 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Acute kidney injury (AKI) often develops during the administration of liposomal amphotericin B (L-AMB), a broad-spectrum antifungal drug. However, clinical recovery approaches for AKI patients administered L-AMB are not well established. This retrospective analysis used the data obtained from hospitals throughout Japan. AKI was defined as a ≥ 1.5-fold increase within 7 days or ≥0.3 mg/dL increase within 2 days in serum creatinine. AKI recovery was defined as a return to creatinine levels below or equal to those recorded before AKI onset. Ninety patients were assessed for recovery from AKI as per the three stages. The incidence of recovery from AKI regardless of its stage was higher, though not significant, in patients administered ≥10 mL/kg/day fluid for 7 consecutive days from AKI onset (63%) than in those who did not (35%, p = 0.053). However, if limited to AKI stage 1 patients, the former group had a significantly higher incidence of recovery (91%) than the latter group (50%, p = 0.017), even after adjusting for confounding factors (odds ratio: 10.135, 95% confidence interval: 1.148–89.513, p = 0.037). The daily fluid volume administered during the 7 consecutive days from AKI onset positively correlated with the recovery from AKI of all stages (p = 0.043). Daily consecutive fluid infusion from AKI onset may be associated with recovery from stage 1 AKI in patients administered L-AMB, with daily fluid volume positively correlating with the incidence of AKI recovery.
Introduction
Invasive fungal infections frequently occur in immunocompromised and critically ill patients and are associated with high rates of morbidity and mortality [Citation1–5]. Amphotericin B is a broad-spectrum antifungal drug that covers clinically relevant yeasts and molds that cause mycoses, such as aspergillosis, candidiasis, cryptococcosis, and mucormycosis [Citation6]. However, the use of amphotericin B has been limited because of its high incidence of toxicities, including nephrotoxicity, liver disorders, and hypokalemia [Citation6]. Liposomal amphotericin B (L-AMB), which encapsulates amphotericin B in a liposomal membrane, was developed to reduce the toxicity of amphotericin B while maintaining its antifungal activity [Citation6]. However, this specific liposomal formulation causes reduced tissue distribution in the kidneys and drug-associated nephrotoxicity, including excessive renal vasoconstriction and renal tubular damage. [Citation7–9]. Despite the reduced nephrotoxicity, physicians are reluctant to prescribe L-AMB, especially to patients with renal failure, because of the risk of acute kidney injury (AKI) [Citation10,Citation11].
Several studies have reported that preexisting renal function, pretreatment and concomitant use of nephrotoxic or antifungal drugs, and L-AMB dosing are associated with AKI in patients administered L-AMB [Citation10–12]. Based on these findings, if these factors are carefully considered, the occurrence of renal dysfunction could be prevented in patients administered L-AMB. However, a study conducted in a single facility revealed that a high volume of fluid infusions one week before and one week after the initiation of L-AMB treatment was not associated with AKI recovery [Citation10]. Although this study suggests that fluid infusion does not contribute to AKI recovery, it appears that the timing, duration, and volume of fluid infusion must still be further scrutinized in a nationwide study. On the basis of claims and laboratory data obtained from hospitals throughout Japan, we identified patients who developed AKI following L-AMB treatment. Thereafter, we investigated the association of fluid infusion before and from the onset of AKI with AKI recovery outcomes and changes in creatinine levels after AKI onset.
Materials and methods
Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki. The data herein were anonymously processed by the database provider (Medical Data Vision Co., Ltd.) in accordance with the Act on the Protection of Personal Information of Japan and other related regulations. The study was approved by the Nagasaki University School of Medicine Research Ethics Committee (approval number 18033038-5). For the usage of un-linkable de-identified data, informed consent was waived by Nagasaki University School of Medicine Research Ethics Committee according to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labor, and Welfare of Japan.
Data source
This was a retrospective, multicenter, observational study based on electronic medical information data obtained between April 2008 and January 2018. This database, provided by Medical Data Vision Co., Ltd. (MDV), consisted of medical fee reimbursement claims and clinical laboratory results from 345 Japanese hospitals operated on the Diagnosis Procedure Combination (DPC) system. The database included the following baseline patient information: age, sex, diagnosis, and comorbidities at admission, coded using the International Classification of Diseases, 10th Revision (ICD-10). In addition, the dates and dosages of all drugs administered to each patient during hospitalization were recorded. All interventional procedures were decoded using standardized Japanese reimbursement codes. As DPC is an administrative database for inpatients, patient follow-up began on admission day and ended on discharge date, transferred to other hospitals, or death.
