Abstract
This study aimed to explore the prevalence and risk factors of poor sleep quality in patients undergoing continuous ambulatory peritoneal dialysis (CAPD) at the peritoneal dialysis center of the First Affiliated Hospital of Nanchang University. This cross-sectional study was conducted from March 2019 to December 2019. The Pittsburgh Sleep Quality Index (PSQI) was used to evaluate the sleep quality of patients undergoing CAPD. A PSQI score of ≥5 was defined as poor sleep quality, whereas a PSQI of <5 was defined as good sleep quality. Logistic regression analysis was used to analyze risk factors for poor sleep quality. In total, 456 patients undergoing CAPD were investigated. The average PSQI score was 5.0 ± 2.9. Among the participants, 46.3% had poor sleep quality, and 45.6% were female patients. The average age was 49.4 ± 13.3 years. Compared with good sleepers, poor sleepers included a higher proportion of females and calcium–phosphorus (Ca × P) product, longer dialysis durations, lower total endogenous creatinine clearance rates, less residual renal function, and lower albumin levels. Multivariate logistic regression analysis showed that a long dialysis duration, low albumin level, and high Ca × P product were independent risk factors for poor sleep quality in patients undergoing CAPD. Odds ratios (95% confidence interval) for these risk factors were 1.01 (1.00–1.02), 0.95 (0.91–1.00), and 1.02 (1.00–1.03), respectively. Interventions aimed at improving albumin and Ca × P product levels may improve quality of life for CAPD patients.
Introduction
Sleep disorders are common complications of end-stage renal disease (ESRD), and they are the main factors leading to death and decline in quality of life in patients with ESRD [Citation1,Citation2]. It is estimated that about 45%–85% of patients undergoing hemodialysis and 43%–80% of patients undergoing continuous ambulatory peritoneal dialysis (CAPD) have various sleep disorders [Citation1,Citation3–6]. Sleep disorders include insomnia, sleep apnea syndrome, hypopnea syndrome, central sleep apnea, central drowsiness, diurnal sleep awakening disorder, unconscious sleep, and restless leg syndrome [Citation1,Citation7–9]. Most dialysis patients have at least one sleep disorder [Citation7]. The main manifestations are difficulty falling asleep, sleep maintenance disorder, early awakening, decreased sleep quality, and decreased total sleep time.
The causes of poor sleep quality in patients undergoing CAPD are not fully understood and may be multifactorial. Previous studies have shown that male sex [Citation10], old age [Citation11], depression [Citation12], anemia [Citation13], uremic pruritus [Citation14], low serum vitamin D [Citation15], and duration of dialysis [Citation16] all contributed to sleep disturbances in dialysis patients. In addition, Li et al. [Citation4] found that high calcium–phosphorus (Ca × P) product, low subjective global assessment scores, and high malnutrition–inflammation scores predicted poor sleep quality in patients with CAPD. However, due to discrepancies in race, region, center, and socioeconomic status, there may be differences in the management of patients undergoing peritoneal dialysis (PD), which eventually lead to inconsistencies in the prevalence and risk factors of poor sleep quality. Furthermore, related research on poor sleep quality is limited to the underdeveloped areas of China.
Since poor sleep quality are the main complaints and strong predictors of mortality of patients undergoing PD, it is necessary to determine coexisting clinical and modifiable biochemical disorders. Therefore, this study aimed to investigate the prevalence and potential risk factors of poor sleep quality in patients undergoing PD in Southeast China.
Materials and methods
Study population
To investigate the prevalence and risk factors of poor sleep quality at the First Affiliated Hospital of Nanchang University, a survey was conducted from March 2019 to December 2019. A total of 456 patients undergoing CAPD were enrolled in this study. The inclusion criteria included age of ≥18 years and CAPD of ≥3 months. Exclusion criteria included mental illness, malignant history, cardiovascular history, and trauma or surgery in the month prior to the study. The study was approved by the Human Ethics Committee of Nanchang University (Application ID: [2019] 032), and written informed consent was obtained from each participant.
Assessment of sleep quality
The Pittsburgh Sleep Quality Index (PSQI) [Citation17] is a common tool for assessing sleep problems associated with anxiety, stress, depression, and schizophrenia. It is divided into seven parts: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disorders, the use of sleep medications, and daytime dysfunction caused by sleep disorders. The score of each part is 0–3, and the highest total score is 21. A score of <5 indicates good sleep quality, and >5 indicates poor sleep quality. A higher score indicates worse sleep quality.
Questionnaires
Questionnaires were administered at the outpatient visits of patients who were regularly followed up. Otherwise, nurses telephoned the patients to gather information. At the outpatient visit, the questionnaires were issued, explained, and recycled by PD nurses. The questionnaires were filled out by the patients or nurses, if patients were illiterate, when they were available to answer questions without interfering with the answers of the participants. Finally, data were independently extracted by two PD doctors.
