Abstract
Background
Both sepsis and AKI are diseases of major concern in intensive care unit (ICU). This study aimed to evaluate the excess mortality attributable to sepsis for acute kidney injury (AKI).
Methods
A propensity score-matched analysis on a multicenter prospective cohort study in 18 Chinese ICUs was performed. Propensity score was sequentially conducted to match AKI patients with and without sepsis on day 1, day 2, and day 3–5. The primary outcome was hospital death of AKI patients.
Results
A total of 2008 AKI patients (40.9%) were eligible for the study. Of the 1010 AKI patients with sepsis, 619 (61.3%) were matched to 619 AKI patients in whom sepsis did not develop during the screening period of the study. The hospital mortality rate of matched AKI patients with sepsis was 205 of 619 (33.1%) compared with 150 of 619 (24.0%) for their matched AKI controls without sepsis (p = 0.001). The attributable mortality of total sepsis for AKI patients was 9.1% (95% CI: 4.8–13.3%). Of the matched patients with sepsis, 328 (53.0%) diagnosed septic shock. The attributable mortality of septic shock for AKI was 16.2% (95% CI: 11.3–20.8%, p < 0.001). Further, the attributable mortality of sepsis for AKI was 1.4% (95% CI: 4.1–5.9%, p = 0.825).
Conclusions
The attributable hospital mortality of total sepsis for AKI were 9.1%. Septic shock contributes to major excess mortality rate for AKI than sepsis.
Registration for the multicenter prospective cohort study
registration number ChiCTR-ECH-13003934
Introduction
The rising prevalence and mortality of sepsis and acute kidney injury (AKI) are main threats to the survival of critically ill patients worldwide. Both sepsis and AKI are diseases of major concern in intensive care unit (ICU) [Citation1–3]. In critically ill patients, a variety of factors can cause AKI [Citation4], but sepsis is the most common trigger of AKI [Citation5]. Approximately, 40–50% of cases of the development of AKI is associated with sepsis [Citation6]. Septic AKI is distinct from nonseptic AKI, with differences in pathogenesis, clinical characteristics and outcomes [Citation7–9]. Limited understanding of pathophysiologic mechanisms has prevented the evolution of effective therapies of sepsis and AKI. The mortality rate of septic AKI is up to 30–60%, depending on severity of illness [Citation6,Citation10]. Up to now, no study assessed the accurate contribution of sepsis for mortality of AKI patients. It is assumed that whether sepsis develops before, simultaneously or after AKI, it would contribute excess mortality for AKI. Therefore, we conducted the propensity score-matched analysis between AKI patients with and without sepsis to evaluate the attributable mortality of sepsis for AKI patients.
Materials and methods
Study setting and population
This is a retrospective propensity score-matched analysis based on database of a prospective cohort study about sepsis epidemiology sponsored by China Critical Care Sepsis Trial (CCCST) workgroup, which was performed in 18 Chinese trial sites of 16 hospitals between January 1, 2014, and August 31, 2015. The database included adult patients of 4910 who stayed longer than 24 h in ICU. We screened and included patients who diagnosed AKI in the 4910 patients within 5 days after ICU admission. Then, we excluded patients from the AKI patients. The exclusion criteria included (1) operated with nephrectomy or kidney transplantation; (2) acquired insufficient data. A complete list of trial sites is provided in the Supplementary File. The protocol of study was registered on August 3, 2013. The study was approved in all participating ICUs by their Hospital Human Ethics Committee. The chief ethics number was 2013FXHEC-KY2018. The registration number was ChiCTR-ECH-13003934. A preprint of the manuscript was online. An article about the risk factors, clinical features and outcome of new-onset AKI on this database was previously published. Now we focused AKI with sepsis and explored the attributable mortality of sepsis for AKI patients [Citation11]. Informed consent from patients or their next of kin was obtained before patients joined in the study. All enrolled patients adhere to the following management principles: active treatment of primary disease and complications; and the same principles of treatment with antibiotics, nutritional metabolism and organ support.