Study design
In line with the selection criteria described by Takazono et al. [Citation12], we identified patients who developed AKI after receiving the first administration of L-AMB. Thereafter, patients who met the following exclusion criteria were removed: (1) age <18 years; (2) zero and/or single serum creatinine record available during the specified periods to determine the development of AKI and baseline creatinine levels; (3) nonzero weight and/or height record unavailable; (4) a mean daily dose of L-AMB of >6 mg/kg/day per body weight; (5) renal replacement therapy performed on the day of or prior to L-AMB treatment initiation; and (6) AKI developed prior to L-AMB treatment initiation. Furthermore, to determine the changes in serum creatinine levels after AKI onset, patients with no recordings of serum creatinine and/or renal replacement therapy during the evaluation period were excluded. For each patient, the evaluation period started on the day after AKI and ended 30 days after AKI, the day of discharge, death, or the day before L-AMB re-administration, whichever came first. Finally, to determine the incidence of 30-day AKI recovery, we excluded patients with no serum creatinine record between 7 days after AKI and the end of the evaluation period, as well as unrecovered patients, if they were discharged within 29 days or re-administered L-AMB within 30 days of AKI onset.
Definitions
The duration of L-AMB therapy was defined as the time from treatment initiation to discontinuation, with an administration interval of ≥8 days. AKI was defined as a ≥1.5-fold increase within 7 days or ≥0.3 mg/dL increase within 2 days in serum creatinine level [Citation13]. AKI was assigned to one of three stages: stage 1, ≥1.5- to <2-fold increase or ≥0.3 mg/dL increase in Cr; stage 2, ≥2- to <3-fold increase in Cr; stage 3, ≥3-fold increase in Cr, ≥4.0 mg/dL of Cr [Citation13]. AKI recovery was defined as a return to creatinine levels below or equal to those recorded before the onset of AKI, the minimum creatinine level within 7 days or 2 days before the onset of AKI, with ≥1.5-fold increase or ≥0.3 mg/dL increase, respectively. For the interventions investigated, the daily fluid infusion was determined by daily volume of extracellular replacement fluid per weight, such as saline and balanced crystalloid solutions (the equilibrium buffer solutions contained sodium lactate, sodium acetate, or sodium hydrogen carbonate), excluding parenteral nutrition with chloride. We defined liberal fluid management group as patients infused with daily fluid volume per weight being more than or equal to a specified threshold for a specified period. Patients infused less fluid than the specified threshold on at least one day during the corresponding period were included in the conservative fluid management group. We defined the baseline threshold for daily fluid volume per weight as ≥10 mL/kg/day and fluid administration period as 7 days from the onset of AKI unless otherwise specified. The last nonzero weight recorded on the day of or within 1 year prior to L-AMB treatment initiation was used to determine fluid volume per weight. Discontinuation of L-AMB treatment was defined as the termination of L-AMB administration until or within 3 days after the onset of AKI. Baseline creatinine was defined as the minimum serum creatinine level recorded between 180 days and 7 days before the initiation of L-AMB administration.
With the last nonzero weight and height recorded on the day of or within 1 year prior to L-AMB treatment initiation, the conversion to corresponding estimated glomerular filtration rate (eGFR) was derived using a Japanese-specific formula [Citation14]:
where body surface area (m2) was derived as
In addition, the change in minimum creatinine level in a given period after AKI onset was defined as the difference between the creatinine level on the day of AKI onset and the minimum recorded creatinine level in the period, including the day of AKI onset.
Comorbidities and fungal infections were identified using the corresponding ICD-10 codes that were registered in the month of L-AMB treatment initiation. Diabetes mellitus was defined using the ICD10 classification code E10-E14, and chronic kidney disease was defined using the ICD10 code N18. Congestive heart failure was defined using the ICD10 codes described by Quan et al. [Citation15]. Treatment with catecholamine, nephrotoxic drugs, and hypokalemia (<3.5 mEq/L serum potassium) were identified between 7 days before the day of L-AMB treatment initiation and the day before AKI recovery or the end day of AKI recovery evaluation period (30 days after AKI onset, the day of discharge, death, or the day before L-AMB re-administration, whichever came first).
