium salts (WMD = −0.44, 95%CI = −0.73, −0.15, p = 0.003). However, no significant difference was identified among LC vs. placebo and LC vs. sevelamer groups.
3.3.1.5. Serum intact parathyroid hormone (iPTH)
As shown in , there were 6, 6, 1, and 2 studies reported in serum iPTH comparison for LC vs. placebo, LC vs. calcium salts, LC vs. non-LC PBs, and LC vs. sevelamer. Serum iPTH levels were significantly lower in patients treated with LC vs. placebo (WMD = −92.57, 95%CI = −181.17, −3.96, p = 0.041), while no significant difference was observed among LC vs. calcium salt (WMD = 47.99, 95%CI = −13.02, 108.99, p = 0.123) and LC vs. sevelamer (WMD = 12.03, 95%CI = −17.06, 41.13, p = 0.418) groups.
3.3.1.6. Serum alkaline phosphatase (ALP)
As shown in , there were 4, 2, and 1 studies reported in ALP comparison for LC vs. calcium salts, LC vs. non-LC PBs, and LC vs. sevelamer. A significantly higher levels of serum ALP was detected in patients treated with LC in comparison to non-LC PBs (WMD = 7.89, 95%CI = 0.87, 14.91, p = 0.028), without considerable heterogeneity between studies (I2 = 0.0%, p = 0.354).
3.4. Adverse events
3.4.1. Treatment-related adverse events (TRAEs)
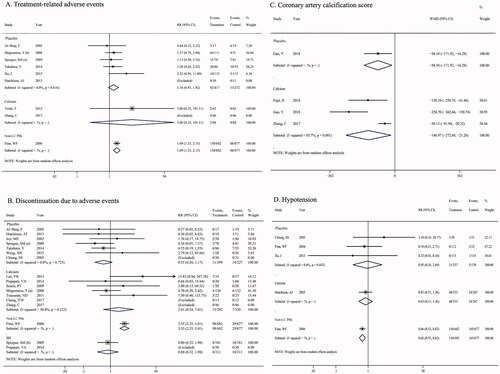
There were no significant differences in the incidence of TRAEs via LC treatment induced in comparison to placebo (RR = 1.34, 95%CI = 0.93, 1.92; p = 0.112), and calcium salts (RR = 5.00, 95% CI = 0.25, 101.11; p = 0.294, ). However, one study reported the effect of LC and non-LC PBs on the adverse events, and a significantly greater adverse events ratio was found in patients treated with LC vs. non-LC PBs (RR = 1.69, 95% CI = 1.33, 2.15; p < 0.001).
3.4.2. Discontinuation due to adverse events (DtAEs)
Similarly, a significantly higher DtAEs with LC treatment compared with non-LC PBs was identified (RR = 3.35, 95% CI = 2.25, 5.01; p < 0.001, ), while no remarkable difference existed among LC vs. placebo (p = 0.123), LC vs. calcium salts (p = 0.295), and LC vs. sevelamer groups (p = 0.630).
3.4.3. Side effects of medications
Patients treated with LC had a lower score of coronary artery calcification in comparison to placebo (WMD = −94.10, 95%CI = –171.92, −16.28, p = 0.0188) or calcium (WMD = −146.97, 95%CI = –272.68, −21.26, p = 0.022, ), a reduction ratio of hypotension (RR = 0.66, 95% CI = 0.53, 0.82; p < 0.001, ) and abdominal pain (RR = 0.73, 95% CI = 0.59, 0.91; p = 0.004, Supplementary Figure 1A) compared with non-LC PBs, a decreased risk of diarrhea in comparison to placebo (RR = 0.32, 95% CI = 0.17, 0.60; p = 0.001) or non-LC PBs (RR = 0.75, 95% CI = 0.63, 0.90; p = 0.001, Supplementary Figure 1B), a reduced risk of dyspepsia (RR = 0.21, 95% CI = 0.07, 0.59; p = 0.003, Supplementary Figure 1C) and pruritus (RR = 0.15, 95% CI = 0.06, 0.37; p < 0.001, Supplementary Figure 1D) in comparison to placebo. No significant differences were observed in the risk of nausea (Supplementary Figure 1E), and vomiting (Supplementary Figure 1F).
3.5. Patient-level outcomes
3.5.1. Mortality
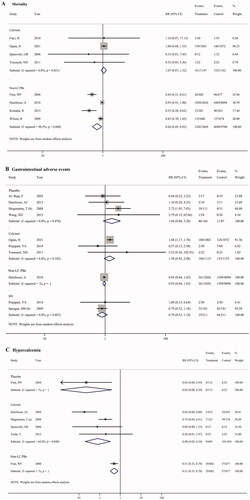
Meta-analysis of four studies showed a significantly lower risk in mortality with LC treatment in comparison to non-LC PBs (RR = 0.64, 95% CI = 0.45, 0.92; p = 0.016, ). However, no significant difference in mortality risk was identified between LC and calcium salt groups ().