Clinical endpoint and definitions
The primary endpoint was hospital mortality of AKI patients. The diagnosis of sepsis and septic shock was according to the sepsis 3.0 definition of the American College of Chest Physicians/Society of Critical Care Medicine criteria [Citation12]. The definitions of AKI and AKI classification were depended on the serum creatinine and urine output criteria proposed by Kidney Disease: Improving Global Outcomes (KDIGO) [Citation13]. AKI with sepsis is defined as patients who develop AKI and sepsis, whether sepsis develops before, simultaneously or after AKI diagnosis. In this study, we focused AKI and sepsis diagnosed in 5 days after ICU admission. Renal replacement therapy (RRT) was initiated according to medical necessity of patients and decision of the treating clinician. The baseline creatinine was defined as follows: if at least five values were available the median of all values available from six months to six days prior to enrollment was used. Otherwise, the lowest value in the five days prior to enrollment was used. If no pre-enrollment creatinine was available or the emergency patient’s serum creatinine was abnormal at the time of admission, the baseline creatinine was estimated using the Modification of Diet in Renal Disease (MDRD) equation assuming that baseline eGFR is 75 mL/min per 1.73 m2 [Citation14].
Data collection
The information collected included demographic characteristics, chronic illnesses, diagnosis, pre-ICU medications and treatment (whether or not used nephrotoxic drugs, nephrotoxic drugs included angiotensin converting enzyme inhibitors, non-steroidal anti-inflammatory drug, Amikacin and Amphotericin B), the reason for ICU admission, acute physiology and chronic health evaluation (APACHE II) on the day of ICU admission, baseline serum creatinine, creatinine values every 12 h and hourly urine output on ICU admission and thereafter until transferred out of ICU, use of mechanical ventilation, as well as serum lactate, use of vasoactive drugs, sequential organ failure assessment (SOFA) score every day in the first 7 days after ICU admission. We also collected time of diagnosing sepsis, septic shock and AKI after ICU admission, AKI classification, ICU stay, hospital stay, hospital mortality and 30-day mortality.
Propensity score matching
A patient in the exposure group with is matched with a comparable patient who has the most similar propensity score. Propensity score matching was constructed for AKI patients with and without sepsis (1:1) at five different time points: day 1, day 2, day 3, day 4 and day 5 after ICU admission (). The propensity score based on baseline characteristics and clinical covariates was used to adjust the differences in matched patients with and without sepsis, which was constructed depending on logistic regression and including variables of age, sex, body mass index (BMI), chronic illnesses (chronic obstructive pulmonary disease (COPD) or asthma, cardiovascular disease, chronic liver disease, cancer, diabetes, hypertension, chronic kidney disease (CKD)), AKI classification, nonrenal SOFA score, and mechanical ventilation. Nonrenal SOFA score was remarkably correlated linearly with APACHE II. AKI patients with sepsis were matched 1:1 with controls of AKI patients without sepsis. Then, the hospital mortality in each matched AKI group was calculated and attributable mortality of total sepsis for AKI was estimated.