Statistical analysis
Fisher’s exact test was used to compare the incidence of AKI recovery between the liberal and the conservative fluid management groups. This test was also used to compare the incidence of AKI recovery between patients administered L-AMB who recovered from AKI and patients administered L-AMB who did not recover from AKI. To exclude the effect of confounding factors on AKI recovery, logistic regression analysis was conducted using the occurrence of AKI recovery as the dependent variable. We selected 15 independent variables from the patient characteristics that were considered to be associated with AKI recovery in patients administered L-AMB or that were identified as the factors related to AKI (any stage or stage 2 or 3) in patients administered L-AMB (i.e., treatment with angiotensin-converting enzyme inhibitor/angiotensin receptor blocker [ACE inhibitors/ARB], carbapenem, catecholamine, or immunosuppressants, hypokalemia, and L-AMB average daily dose) [Citation12]. These variables included liberal fluid management, age, sex, comorbidities (diabetes mellitus, congestive heart failure), catecholamine treatment, hypokalemia (<3.5 mEq/L serum potassium), baseline eGFR, L-AMB average daily dose (continuous variable), and treatment with nephrotoxic drugs (vancomycin, aminoglycoside, carbapenem, immunosuppressants, diuretics, or ACE inhibitors/ARB). Variables were subjected to a univariate binomial logistic regression analysis. Variables with a p-value of <0.1 in univariate logistic regression analysis and liberal fluid management were subjected to multivariate logistic regression analysis. Odds ratio (OR), 95% confidence interval (CI), and variance inflation factor (VIF) were calculated. The correlation between the incidence of AKI recovery and fluid infusion volume was determined using the Cochran–Armitage trend test. Finally, Welch’s t-test was used to compare the decline in creatinine levels after AKI onset between the liberal fluid management group (from 1 day to 6 days after AKI onset) and the conservative fluid management group. Statistical significance was set at p-value of <0.05.
Results
Study population and patient characteristics
By applying the criteria and definitions described in the Materials and methods, 189 patients were identified as having developed AKI following L-AMB administration. Of these patients, we evaluated 90 who were administered L-AMB and developed AKI to assess AKI recovery (). As shown in , 62% of the patients were male and 38% were female. The mean age was approximately 65 years, and the mean body mass index (BMI) was 22.0 ± 4.1 kg/m2. The prevalence of comorbidities was as follows: diabetes mellitus, 37%; chronic kidney disease, 8%; and congestive heart failure, 27%. Twenty percent of the patients received treatment with catecholamine, while 80% of the patients suffered from hypokalemia. The mean baseline eGFR was 101.8 ± 42.8 mL/min. More than half of the patients received treatment with several nephrotoxic drugs, such as vancomycin (52%), carbapenem (70%), and diuretics (69%). More than half of the patients were administered L-AMB in the hematology department (56%). Of the fungal infections identified, aspergillosis was the most commonly recognized (29%) among the patients. The mean daily dose was 2.7 ± 0.9 mg/kg/day and the duration of L-AMB administration was approximately 20 days.
Figure 1. Flow chart for patient selection. AKI: acute kidney injury; L-AMB: liposomal amphotericin B.
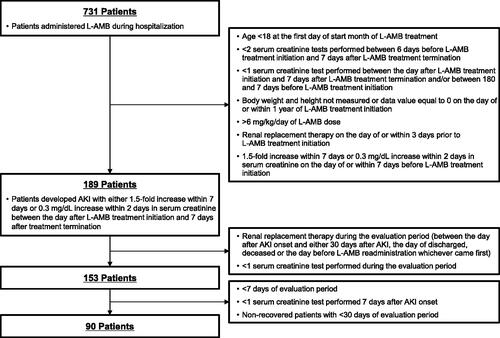
Table 1. Patient characteristics of liberal and conservative fluid management groups.
Of the 90 patients, 16 (18%) were categorized as a liberal fluid management group. BMI was lower in the liberal fluid management group (20.3 ± 2.6 kg/m2) than the conservative fluid management group (22.3 ± 4.3 kg/m2, p = 0.022). The proportion of patients treated with ACE inhibitors/ARB was lower in the liberal fluid management group (0%) than in the conservative fluid management group (23%, p = 0.035). No significant differences were found between the two groups according to sex, age, comorbidities, catecholamine treatment, hypokalemia, eGFR at baseline, average daily dose and duration of L-AMB treatment, treatment department, and diagnosis.