Basic information collection
Demographic, clinical and laboratory information were extracted from the patients’ medical records, retrospectively. The data included sex, age, duration of CAPD, Charlson Comorbidity Index (CCI) score, body mass index (BMI), diabetes, hypertension, total Kt/V, total endogenous creatinine clearance rate (Ccr), estimated glomerular filtration rate (eGFR), hemoglobin, creatinine, uric acid, albumin, total cholesterol, triglyceride, Ca × P product, intact parathyroid hormone (iPTH), and C-reactive protein. Baseline residual renal function (RRF) was assessed by eGFR using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation [Citation18].
Statistical analysis
SPSS (version 22.0; IBM Corp., Armonk, NY, USA) was used for the statistical analysis. A p-value of <0.05 was regarded as statistically significant. Categorical variables were expressed as frequencies and percentages, and continuous variables were expressed as means and standard deviations or medians and interquartile ranges. Student’s t test, Mann-Whitney U test, and chi-squared test were used for comparisons. Univariate and multivariate logistic analyses were also performed. Covariates considered to be clinically significant or with a p-value of <0.05 in the univariate logistic regression were chosen for multivariate logistic regression.
Results
Baseline characteristics of participants undergoing CAPD
Among the 456 participants in the study, the average PSQI score was 5.0 ± 2.9. While 245 patients were good sleepers (PSQI < 5), 211 were poor sleepers (PSQI ≥ 5) (). The prevalence of poor sleep quality was 46.3%, the mean age was 49.4 ± 13.3 years, and 45.6% were female. The baseline eGFR was 1.05 mL/min/1.73 m2 (range, 0.00–3.12). The baseline characteristics of the participants, stratified by PSQI score, are shown in . The average PSQI scores of poor sleepers and good sleepers were 7.4 ± 2.6 and 3.0 ± 0.9, respectively. The duration of CAPD was significantly longer in poor sleepers (33.2 vs. 24.9 months; p < 0.001). The mean values of total Ccr, eGFR, albumin, and the proportion of men were significantly lower in poor sleepers. The mean Ca × P product of poor sleepers was higher than that of good sleepers at baseline (p = 0.014).
Figure 1. Enrollment flow chart of this study. PD: Peritoneal dialysis; PSQI: Pittsburgh Sleep Quality Index.
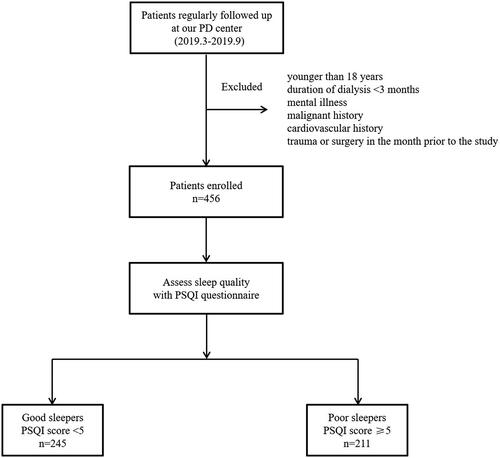
Table 1. Comparison of demographic and laboratory data between good sleepers and poor sleepers.
Risk factors for poor sleep quality in patients undergoing CAPD
shows the risk factors for poor sleep quality in patients undergoing CAPD. Several factors were univariately associated with poor sleep quality, including female sex, duration of CAPD, total Ccr, eGFR, albumin, and Ca × P product. In the multivariable analysis, the duration of CAPD (odds ratio [OR] = 1.01, 95% confidence interval [CI] = 1.00–1.02, p= 0.003), albumin (OR = 0.95, 95% CI = 0.91–1.00, p= 0.034), and Ca × P product (OR = 1.02, 95% CI = 1.00–1.03, p= 0.046) were independently associated with poor sleep quality.
Table 2. Risk factors related to poor sleep quality in patients undergoing CAPD: univariate logistic and multivariate logistic regression analysis.
Discussion
This study shows that the prevalence of poor sleep quality in our center is 46.3%, similar to previous studies [Citation5,Citation6]. In addition, patients with poor sleep quality are usually characterized by female sex, long CAPD duration, low total Ccr, low eGFR, low albumin level, and high Ca × P product. Combined with the results of univariate and multivariate analyses, this study found that a long CAPD duration, low albumin level, and high Ca × P product were independent risk factors for poor sleep quality.