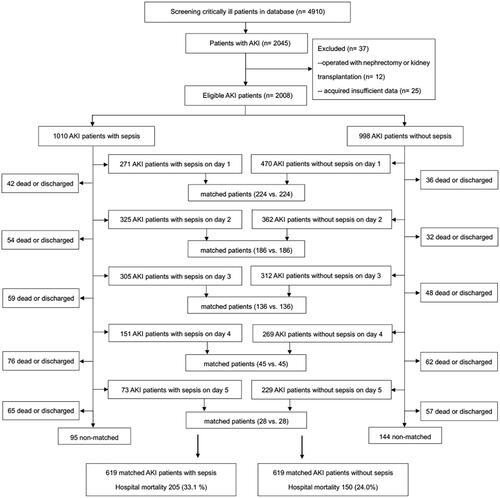
Figure 1. Study flow diagram. The propensity score for AKI with sepsis on the first ICU day (day 1) included patients diagnosed AKI and sepsis on day 1. Excluding patients who were matched on day 1, discharged and dead on day 1, patients of AKI with sepsis on the second ICU day (day 2) were identified in the rest of patients, including patients who diagnosed AKI on day 1 with renal recovery on day 2. Sequentially, excluding patients who were matched on day 2, discharged and dead on day 2, patients of AKI with sepsis on the third ICU day (day 3) were identified, including patients who diagnosed AKI on day 1 or day 2 but with renal recovery on day 3. Patients of AKI with sepsis on days 4-5 were constructed correspondingly. Further, cohort of AKI without sepsis on the first ICU day included patients diagnosed AKI on day 1 who never developed sepsis during the study screening period. Excluding patients who were matched on day 1, discharged and dead on day 1, patients of AKI who never developed sepsis during the study screening time on the second ICU day were identified in the rest of patients, including patients who diagnosed AKI on day 1 with renal recovery on day 2. Sequentially, excluding patients who were matched on day 2, discharged and dead on day 2, patients of AKI who never developed sepsis during the study screening time on the third ICU day were identified, including patients who diagnosed AKI on day 1 or day 2 but with renal recovery on day 3. Patients of AKI without sepsis on days 4-5 were constructed correspondingly. AKI acute kidney injury, ICU intensive care unit.
Matched AKI patients with sepsis and their controls of AKI patients without sepsis were subgrouped by the severity of sepsis (sepsis, septic shock). The severity of sepsis (sepsis, septic shock) was distinguished according to whether the patients met diagnostic criteria of septic shock on the time of diagnosis. The mortality in each matched subgroup was calculated and attributable mortalities of sepsis and septic shock for AKI were estimated, respectively.
Statistical analysis
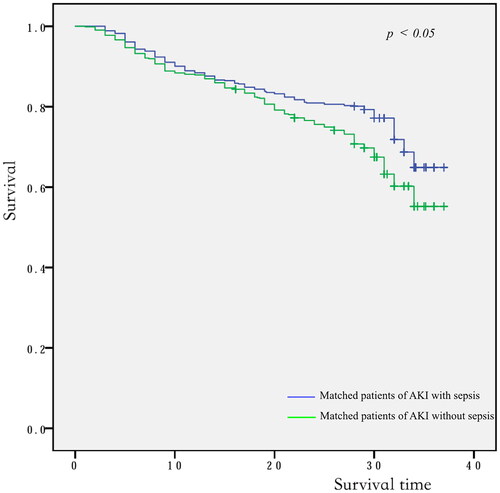
Continuous variables were presented as mean ± standard deviation (SD) or median values (25th and 75th percentiles), categorical variables were presented as percentiles. Continuous data between two groups was compared using the Student’s t-test or Mann–Whitney U-tests, and categorical variables used the Chi square test or Fisher’ s exact test. Propensity score was constructed to match AKI patients with and without sepsis. The caliper width was set to 0.1 of the standard deviation of the logit of the propensity score. Covariate balance before and after matching was examined using standardized differences, with values 0.15 considered as evidence of meaningful differences [Citation15]. Pearson or Spearman correlation test was used to estimate the correlation between two variables. The excess mortality of AKI patients attributable to sepsis was calculated by subtracting the mortality of matched AKI patients without sepsis from the mortality of matched AKI patients with sepsis. 95% confidence interval (CI) for the attributable mortality difference was calculated by Newcombe’s method [Citation16]. The McNemar’s test was used for sensitivity analysis to assess the stability of outcomes [Citation15]. The primary endpoint was further evaluated by Kaplan–Meier curves (log-rank test). For all analyses, statistical significance was indicated by two-sided p < 0.05. SPSS statistics 22 (IBM, Chicago, IL, USA) and R 2.1.2 were used for statistical analyses.
Results
A total of 4910 adult critically ill patients were screened. Of them, 2045 developed AKI within 5 days after ICU admission. After excluding 37 ineligible patients, there were 2008 AKI patients finally included in this study. The characteristics of included patients are performed in . The study flow diagram () illustrates the ICU treatment day on which sepsis was diagnosed and the sequential matching procedure. Supplemental Table 1 shows the characteristics of matched AKI patients with sepsis and their controls without sepsis in five separate time points.