Association between fluid infusions and recovery from AKI in patients administered L-AMB by AKI stage
As shown in , in patients with all stages of AKI, the liberal fluid management group had a higher, though not significant, incidence of renal recovery (63%) than in the conservative fluid management group (35%, p = 0.053). For patients with AKI stage 1, a significantly higher proportion of patients recovered from AKI in the liberal management group (91%) than in the conservative management group (50%, p = 0.017). In patient with AKI stage 2 or 3, the liberal fluid management group did not have a higher incidence of AKI recovery compared to the conservative fluid management group. To exclude the effects of confounding factors on AKI recovery, we conducted logistic regression analysis for patients with all stages of AKI and for patients with AKI stage 1 only. After adjusting for confounding factors, liberal fluid management was not associated with recovery from AKI of all stages (OR: 2.381, 95% CI: 0.701-8.083, p = 1.037) (Supplementary Table 1). For AKI stage 1 patients, univariate logistic regression analysis revealed that liberal fluid management and no treatment with vancomycin were associated with AKI recovery (p < 0.1) (). Using these variables in multivariate regression analysis, we found that liberal fluid management was identified as a factor associated with recovery from AKI stage 1 (OR: 10.135, 95% CI: 1.148–89.513, p = 0.037) (). As for other interventions, we examined L-AMB discontinuation until or within 3 days after the onset of AKI; however, no significant difference in the incidence of AKI recovery was observed (19/49, 39%, discontinued; 17/41, 41%, continued, p = 0.832).
Table 2. Incidence of AKI recovery in patient administered fluid infusion by AKI stage.
Table 3. Logistic regression analysis of the factors associated with AKI recovery from stage 1 AKI in the liberal and the conservative fluid management groups.
Association between daily consecutive fluid infusion timing in relation to the onset of AKI and AKI recovery in patients administered L-AMB
We hereafter assessed the relation between fluid management variations and AKI recovery with patients regardless of their AKI stages due to the limited number of subjects. To examine the association between fluid infusion period or timing and AKI recovery, we examined the incidence of AKI recovery in patients who received fluid infusion 2 days or 7 days before or from AKI onset. As shown in , the incidence of AKI recovery was similar between the liberal fluid management group (7 days before AKI onset) (33%) and the conservative fluid management group (36%). However, liberal fluid management groups (2 days before/from AKI onset) had a slightly higher, though not significant, incidence of AKI recovery (before AKI onset: 50%; from AKI onset: 50%) than respective conservative fluid management groups (before AKI onset: 35%, p = 0.247; from AKI onset: 36%, p = 0.242) ().
Table 4. Incidence of AKI recovery in patients administered fluid infusion before and after AKI onset.
Association between the daily volume of fluid infusion and AKI recovery in patients administered L-AMB
We sought to determine the association between the volume of fluid infusions and the incidence of AKI recovery in patients administered L-AMB. As shown in , we evaluated the incidence of AKI recovery in patients administered daily fluid infusions between ≥5 mL/kg and ≥30 mL/kg consecutively for 2 days or 7 days from AKI onset. In conservative fluid management groups (2 or 7 days from AKI onset), the incidence of AKI recovery remained relatively constant at approximately 39% for all daily fluid volume thresholds. However, for the liberal fluid management group (2 days from AKI onset), a daily volume of ≥25 mL/kg resulted in the highest incidence of AKI recovery (57%), and there was no significant difference between the incidence of AKI recovery and fluid volume increase. For the liberal fluid management group (7 days from AKI onset), higher incidences of AKI recovery were observed when higher daily fluid volumes were administered. Although the number of subjects in the higher fluid volume groups was extremely limited, daily fluid volume was found to be positively correlated with the incidence of AKI recovery (p = 0.043, Cochran–Armitage trend test; <5 mL/kg, 17/49, 35%; ≥5 mL/kg and <10 mL/kg, 9/25, 36%; ≥10 mL/kg and <15 mL/kg, 5/8, 63%; ≥15 mL/kg and <20 mL/kg, 1/2, 50%; ≥20 mL/kg and <25 mL/kg, 1/3, 33%; ≥25 mL/kg and <30 mL/kg, 2/2, 100%; ≥30 mL/kg, 1/1, 100%).