3.5.2. Gastrointestinal adverse events
There was no significant difference in gastrointestinal adverse events with LC treatment in comparison to placebo, calcium salts, non-LC PBs, and sevelamer (), respectively.
3.5.3. Hypercalcemia
There was a significant reduction risk in hypercalcemia with LC treatment in comparison with calcium salts based on meta-analysis of four studies (RR = 0.08, 95% CI = 0.02, 0.34; p = 0.001, ). Similarly, only one study reported the hypercalcemia risk in patients treated with LC vs. placebo (RR = 0.02, 95% CI = 0.00, 0.39; p = 0.009), and LC vs. non-LC PBs (RR = 0.51, 95% CI = 0.33, 0.78; p = 0.002), and a decreased risk also detected in LC group.
3.6. Subgroup analysis
Subgroup analysis was further performed to explore the potential sources of heterogeneity. Factors for the subgroup analysis included age, area, and sample size. When patients were treated with LC compared with placebo, results showed that age, area, and sample size were significant effects on serum phosphate, and Ca × P outcomes (p < 0.05). In addition, when patients treated with LC were compared with calcium salts, results showed that age and area were significant effects on serum calcium, as well as age and sample size on hypercalcemia outcomes (p < 0.05; ).
Table 2. Outcomes of subgroup analyze.
3.7. Publication bias test
Due to the number limitation of literature included in the comparison of other outcomes, the publication bias of blood phosphorus control and total AEs for LC vs. placebo were assessed. Results showed that no publication bias was identified in phosphorus control (p = 0.219) and total adverse events (p = 0.879).
4. Discussion
It remains a question regarding whether LC is a safe and efficacious treatment to patients with CKD. The present meta-analysis included 29 studies to examine the effect of LC in comparison to calcium salts, non-LC PBs, sevelamer, and placebo. Our results showed that LC can better reduce serum phosphorus levels and significantly reduce the risk of adverse events and mortality compared with other drugs, suggesting that LC may be a safe and effective PB for the treatment of hyperphosphatemia in CKD.
Hyperphosphatemia, which is related to increased incidence of cardiovascular disease, is a common complication in patients with CKD [Citation45]. Serum phosphorus control is an important part of CKD treatment [Citation13]. A large observational study reveals that serum phosphate and calcium-phosphate product (Ca × P) are independent risk factors for mortality in dialysis patients [Citation46]. LC is a calcium-free PB, which is widely used in the management of dialysis patients [Citation36]. Our systematic review identified that LC was superior to placebo, control diet, or other oral activated charcoal for serum phosphorus controls and reduction of Ca× P levels. In addition, a recent meta-analysis of patients with CKD treated by calcium or non-calcium-based PBs showed that calcium increased mortality in comparison with non-calcium-based PBs, such as sevelamer and LC [Citation47]. Reportedly, emerging data suggest that calcium-containing agents have become somewhat limited due to the possibility of increasing the risk of vascular calcification and adynamic bone disease [Citation48]. Furthermore, the large amount of calcium salts required for phosphate binding may affect their effectiveness for the related symptomatic hypercalcemia [Citation46]. In this meta-analysis, no significant difference in mortality was identified between LC and calcium salt groups, while there was a significant reduction risk in hypercalcemia and coronary artery calcification with LC treatment in comparison to calcium salt group. Additionally, a remarkably lower risk in mortality with LC treatment in comparison to non-LC PBs was identified, and various adverse effects, such as hypotension, abdominal pain, diarrhea, and cough were reported with non-LC PBs in clinical trials. A previous study suggests that combination therapy with sevelamer and non-LC PBs is effective in decrease serum phosphate and Ca × P product in hyperphosphatemia patients, while gastrointestinal intolerance and compliance are significant side effects with such an approach [Citation46]. LC is poorly absorbed from the gastrointestinal tract and mainly eliminated by the liver. A 6-year follow-up study reported that LC was not associated with adverse reactions in the liver of patients with CKD [Citation49,Citation50]. Taken together, LC might be a safe and effective agent for hyperphosphatemia treatment in patients with CKD.