Table 1. Characteristics of the whole cohort AKI.
Of the 1010 AKI patients with sepsis, 619 (61.3%) were matched to 619 AKI patients in whom sepsis did not develop during the screening period of the study (). The distributions of propensity score before and after matching in cohorts with and without sepsis are shown in . Patient characteristics of the matched pairs are presented in . After matching, the groups were balanced regarding the matched variables with small standardized differences (). RRT was used in 167 (27.0%) and 160 (25.8%) patients in matched AKI patients with and without sepsis, respectively (p = 0.699). The length of ICU and hospital stay was 8.0 days (4.0–15.5 d), 17.0 days (11.0–25.0 d) for AKI patients with sepsis and 6.0 days (4.0–11.0 d), 18.0 days (10.0–28.0 d) for the nonsepsis AKI control patients, respectively (p = 0.001, p = 0.158). The hospital mortality rate of matched AKI patients with sepsis was 205 of 619 (33.1%) compared with 150 of 619 (24.0%) for their matched AKI controls without sepsis (p = 0.001). The attributable mortality of total sepsis for AKI patients was 9.1% (95% CI: 4.8–13.3%). The attributable mortalities of total sepsis and different severity of sepsis (sepsis and septic shock) for AKI are shown in . The Kaplan-Meier curves and comparison of the distribution of the two groups are shown in .
Figure 2. The distributions of propensity score before and after matching in cohorts with and without sepsis: (A) propensity score before matching; (B) propensity score after matching. PS propensity score.
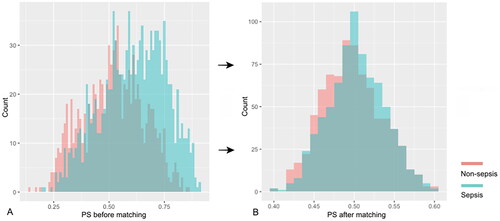
Figure 3. The attributable mortalities of total sepsis and different severity of sepsis (sepsis and septic shock) for AKI. AKI acute kidney injury, AM attributable mortality, HM hospital mortality.
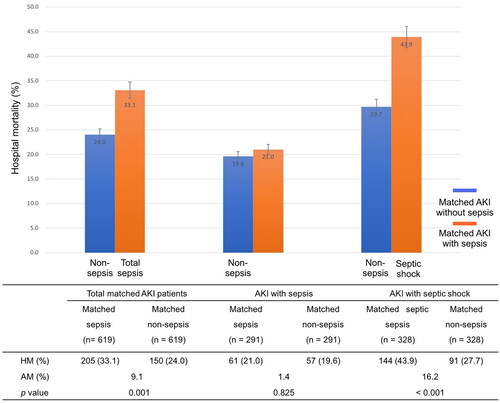
Table 2. A comparison of characteristics between matched AKI patients with and without sepsis.
Matched AKI patients with and without sepsis were subgrouped according to the severity of sepsis (sepsis, septic shock). Of the matched patients with sepsis, 328 (53.0%) diagnosed septic shock. The mortality rate showed remarkably higher in matched AKI patients with septic shock (43.9%) than their controls of patients without sepsis (27.7%). The attributable mortality of septic shock for AKI was 16.2% (95% CI: 11.3–20.8%, p < 0.001). Further, the attributable mortality of sepsis for AKI was 1.4% (95% CI: −4.1–5.9%, p = 0.825), although there was no significant difference of mortality rate observed between matched AKI patients with and without sepsis (21.0% vs. 19.6%).