Table 5. Incidence of AKI recovery in patients infused with the selected daily fluid volume.
Association between the duration of consecutive daily fluid infusion from AKI onset and AKI recovery in patients administered L-AMB
As shown in , we evaluated the incidence of AKI recovery for patients administered daily fluid volumes of ≥10 mL/kg or ≥25 mL/kg for the selected periods from the onset of AKI. Although the incidence of AKI recovery was found to be higher, though not significant, with the liberal fluid management group (≥10 mL/kg or ≥25 mL/kg) than with the respective conservative fluid management group for the evaluated periods, fluid infusions for 7 consecutive days yielded a relatively high incidence of AKI recovery (≥10 mL/kg, 63%; ≥25 mL/kg, 100%) than infusions for 2 consecutive days (≥10 mL/kg, 50%; ≥25 mL/kg, 57%) or 5 consecutive days (≥10 mL/kg, 50%; ≥25 mL/kg, 80%). Thus, despite the extremely limited number of subjects employed for these longer periods, extending the daily fluid infusion period over 7 days from AKI onset was not found to be associated with the incidence of AKI recovery in patients administered L-AMB.
Table 6. Incidence of AKI recovery in patients administered infused fluids for the selected periods.
Changes in the level of serum creatinine in patients administered L-AMB who received daily consecutive fluid infusion
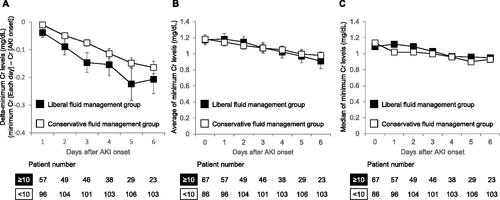
Finally, changes in the minimum creatinine levels from AKI onset to each observation day were assessed for 153 patients; these patients had at least one creatinine record and no renal replacement therapy during the evaluation period. As described above, we compared the minimum creatinine levels from the day of AKI onset between the liberal and conservative fluid management groups to each of the first 6 days following AKI onset. As the unbiased levels of creatinine on AKI onset were obtained until 6 days after AKI onset in the liberal fluid management group, we evaluated minimum creatinine levels until 6 days after AKI onset. The liberal fluid management group have a decline, although not significantly, in delta-minimum creatinine (the difference between minimum creatinine on each day and the creatinine level on AKI onset) as well as the average and median of minimum creatinine for the first 6 days after the onset of AKI relative to corresponding conservative fluid management group (). On day 6 following AKI onset (i.e., after 7 days of consecutive fluid infusion, including the day of AKI onset), the minimum creatinine levels were reduced by 0.21 mg/dL for the liberal fluid management group and 0.16 mg/dL for the conservative fluid management group ().
Figure 2. Change in minimum creatinine levels after the onset of AKI in the liberal and the conservative fluid management groups. (a) Delta-minimum Cr levels. On each day, the levels of minimum Cr were identified as the minimum value of Cr in the liberal fluid management group (≥10 mL/kg/day daily consecutive fluid infusion between AKI onset and each observation day) and the conservative fluid management group (<10 mL/kg/day fluid infusion for at least one day during the corresponding period). The levels of delta-minimum Cr were calculated as the difference between minimum Cr on each day and the Cr level on AKI onset. (b, c) Average (b) and median (c) of minimum Cr levels. As the unbiased levels of Cr on AKI onset were obtained until 6 days after AKI onset in the liberal fluid management group, we evaluated minimum Cr levels until 6 days after AKI onset. Error bars represent the standard error of the mean. Welch’s t-test was employed to compare the groups (a, b). AKI: acute kidney injury; Cr: creatinine; N: number.