Many factors presumably contribute to the high prevalence of sleep disturbances in patients undergoing PD, including demographic, clinical, psychological, metabolic, and nutritional abnormalities. In the present study, patients with poor sleep quality tended to be female, which is inconsistent with a previous study [Citation10]. A plausible explanation is that during times of hormonal changes, women are at an increased risk of sleep disturbances [Citation19]. Another study showed that women were more prone to anxiety and depression [Citation20]. Moreover, RRF was poorer in poor sleepers. In general, a decline in RRF is accompanied by an increased risk of metabolic and cardiovascular complications, mineral and bone disorders, and poor nutritional status [Citation21–23]. In addition to the aforementioned confounding factors that may affect sleep quality, deterioration of RRF could lead to uremia-induced neuropathy or myopathy, altered chemosensitivity, and hypervolemia, resulting in sleep disorders [Citation24].
Our study demonstrated a significantly higher risk of poor sleep quality in patients with a longer dialysis duration and a higher Ca × P product. These findings are consistent with those of the previous studies. Generally, long dialysis duration is often accompanied by inadequate dialysis [Citation25], malnutrition [Citation26], depression [Citation27], or abnormal calcium and phosphorus metabolism [Citation11], which are well-known risk factors for sleep disturbances. A high Ca × P product may be related to secondary hyperparathyroidism, uremic pruritus, bone pain, coronary artery calcification, and increased cardiovascular diseases [Citation11,Citation28], which may further affect the sleep quality of patients undergoing CAPD.
Nutritional status is a risk factor for poor sleep quality in patients with CAPD. Serum albumin level, a well-known nutritional indicator, was significantly lower in poor sleepers than in good sleepers in the present study. In the multivariate logistic regression analysis, albumin level was negatively correlated with poor sleep quality. In a study conducted by Li et al. [Citation4], the PSQI score was found to be negatively correlated with serum albumin and subjective global assessment score, but positively correlated with malnutrition–inflammation score. Both were measures of protein-energy wasting in CAPD patients. Similar findings have been reported previously [Citation6,Citation29,Citation30]. The possible mechanisms by which nutritional status affects sleep quality are as follows: first, nutrition can directly or indirectly contribute to poor sleep quality by affecting the hormones and inflammation status [Citation31]; second, serum albumin level was negatively related to the intensity of uremic pruritus, which affects the quality of sleep in patients undergoing CAPD [Citation14]; and third, low serum albumin level is an important determinant of overhydration in CAPD patients [Citation32], which contributes to obstructive sleep apnea, not only by its effects on upper airway collapsibility but also by potentially affecting ventilatory instability [Citation24].
Effective intervention and treatment of risk factors for poor sleep quality may improve the sleep quality of patients undergoing CAPD. This study confirms that a long dialysis duration, low albumin level, and high Ca × P product are the main factors affecting sleep quality in patients undergoing CAPD. Therefore, these factors can become new predictors of poor sleep quality and have important clinical significance. However, this study has some limitations. First, the PSQI questionnaire is a commonly used tool to evaluate only sleep quality, and there may be some errors due to recall bias and subjective judgment. Second, the present study was a cross-sectional study, the sample size was insufficient, and other potential risk factors that may affect sleep quality were not evaluated, such as monocytes, neutrophils, oxidative stress, and other inflammatory cells or factors related to poor sleep quality. Third, this study did not fully assess the nutritional status of patients or the presence of depression and other common factors affecting sleep quality.
Conclusions
In conclusion, this study showed that long dialysis duration, low albumin level, and high Ca × P product are the main predictors of poor sleep quality. Our findings may be helpful for the treatment of poor sleep quality in patients undergoing CAPD.
Author contributions
CXY designed the study. YJM and XJZ analyzed and interpreted the data regarding the PD patients. CXY and CFZ were the major contributors to the writing of the manuscript. YM revised the manuscript accordingly. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Raw data were generated at the First Affiliated Hospital of Nanchang University, China. The derived data supporting the findings of this study are available from the corresponding author, YM, upon request.
Additional information
Funding
References
- So JY, Warburton KM, Rosen IM. A guide to management of sleepiness in ESKD. Am J Kidney Dis. 2020;75(5):782–792.
- De Silva I, Evangelidis N, Hanson CS, SONG-HD, SONG-PD initiative, et al. Patient and caregiver perspectives on sleep in dialysis. J Sleep Res. 2021;30(4):e13221.
- Elder SJ, Pisoni RL, Akizawa T, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the dialysis outcomes and practice patterns study (DOPPS). Nephrol Dialysis Transplant. 2007;23(3):998–1004.
- Li J, Guo Q, Ye X, et al. Prevalence and risk factors of sleep disturbance in continuous ambulatory peritoneal dialysis patients in Guangzhou, Southern China. Int Urol Nephrol. 2012;44(3):929–936.
- Guney I, Biyik M, Yeksan M, et al. Sleep quality and depression in peritoneal dialysis patients. Renal Failure. 2008;30(10):1017–1022.
- Li H, Li X, Feng S, et al. Sleep disorders and its related risk factors in patients undergoing chronic peritoneal dialysis. Chin Med J (Engl). 2014;127(7):1289–1293.