Discussion
Sepsis and AKI are inextricably common diseases in critically ill patients. Sepsis is a leading cause of AKI, and AKI is a frequent complication of sepsis [Citation4,Citation5]. Many researches’ results convincingly showed the ‘intimate’ bond between sepsis and AKI in ICU patients. For instance, AKI in up to half of septic patients was reported in the BEST Kidney and FINNAKI studies [Citation6,Citation17]. Study by Vaara ST, et al. [Citation18] estimated the attributable mortality of AKI. Kelly F, et al. [Citation19] examined long-term mortality in sepsis patients compared to hospitalized non-sepsis controls. However, none of studies accurately calculated the attributable mortality of sepsis for AKI. For this purpose, this sequentially propensity-matched analysis calculated the attributable mortality of sepsis for AKI. Propensity score balanced the baseline characteristics and clinical covariates of diabetes mellitus, COPD, cardiovascular disease, etc in two groups of AKI patients with and without sepsis. The main findings show that the estimated excess hospital mortality statistically attributable to sepsis for AKI was 9.1 percentage points (95% CI: 4.8–13.3% percentage points). Septic shock contributed to major excess mortality for AKI than sepsis. The findings further strengthen the role of sepsis as a significant leading cause for mortality of AKI.
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Abundant releases of inflammatory cytokines and leukocyte activation may result in capillary plugging and micro-thrombi, as well as production of reactive oxygen species and induction of nitric oxide synthase, which further destroy the endothelial barrier and the glycocalyx leading to organ edema and systemic hypovolemia [Citation20–22]. Overall, pathogenesis of sepsis includes macrovascular and microvascular dysfunction, immunologic and autonomic dysregulation, septic AKI may occur in the absence of renal hypoperfusion and hemodynamic derangement and even in the presence of normal or increased global renal blood flow [Citation5,Citation23–25]. Premature cell senescence plays a critical role in septic AKI. Study by Chaojin Chen and colleagues indicated that LXA4 exerted its renoprotective effects by blocking the crosstalk between inflammation and premature senescence in a PPAR-dependent manner [Citation26]. It is different from the major causes of nonseptic AKI which are renal hypoperfusion and associated ischemia in the critically ill patients [Citation7,Citation27,Citation28]. This study shows sepsis increase excess mortality compared to nonseptic pathogenic factors such as trauma and cardiac insufficiency, etc in matched clinical conditions.
Sepsis may have specific prognostic implications when it compared with other AKI causes. There was study showed an overall mortality of 27% in post-traumatic AKI was comparable with what had been observed in a general ICU population [Citation29]. Emergency surgery increased the risk of postoperative sepsis over 6-fold after regarding the confounders in aged patients [Citation30]. Some studies in patients developing AKI after cardiac surgery showed short-term mortality of cardiac surgery-associated AKI (CSA-AKI) was reported between 15–30% [Citation31]. Clinical and prognostic relevance of AKI in the setting of ST-elevation acute myocardial infarction (STEMI) complicated by cardiogenic shock (CS) was evaluated by study of Marenzi G, et al. [Citation32]. Ninety-seven consecutive STEMI patients with CS were included and 52 (55%) patients developed AKI. Patients developing AKI had a high mortality rate of 50 percentage points. In the BEST Kidney trial analysis [Citation16], septic AKI were 50% higher of the odds of dying in hospital in compared with non-septic AKI. Visibly, the composition of the non-septic group and its proportion of conditions with poor prognosis (such as CS) greatly influence the different prognosis between septic and nonseptic AKI.
The various mortalities of septic AKI were reported in different studies. Our study showed a hospital mortality of 33.1% in matched AKI patients with sepsis compared with 24.0% of their matched AKI controls without sepsis. Angus et al. examined 192,980 patients with severe sepsis from seven US states [Citation33]. They found AKI occurred in 22% and was associated with a mortality of 38.2%. The SOAP cohort study recruited 3147 patients [Citation34]. Of them, AKI occurred in 51% of sepsis cases and was related to an ICU mortality of 41%. Different from studies above, this study highlights the attributable mortality of sepsis for AKI, and a close link was observed between increased mortality and sepsis severity. There are a lot of studies focused on septic AKI, we should also be concerned for the AKI patients who developed sepsis after AKI. Whether sepsis developed before, simultaneously or after AKI, it would deteriorate organ function and contribute excess mortality for AKI. Furthermore, septic shock contributed to major excess mortality for AKI than sepsis. The seriousness of this condition emphasized the need for prompt and appropriate intervention. Prevention of sepsis development and progress to septic shock may represent a potential key therapeutic target for AKI and decrease mortality.