Discussion
In the present study, we retrospectively evaluated patients who developed AKI following treatment with L-AMB by using a dataset obtained from 345 medical facilities in Japan. In addition, we sought to determine the association between the interventions and AKI recovery. Previously, Yamazaki et al. suggested that intravenous fluid infusion one week before and after the initiation of L-AMB administration did not contribute to AKI recovery. Thus, we investigated the association between fluid infusion and AKI recovery when an infusion was administered before or from AKI onset (). Accordingly, we observed a higher, although not significant, incidence of AKI recovery for the liberal fluid management group (a 1.8-fold increase, 63 vs. 35%, p = 0.053). However, if limited to AKI stage 1 patients, the liberal fluid management group had a significantly higher recover rate (a 1.8-fold increase, 91 vs. 50%, p = 0.017) even after adjusting for confounding factors (OR: 10.135, 95% CI: 1.148–89.513, p = 0.037). On the other hand, though the number of subjects was limited, the liberal fluid management group did not have a higher incidence of AKI recovery for patients with AKI stage 2 or 3. This difference in prognosis may be due to the differences in the severity of underlying conditions including the use of catecholamine between AKI stage 1 patients (6/51, 12%) and AKI stage 2 or 3 patients (12/39, 31%, p = 0.034). The non-recovered patients in the liberal fluid management group may have partially recovered from AKI as they exhibited more reductions in minimum creatinine level from AKI onset though there were no significant differences (). Furthermore, despite the limited number of subjects, a positive correlation was found between daily fluid volume for 7 consecutive days from AKI onset and incidence of AKI recovery (p = 0.043, Cochran–Armitage trend test), with an approximate 2.6-fold increase in the incidence of recovery from AKI of all stages for liberal fluid management group (≥25 mL/kg) relative to the conservative fluid management group. (). Extending the fluid infusion period beyond 7 days with a fixed volume did not result in a higher incidence of AKI recovery (), daily high-volume fluid infusion for 1 week from AKI onset may be associated with recovery from AKI.
To evaluate the association of consecutive fluid infusion over a period of time with AKI recovery, a liberal fluid management group was derived by selecting patients whose fluid infusion volumes were more than or equal to the threshold each day during a specified period and not patients whose mean fluid volume over the corresponding period was more than or equal to the threshold. In other words, the former definition specifically excludes patients that were intermittently infused with large volumes of fluid a few days out of the evaluation period and no fluid administration on the remaining days. We employed this definition of fluid management because the frequency of fluid infusion could be an important factor for AKI recovery. For instance, patients with a daily fluid infusion of ≥10 mL/kg/day for 7 consecutive days from AKI onset had an incidence of AKI recovery of 63% (10/16), whereas patients who received a mean fluid infusion volume ≥10 mL/kg/day for 7 days had an incidence of AKI recovery of 45% (14/31); the latter was however 27% (4/15) when patients administered daily fluid infusion were excluded. It should also be noted that fluids were confined to extracellular fluids, such as isotonic saline, which excluded parenteral nutrition with chloride as it was determined as a known risk factor for AKI [Citation16].
The implication that daily high-volume fluid infusion from AKI onset may be associated with AKI recovery is consistent with standard interventions, such as salt loading against amphotericin B-associated nephrotoxicity [Citation17]. However, such implications differ from the findings of Yamazaki et al., who reported that one week of fluid infusion before and after L-AMB administration initiation did not significantly improve the incidence of AKI recovery. This discrepancy between the two studies may be due to differences in the timing of fluid infusion, frequency of fluid infusion, and types of infused fluids.
Notably, if no interventions are found to be associated with AKI recovery after AKI onset, preventative measures will become more critical. Herein, liberal fluid management did not increase the incidence of recovery from stage 2 or 3 AKI; however, the risk factors associated with L-AMB-induced stage 2 or 3 AKI have been previously identified [Citation12]. Special attention should be paid to patients with these risk factors: Hypokalemia before L-AMB therapy; prior treatment with angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers or carbapenem; concomitant administration of catecholamines; and higher mean daily dose of L-AMB administration [Citation12].
In patients with L-AMB-induced nephrotoxicity, adequate hydration and careful electrolyte supplementation could prevent or alleviate a reduction in eGFR or an increase in serum creatinine, without affecting on tubular toxicity [Citation18,Citation19]. The types of fluids may not have a remarkable effect on preventing or attenuating L-AMB-induced AKI. In this study, the incidence of AKI recovery was higher, although not significant, in patients receiving liberal fluid management with saline and/or balanced crystalloid solutions (8/11, 73%) than those receiving conservative fluid management with saline and/or balanced crystalloid solutions (7/17, 41%, p = 0.137), while the incidence of AKI recovery was similar between patients receiving liberal fluid management with saline alone (2/5, 40%) and those receiving conservative fluid management with saline alone (19/54, 35%, p = 1.000). Another group demonstrated that the combination of sodium bicarbonate and normal saline compared to normal saline alone appears to have no superiority in preventing or attenuating amphotericin B nephrotoxicity in patients with hematological malignancies [Citation20].