In this meta-analysis, we did not focus on the effect of comorbid drugs on the phosphorus lowering effect of phosphorus binders because most articles did not list the comorbid drugs with phosphorus binders. Recent studies have found that proton pump inhibitors (PPI) significantly attenuate the phosphorus reduction effect of LC, although it does not significantly affect the phosphorus reduction effect of ferric citrate hydrate (FCH) and sucroric oxyhydroxide (SFOH) [Citation51]. It has been reported that PPI also affects the phosphorus reducing effect of calcium [Citation52]. This suggests that we need to pay attention to the effect of comorbid drugs on phosphorus binders in future similar meta-analyses.
The heterogeneity test showed an obvious heterogeneity among studies for some variables. Thus, the random effect model was chosen to assess the pooled effect. The significant heterogeneity might be related to the various baseline characteristics of enrolled patients. For instance, patients involved in this study come from diverse regions of the world, and the age distribution ranged from 6 to 83 years old, mostly with middle-aged patients. Moreover, different stages of patients with CKD who underwent different dialysis modalities were included, such as early stage of CKD, end-stage renal disease, hemodialysis, peritoneal dialysis, or non-dialysis. In addition, the sample size might be another reason for the observed heterogeneity. For instance, 2026 patients treated with LC and 8094 patients treated with non-LC PBs were registered in the study by Randen et al, whereas only 17 patients treated with calcium and 17 patients treated with oral activated charcoal were registered in the study by Hutchison et al. [Citation24].
5. Conclusion
In conclusion, compared with calcium salts, non-LC PBs, sevelamer, and placebo, LC showed higher ability in serum phosphorus controls, reduced risk in gastrointestinal adverse events, coronary artery calcification, and mortality for patients with CKD. LC seemed to be a safe and effective agent in CKD treatment. However, future meta-analyses with a larger number of eligible primary articles still need to be carried out for more reliable results.
6. Limitations and perspectives
There are some limitations in this study: (1) publication bias test for most variables comparison except phosphorus control and total adverse events were not performed due to the less included studies; (2) the follow-up period included in the study were inconsistently ranged from four weeks to 5 years, and there was still no literature available to systematically assess the short-, medium- and long-term efficacy and safety of LC for CKD; (3) For some variables comparison, the less included study and small sample size might affect the results of meta-analysis, such as the comparison of total adverse events incidence between LC and non-LC PBs, only one study reported the effect of LC and non-LC PBs on the adverse events, and a significantly greater adverse events ratio was found with LC treatment. Hence, the analysis based on more studies with high quality was needed to confirm the clinical effect of LC in CKD treatment. In addition, this meta-analysis had not been registered online in advance, but the study was carried out and the article was written strictly according to the PRISMA statement.
Supplemental Material
Download JPEG Image (412.8 KB)Supplemental Material
Download PDF (59.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Michishita R, Matsuda T, Kawakami S, et al. Hypertension and hyperglycemia and the combination thereof enhanced the incidence of chronic kidney disease (CKD) in middle-aged and older males. Clin Exp Hypertens. 2017;39(37):1.
- Sharbaf FG, Assadi F. Effect of allopurinol on the glomerular filtration rate of children with chronic kidney disease. Pediatr Nephrol. 2018; 33(3):1–5.
- Vervloet MG, Ballegooijen AJV. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018;93(5):1060–1072.
- Haider DG, Lindner G, Wolzt M, et al. Hyperphosphatemia is an independent risk factor for mortality in critically ill patients: results from a cross-sectional study. PLOS One. 2015;10(8):e0133426.
- Shang D, Xie Q, Ge X, et al. Hyperphosphatemia as an independent risk factor for coronary artery calcification progression in peritoneal dialysis patients. BMC Nephrol. 2015;16(1):1–9.
- Francesco L, Lucia DV, Leano V, et al. Phosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: a comparison of safety profiles. Expert Opin Drug Saf. 2014;13(5):551–561.
- Stevens LA, Ognjenka D, Savannah C, et al. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol. 2004;15(3):770–779.
- Hans-Gernot A, Johan B, Rolfdieter K, et al. Two-year comparison of sevelamer and calcium carbonate effects on cardiovascular calcification and bone density. Nephrol Dial Transplant. 2005;20(8):1653–1661.
- Navaneethan SD, Palmer SC, Craig JC, et al. Benefits and harms of phosphate binders in CKD: a systematic review of randomized controlled trials. Am J Kidney Dis. 2009;54(4):619–637.
- Jamal SA, Ben V, Paolo R, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382(9900):1268–1277.
- Damment SJ, Pennick M. Clinical pharmacokinetics of the phosphate binder lanthanum carbonate. Clin Pharmacokine. 2008;47(9):553–563.