This study still has some limitations. The database was prospectively collected and detailed clinical data allowed us to construct careful sequential matching. However, the study was analyzed retrospective, which may make some hidden biases. Secondly, we studied AKI and sepsis in the first 5 days after ICU admission, there were still a minority of patients develop AKI and/or sepsis after 5 days, this may slightly affact the results of excess mortality attributable to sepsis for AKI patients. Nevertheless, development of AKI and sepsis after day 5 is uncommon [Citation18]. Furthermore, the majority of AKI and sepsis were diagnosed in the first 2 days in this study.
Conclusion
The attributable hospital mortality of total sepsis for AKI were 9.1% (95% CI 4.8-13.3%). Septic shock contributes to more excess mortality rate for AKI than sepsis.
Ethical approval
The study was approved in all participating ICUs by their Hospital Human Ethics Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number 2013FXHEC-KY2018) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Geolocation information
Beijing, China.
Supplemental Material
Download PDF (300.5 KB)Acknowledgements
(1) We thank the 18 trial sites of 16 hospitals in the China Critical Care Sepsis Trial (CCCST) workgroup, including Department of Critical Care Medicine, Fuxing Hospital, Capital Medical University, Beijing, China; Department of Critical Care Medicine, West China Hospital, Sichuan University, Sichuan, China; Department of Medical Intensive Care Unit, Peking Union Medical College Hospital, Beijing, China; Department of Critical Care Medicine, Guangdong Geriatric Institute, Guangdong General Hospital, Guangdong, China; Department of Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China; Department of Surgical Intensive Care Unit, Department of Anesthesiology, ZhongShan Hospital, FuDan University, Shanghai, China; Department of Intensive Care Unit, The First Hospital of Jilin University, Changchun, China; Department of Critical Care Medicine, China-Japan Friendship Hospital, Beijing, China; Department of Critical Care Medicine, Beijing Friendship Hospital, Capital Medical University, Beijing China; Department of Surgical Intensive Care Unit, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China; Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China; Department of Critical Care Medicine, General Hospital of Ningxia Medical University, Ningxia, China; Department of Critical Care Medicine, Xiangya Hospital, Central South University, Changsha, China; Department of Critical Care Medicine, Beijing Tongren Hospital, Capital Medical University, Beijing, China; Department of Critical Care Medicine, Peking University Third Hospital, Beijing, China; Department of Surgical Intensive Care Unit, Xuanwu Hospital, Capital Medical University, Beijing, China; Department of Critical Care Medicine, Beijing Tiantan Hospital, Capital Medical University, Beijing, China. In addition, we especially thank Professor Xiaoxia Peng in Centre for Clinical Epidemiology and Evidence-based medicine, Beijing Children’s Hospital, Capital Medical University, National Centre for Children Health for technical support in applying for the project.
(2) A preprint of the manuscript was online. The online platform: Research Square; Online posting’s date: 22 Jul, 2021; Internet link: https://doi.org/10.21203/rs.3.rs-699907/v1.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data not available due to ethical restrictions.
Additional information
Funding
References
- Bagshaw SM, George C, Bellomo R, and the ANZICS Database Management Committee Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12(2):R47.
- Fleischmann-Struzek C, Mikolajetz A, Schwarzkopf D, et al. Challenges in assessing the burden of sepsis and understanding the inequalities of sepsis outcomes between national health systems: secular trends in sepsis and infection incidence and mortality in Germany. Intensive Care Med. 2018;44(11):1826–1835.
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–1964.
- Case J, Khan S, Khalid R, et al. Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract. 2013;2013:479730.
- Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43(6):816–828.
- Poukkanen M, Vaara ST, Pettilä V, FINNAKI study group, et al. Acute kidney injury in patients with severe sepsis in finnish intensive care units. Acta Anaesthesiol Scand. 2013;57(7):863–872.