In this study, the proportion of patients treated with vancomycin was higher in the group that did not recover from AKI (63%) than in the group that recovered from AKI (36%, p = 0.018). Furthermore, concomitant administration of vancomycin was negatively associated with recovery from AKI of all stages after adjusting for confounding factors (Supplementary Table 1) though it was not the case for stage 1 AKI. Vancomycin-induced renal toxicity may be due to oxidative effects on the proximal renal tubule, which results in renal tubular ischemia, or allergic interstitial nephritis [Citation21], which is different from the mechanism underlying L-AMB-induced renal toxicity (i.e., tubular injury or renal vasoconstriction) [Citation22,Citation23]. Therefore, careful concomitant treatment with vancomycin may be needed to attenuate AKI in patients administered L-AMB.
Our study had several limitations. First, several data could not be obtained from the database, which limited the study design. Because the database did not include follow-ups after discharge or hospital transfer, the renal condition of patients was difficult to assess for a sufficiently long period. During the assessment of the incidence of AKI recovery, patients were excluded from the sample if they were discharged within 29 days or re-administered L-AMB within 30 days after AKI onset. Despite the inconsistent frequencies of serum creatinine measurements across patients, AKI recovery was only evaluated using the available records. Furthermore, we could not evaluate AKI by assessing reductions in urine volume, as this parameter could not be obtained from the database employed. Second, the generalizability of the findings presented herein requires further discussion. This is because the database did not contain patients admitted to university hospitals, where infectious disease experts were more likely to work or where the facilities had less than 200 beds. Additionally, in this study, patients who underwent renal replacement therapy (RRT), including dialysis and continuous renal replacement therapy, were excluded. Therefore, RRT was not considered an endpoint of AKI recovery. Accordingly, our study findings may not be fully representative of patients treated throughout Japan. Finally, further verification is required. Due to its retrospective design, this study could not prove a cause-effect relationship between fluid infusion and recovery from L-AMB-induced AKI. A clear dose-response effect regarding fluid volume or duration was not observed, which limits the enthusiasm regarding the findings. The introduction of liberal and conservative fluid management as dichotomous groups does not fully capture the complex and dynamic fluid infusion pattern over the evaluation period in real clinical settings. Confounders that increased fluid volumes may represent lower levels of serum creatinine, rather than a real representation of improved AKI recovery. The incidence of AKI following the L-AMB treatment initiation may not have been L-AMB-induced. Moreover, the sample size in several groups, especially patients administered with larger volumes of fluid infusion, is extremely small. Therefore, further studies with a large number of subjects from real-world databases and prospective studies are necessary to verify the results obtained herein.
In conclusion, 7 consecutive days of daily fluid infusion from the onset of AKI may be associated with recovery from stage 1 AKI in patients administered L-AMB. For those administered fluid infusions for 7 days from AKI onset, daily fluid volume was found to be positively correlated with the incidence of AKI recovery.
Author contributions
M. Tashiro, Y. Obata, T. Takazono, T. Wakamura, T. Miyazaki, T. Nishino, and K. Izumikawa contributed to the study conception and design. Data analysis was performed by Y. Shiozawa and A. Tsuyuki. M. Tashiro drafted the manuscript. M. Tashiro, Y. Obata, T. Takazono, Y. Ota, T. Wakamura, T. Miayazaki, T. Nishino, and K. Izumikiawa reviewed and edited the manuscript. Data analysis was also conducted by Akinori Takahashi, Masato Homma, and Kumiko Sato from Deloitte Tohmatsu Consulting LLC. Sumitomo Dainippon Pharma Co., Ltd. did not contribute in the data analysis. All authors discussed the results and approved the final manuscript.
Supplemental Material
Download PDF (198.7 KB)Acknowledgements
We thank Kenji Baba from Sumitomo Dainippon Pharma Co., Ltd. for supporting the creation of the study plan. Medical writing was supported by Deloitte Tohmatsu Consulting LLC, and proofreading was supported by Editage.
Disclosure statement
No potential conflict of interest was reported by the author(s). T. Wakamura is a full-time employee of Sumitomo Dainippon Pharma Co., Ltd.; and Y. Shiozawa and A. Tsuyuki are full-time employees of Deloitte Tohmatsu Consulting LLC.