- Zhao Y, Zhang Y, Yang Z, et al. Sleep disorders and cognitive impairment in peritoneal dialysis: a multicenter prospective cohort study. Kidney Blood Press Res. 2019;44(5):1115–1127.
- Stergiannis P, Govari M, Jahaj E, et al. Sleep disorders and restless legs syndrome in hemodialysis patients in Greece: a cross-sectional study. Adv Exp Med Biol. 2020;1195:155–162.
- Scherer JS, Combs SA, Brennan F. Sleep disorders, restless legs syndrome, and uremic pruritus: Diagnosis and treatment of common symptoms in dialysis patients. Am J Kidney Dis. 2017;69(1):117–128.
- Lee YC, Hung SY, Wang HK, et al. Male patients on peritoneal dialysis have a higher risk of sleep apnea. J Clin Sleep Med. 2019;15(7):937–945.
- Wang Y, Zhu J, Cao J, et al. Remote diagnosis system of uremia complicated with sleep disorder and effectiveness of nursing intervention. Contrast Media Mol Imaging. 2021;2021:1–6.
- Tu CY, Chou YH, Lin YH, et al. Sleep and emotional disturbance in patients with non-dialysis chronic kidney disease. J Formos Med Assoc. 2019;118(6):986–994.
- Zhang H, Yang Y, Huang J, et al. Correlates of objective sleep quality in older peritoneal dialysis patients. Ren Fail. 2021;43(1):180–187.
- Min JW, Kim SH, Kim YO, et al. Comparison of uremic pruritus between patients undergoing hemodialysis and peritoneal dialysis. Kidney Res Clin Pract. 2016;35(2):107–113.
- Han B, Zhu FX, Shi C, et al. Association between serum vitamin D levels and sleep disturbance in hemodialysis patients. Nutrients. 2017;9(2):139.
- Pai MF, Hsu SP, Yang SY, et al. Sleep disturbance in chronic hemodialysis patients: the impact of depression and anemia. Ren Fail. 2007;29(6):673–677.
- Mollayeva T, Thurairajah P, Burton K, et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73.
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379(9818):815–822.
- Pengo MF, Won CH, Bourjeily G. Sleep in women across the life span. Chest 2018;154(1):196–206.
- Chueh KH, Chen KR, Lin YH. Psychological distress and sleep disturbance among female nurses: anxiety or depression? J Transcult Nurs. 2021;32(1):14–20.
- Wu T, Qi Y, Ma S, et al. Efficacy of roxadustat on anemia and residual renal function in patients new to peritoneal dialysis. Ren Fail. 2022;44(1):529–540.
- Wang AY, Brimble KS, Brunier G, et al. ISPD cardiovascular and metabolic guidelines in adult peritoneal dialysis patients part I - assessment and management of various cardiovascular risk factors. Perit Dial Int. 2015;35(4):379–387.
- Sikorska D, Pawlaczyk K, Olewicz-Gawlik A, et al. The importance of residual renal function in peritoneal dialysis. Int Urol Nephrol. 2016;48(12):2101–2108.
- Lin CH, Lurie RC, Lyons OD. Sleep apnea and chronic kidney disease: a state-of-the-Art review. Chest 2020;157(3):673–685.
- Li PK, Chow KM, Van de Luijtgaarden MW, et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13(2):90–103.
- Orozco-Gonzalez CN, Cortes-Sanabria L, Cueto-Manzano AM, et al. Prevalence of pica in patients on dialysis and its association with nutritional status. J Ren Nutr. 2019;29(2):143–148.
- Lin J, Guo Q, Ye X, et al. The effect of social support and coping style on depression in patients with continuous ambulatory peritoneal dialysis in Southern China. Int Urol Nephrol. 2013;45(2):527–535.
- Rivara MB, Ravel V, Kalantar-Zadeh K, et al. Uncorrected and albumin-corrected calcium, phosphorus, and mortality in patients undergoing maintenance dialysis. JASN. 2015;26(7):1671–1681.
- Erdogan A, Dervisoglu E, Kutlu A. Sleep quality and its correlates in patients on continuous ambulatory peritoneal dialysis. Scand J Urol Nephrol. 2012;46(6):441–447.
- Ezzat H, Mohab A. Prevalence of sleep disorders among ESRD patients. Ren Fail. 2015;37(6):1013–1019.
- Zhao M, Tuo H, Wang S, et al. The effects of dietary nutrition on sleep and sleep disorders. Mediators Inflamm. 2020;2020:3142874.
- FornazariÄ D, AntoniÄ M, Knap B. Volume status and arterial stiffness evaluation in peritoneal dialysis patients. Clin Nephrol. 2021;96(Suppl 1):74–79.