- Michael P, Lynne P, Kerry D, et al. Lanthanum carbonate reduces urine phosphorus excretion: evidence of high-capacity phosphate binding. Ren Fail. 2012;34(3):263–270.
- Xu J, Zhang YX, Yu XQ, et al. Lanthanum carbonate for the treatment of hyperphosphatemia in CKD 5D: a multicenter, double-blind, randomized, controlled trial in mainland China. BMC Nephrol. 2013;14(1):29.
- Green S. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Naunyn-Schmiedebergs Archiv Für Experimentelle Pathologie Und Pharmakologie. 2011;5(2):S38.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Al-Baaj F, Speake M, Hutchison AJ. Control of serum phosphate by oral lanthanum carbonate in patients undergoing haemodialysis and continuous ambulatory peritoneal dialysis in a short-term, placebo-controlled study. Nephrol Dial Transplant. 2005; 20(4):775–782.
- Chang YM, Tsai SC, Shiao CC, Liou HH, et al. Effects of lanthanum carbonate and calcium carbonate on fibroblast growth factor 23 and hepcidin levels in chronic hemodialysis patients. Clin Exp Nephrol. 2017;21(5):908–916.
- Chiang SS, Chen JB, Yang WC. Lanthanum carbonate (fosrenol) efficacy and tolerability in the treatment of hyperphosphatemia patients with end-stage renal disease. Clin Nephrol. 2005;63(06):461–470.
- Finn WF. Lanthanum carbonate versus standard therapy for the treatment of hyperphosphatemia: safety and efficacy in chronic maintenance hemodialysis patients. Clin Nephrol. 2006;65(03):191–202.
- Finn WF, Joy MS, Hladik G, et al. Efficacy and safety of lanthanum carbonate for reduction of serum phosphorus in patients with chronic renal failure receiving hemodialysis. Clin Nephrol. 2004;62(09):193–201.
- Fujii H, Kono K, Nakai K, et al. Effects of lanthanum carbonate on coronary artery calcification and cardiac abnormalities after initiating hemodialysis. Calcif Tissue Int. 2018;102(3):310–320.
- Gao Y, Wang G, Li Y, et al. Effects of oral activated charcoal on hyperphosphatemia and vascular calcification in Chinese patients with stage 3-4 chronic kidney disease. J Nephrol. 2019;32(2):265–272.
- Hutchison A, Whelton A, Thadhani R, et al. Long-term mortality and bone safety in patients with end-stage renal disease receiving lanthanum carbonate. Nephron. 2018;140(4):265–274.
- Hutchison AJ, Gill M, Copley JB, et al. Lanthanum carbonate versus placebo for management of hyperphosphatemia in patients undergoing peritoneal dialysis: a subgroup analysis of a phase 2 randomized controlled study of dialysis patients. BMC Nephrol. 2013;14(1):40.
- Hutchison AJ, Maes B, Vanwalleghem J, et al. Efficacy, tolerability, and safety of lanthanum carbonate in hyperphosphatemia: a 6-month, randomized, comparative trial versus calcium carbonate. Nephron Clin Pract. 2005;100(1):c8–c19.
- Joy MS, Finn WF. Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: a new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis. 2003;42(1):96–107.
- Kalil RS, Michael F, William S, et al. Dissociation between progression of coronary artery calcification and endothelial function in hemodialysis patients: a prospective pilot study. Clin Nephrol. 2012;78(1):1–9.
- Komaba H, Kakuta T, Suzuki H, et al. Survival advantage of lanthanum carbonate for hemodialysis patients with uncontrolled hyperphosphatemia. Nephrol Dial Transplant. 2015;30(1):107–114.
- Kyu LY, Young HC, SugKyun S, et al. Effect of lanthanum carbonate on phosphate control in continuous ambulatory peritoneal dialysis patients in Korea: a randomized prospective study. Clin Nephrol. 2013;79(2):136–142.
- Prajapati VA, Galani VJ, Shah PR. A comparative study of phosphate binders in patients with end stage kidney disease undergoing hemodialysis . Saudi J Kidney Dis Transpl. 2014;25(3):530–538.
- Scaria PT, Gangadhar R, Pisharody R. Effect of lanthanum carbonate and calcium acetate in the treatment of hyperphosphatemia in patients of chronic kidney disease. Indian J Pharmacol. 2009;41(4):187–191.
- Shigematsu T; Lanthanum Carbonate Group. Multicenter prospective randomized, double-blind comparative study between lanthanum carbonate and calcium carbonate as phosphate binders in Japanese hemodialysis patients with hyperphosphatemia. Clin Nephrol. 2008;70(5):404–410.