- Gomez H, Ince C, Backer DD, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11.
- Mehta RL, Bouchard J, Soroko SB, Program to Improve Care in Acute Renal Disease (PICARD) Study Group, et al. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Med. 2011;37(2):241–248.
- Peters E, Antonelli M, Wittebole X, et al. A worldwide multicentre evaluation of the influence of deterioration or improvement of acute kidney injury on clinical outcome in critically ill patients with and without sepsis at ICU admission: results from the intensive care over nations audit. Crit Care. 2018;22(1):188.
- Mårtensson J, Bellomo R. Sepsis-induced acute kidney injury. Crit Care Clin. 2015;31(4):649–660.
- Jiang YJ, Xi XM, Jia HM, et al. Risk factors, clinical features and outcome of new-onset acute kidney injury among critically ill patients: a database analysis based on prospective cohort study. BMC Nephrol. 2021;22(1):289.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810.
- Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649–672.
- Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25.
- Lee J, Little TD. A practical guide to propensity score analysis for applied clinical research. Behav Res Ther. 2017;98:76–90.
- Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Statist Med. 1998;17(22):2635–2650.
- Bagshaw SM, Uchino S, Bellomo R, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–439.
- Vaara ST, Pettilä V, Kaukonen KM, Finnish Acute Kidney Injury Study Group, et al. The attributable mortality of acute kidney injury: a sequentially matched analysis. Crit Care Med. 2014;42(4):878–885.
- Kelly F, Lauralyn M, Christopher JD, et al. Sepsis-associated mortality, resource use, and healthcare costs: a propensity-matched cohort study. Crit Care Med. 2021;49(2):215–227.
- Chelazzi C, Villa G, Mancinelli P, et al. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care. 2015;19(1):26.
- Tsukahara Y, Morisaki T, Kojima M, et al. iNOS expression by activated neutrophils from patients with sepsis. ANZ J Surg. 2001;71(1):15–20.
- Guerci P, Ergin B, Ince C. The macro- and microcirculation of the kidney. Best Pract Res Clin Anaesthesiol. 2017;31(3):315–329.
- Langenberg C, Wan L, Egi M, et al. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69(11):1996–2002.
- Prowle JR, Molan MP, Hornsey E, et al. Measurement of renal blood flow by phase- contrast magnetic resonance imaging during septic acute kidney injury: a pilot investigation. Crit Care Med. 2012;40(6):1768–1776.
- Murugan R, Karajala-Subramanyam V, Lee M, Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–535.
- Chen CJ, Qiu RZ, Yang J, et al. Lipoxin A4 restores septic renal function via blocking crosstalk between inflammation and premature senescence. Front Immunol. 2021;12:637753.
- Honore PM, Jacobs R, Joannes-Boyau O, et al. Septic AKI in ICU patients. Diagnosis, pathophysiology, and treatment type, dosing, and timing: a comprehensive review of recent and future developments. Ann Intensive Care. 2011;1(1):32.
- Gül F, Arslantaş MK, Cinel İ, et al. Changing definitions of sepsis. Turk J Anaesthesiol Reanim. 2017;45(3):129–138.
- Søvik S, Isachsen MS, Nordhuus KM, et al. Acute kidney injury in trauma patients admitted to the ICU: a systematic review and meta-analysis. Intensive Care Med. 2019;45(4):407–419.
- Peng XR, Chen CJ, Chen JJ, et al. Tree-based, two-stage risk factor analysis for postoperative sepsis based on sepsis-3 criteria in elderly patients: a retrospective cohort study. Front Public Health. 2022;10:1006955.
- Xu JR, Jiang WH, Shen B, et al. Acute kidney injury in cardiac surgery. Contrib Nephrol. 2018;193:127–136.
- Marenzi G, Assanelli E, Campodonico J, et al. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med. 2010;38(2):438–444.
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- Vincent JL, Sakr Y, Sprung CL, Sepsis Occurrence in Acutely Ill Patients Investigators, et al. Sepsis in european intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353.