Data availability statement
The data that support the findings of this study are available from Medical Data Vision Co., Ltd., but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The data, however, are available from the authors upon reasonable request and with permission from the Medical Data Vision Co., Ltd. (https://www.mdv.co.jp/).
Additional information
Funding
References
- Kleinberg M. Aspergillosis in the CLEAR outcomes trial: working toward a real-world clinical perspective. Med Mycol. 2005;43(Suppl 1):S289–S294.
- Brown JM. Fungal infections in bone marrow transplant patients. Curr Opin Infect Dis. 2004;17(4):347–352.
- Marr KA, Carter RA, Crippa F, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909–917.
- Morgan J, Wannemuehler KA, Marr KA, et al. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol. 2005;43(Suppl 1):S49–S58.
- O’Brien SN, Blijlevens NMA, Mahfouz TH, et al. Infections in patients with hematological cancer: recent developments. Hematology Am Soc Hematol Educ Program. 2003;2003(1):438–472.
- Stone NR, Bicanic T, Salim R, et al. Liposomal amphotericin B (AmBisome(®)): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs. 2016;76(4):485–500.
- Barrett JP, Vardulaki KA, Conlon C, Amphotericin B Systematic Review Study Group, et al. A systematic review of the antifungal effectiveness and tolerability of amphotericin B formulations. Clin Ther. 2003;25(5):1295–1320.
- Karimzadeh I, Khalili H, Farsaei S, et al. Role of diuretics and lipid formulations in the prevention of amphotericin B-induced nephrotoxicity. Eur J Clin Pharmacol. 2013;69(7):1351–1368.
- Loo AS, Muhsin SA, Walsh TJ. Toxicokinetic and mechanistic basis for the safety and tolerability of liposomal amphotericin B. Expert Opin Drug Saf. 2013;12(6):881–895.
- Yamazaki H, Kondo T, Aoki K, et al. Occurrence and improvement of renal dysfunction and serum potassium abnormality during administration of liposomal amphotericin B in patients with hematological disorders: a retrospective analysis. Diagn Microbiol Infect Dis. 2018;90(2):123–131.
- Saito Y, Horita E, Uesaka T, et al. Factor analysis in decreased expression of renal function by liposomal-amphotericin B. J Pharm Health Care Sci. 2014;40(2):94–99.
- Takazono T, Tashiro M, Ota Y, et al. Factor analysis of acute kidney injury in patients administered liposomal amphotericin B in a real-world clinical setting in Japan. Sci Rep. 2020;10(1):15033.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139.
- Krajewski ML, Raghunathan K, Paluszkiewicz SM, et al. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102(1):24–36.
- Mayer J, Doubek M, Doubek J, et al. Reduced nephrotoxicity of conventional amphotericin B therapy after minimal nephroprotective measures: animal experiments and clinical study. J Infect Dis. 2002;186(3):379–388.
- Karimzadeh I, Farsaei S, Khalili H, et al. Are salt loading and prolonging infusion period effective in prevention of amphotericin B-induced nephrotoxicity. Expert Opin Drug Saf. 2012;11(6):969–983.
- Girmenia C, Cimino G, Di Cristofano F, et al. Effects of hydration with salt repletion on renal toxicity of conventional amphotericin B empirical therapy: a prospective study in patients with hematological malignancies. Support Care Cancer. 2005;13(12):987–992.
- Karimzadeh I, Sepehr-Sobhani A, Khoshnoud MJ, et al. Comparison of intravenous sodium bicarbonate and sodium chloride combination versus intravenous sodium chloride hydration alone in reducing amphotericin B nephrotoxicity: a randomized clinical trial. Res Pharm Sci. 2020;15(6):583–591.
- Vora S. Acute renal failure due to vancomycin toxicity in the setting of unmonitored vancomycin infusion. Proc (Bayl Univ Med Cent). 2016;29(4):412–413.
- Zager RA, Bredl CR, Schimpf BA. Direct amphotericin B-mediated tubular toxicity: assessments of selected cytoprotective agents. Kidney Int. 1992;41(6):1588–1594.
- Heyman SN, Stillman IE, Brezis M, et al. Chronic amphotericin nephropathy: morphometric, electron microscopic, and functional studies. JASN. 1993;4(1):69–80.