- Takashi S. Lanthanum carbonate effectively controls serum phosphate without affecting serum calcium levels in patients undergoing hemodialysis. Ther Apher Dial. 2010;12(1):55–61.
- Sprague SM, Ross EA, Nath SD, et al. Lanthanum carbonate vs. sevelamer hydrochloride for the reduction of serum phosphorus in hemodialysis patients: a crossover study. Clin Nephrol. 2009;72(4):252–258.
- Takahara Y, Matsuda Y, Takahashi S, et al; Lanthanum Carbonate Study Group. Efficacy and safety of lanthanum carbonate in pre-dialysis CKD patients with hyperphosphatemia: a randomized trial. Clin Nephrol. 2014;82(2014)(09):181–190.
- Tatsunori T, Keiichi F, Shouichi F, et al. Effect of lanthanum carbonate vs. calcium carbonate on serum calcium in hemodialysis patients: a crossover study. Clin Nephrol. 2012;78(3):216–223.
- Toussaint ND, Lau KK, Polkinghorne KR, et al. Attenuation of aortic calcification with lanthanum carbonate versus calcium-based phosphate binders in haemodialysis: a pilot randomized controlled trial. Nephrol. 2011;16(3):290–298.
- Wang X-H, Zhang X, Mu C-J, et al. Effects of lanthanum carbonate on vascular calcification in elderly maintenance hemodialysis patients. J Huazhong Univ Sci Technol. 2015;35(4):508–513.
- Wilson R, Zhang P, Smyth M, et al. Assessment of survival in a 2-year comparative study of lanthanum carbonate versus standard therapy. Curr Medi Res Opin. 2009;25(12):3021–3028.
- Zhang C, Wang S, Zhao S, et al. Effect of lanthanum carbonate on coronary artery calcification and bone mineral density in maintenance hemodialysis patients with diabetes complicated with adynamic bone disease: a prospective pilot study. Med. 2017;96(45):e8664.
- Spasovski GB, Aleksandar S, Saso G, et al. Evolution of bone and plasma concentration of lanthanum in dialysis patients before, during 1 year of treatment with lanthanum carbonate and after 2 years of follow-up. Nephrol Dial Transplant. 2006;21(8):2217–2224.
- Sprague SM, Abboud H, Qiu P, et al. Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: a randomized trial. CJASN. 2009;4(1):178–185.
- Ogata H, Fukagawa M, Hirakata H, LANDMARK Investigators and Committees, et al. Effect of treating hyperphosphatemia with lanthanum carbonate vs calcium carbonate on cardiovascular events in patients with chronic kidney disease undergoing hemodialysis: the LANDMARK randomized clinical trial. JAMA. 2021;325(19):1946–1954.
- Mario C, Sandro M, Vincent B. The treatment of hyperphosphataemia in CKD: calcium-based or calcium-free phosphate binders? Nephrol Dial Transplant. 2011;26(2):402–407.
- Sturtevant JM, Hawley CM, Reiger K, et al. Efficacy and side-effect profile of sevelamer hydrochloride used in combination with conventional phosphate binders. Nephrology. 2004;9(6):406–413.
- Sekercioglu N, Thabane L, Martínez JP, et al. Comparative effectiveness of phosphate binders in patients with chronic kidney disease: a systematic review and network meta-analysis. PLOS One. 2016; 11(6):e0156891.
- Mehrotra R, Martin KJ, Fishbane S, et al. Higher-strength lanthanum carbonate provides serum phosphorus control with a low tablet burden and is preferred by patients and physicians: a multicenter study. CJASN. 2008; 3(5):1437–1445.
- Hutchison AJ, Barnett ME, Krause R, et al. Lanthanum carbonate treatment, for up to 6 years, is not associated with adverse effects on the liver in patients with chronic kidney disease stage 5 receiving hemodialysis. Clin Nephrol. 2009;71(3):286–295.
- Bervoets A, Behets G, Roels F, et al. Hepatocellular transport and gastrointestinal absorption of lanthanum in chronic renal failure. Kidney Int. 2009;75(4):389–398.
- Minakuchi H, Yoshida T, Kaburagi N, et al. Proton pump inhibitors may hinder hypophosphatemic effect of lanthanum carbonate, but not of ferric citrate hydrate or sucroferric oxyhydroxide, in hemodialysis patients. Ren Fail. 2020;42(1):799–806.
- Tatsuzawa M, Ogawa R, Ohkubo A, et al. Influence of proton pump inhibitors and histamine H2 receptor antagonists on serum phosphorus level control by calcium carbonate in patients undergoing hemodialysis: a retrospective medical chart review. J Pharm Health Care Sci. 2016;2:34.