Dear Editor,
Renal anemia develops when the glomerular filtration rate (GFR) decreases to less than 60 mL/min [Citation1]. Erythropoietin (EPO) is an erythropoiesis-stimulating hormone primarily produced by renal erythropoietin-producing (REP) cells, located between the tubules and capillaries in the renal tubulointerstitium, in response to cellular hypoxia, as is found in the anemic state [Citation2]. In patients with reduced renal function, such as those with chronic kidney disease, serum EPO levels can be within the reference range; however, the levels are unexpectedly low for the severity of anemia [Citation3]. Chronic tubulointerstitial damage is a major pathophysiological mechanism of renal anemia, which is explained by decreased EPO production. However, the development of renal anemia in acute tubulointerstitial damage is not well understood. Herein, we report a case of renal anemia in a patient with vasculitis-induced acute tubulointerstitial nephritis (ATIN) and relatively normal renal function.
A 75-year-old Japanese woman was hospitalized with a fever that had persisted for a month and a half. Her nighttime body temperature was approximately 38 °C, with general fatigue and decreased appetite. She also experienced pain in her temples when chewing food and had conjunctival hyperemia in her right eye. There was no evidence of thickening or tenderness of the bilateral temporal arteries, and no other abnormal findings on physical examination were found. She had no relevant medical history, nor was she taking oral medications/supplements. Laboratory examination showed serum myeloperoxidase antineutrophil cytoplasmic antibody (MPO-ANCA) and C-reactive protein levels as >134 IU/L (reference range <3.5 IU/L) and 9.3 mg/dL (reference range ≤0.14 mg/dL), respectively (). Additionally, the hemoglobin (Hb) level was 10.5 g/dL (reference range 11.5–15.0 g/dL) with a mean corpuscular volume of 92.7 fL (reference range 83.6–98.2 fL), mean corpuscular Hb of 30.6 pg (reference range 27.5–33.2 pg), and mean corpuscular Hb concentration of 33.1 g/dL (reference range 31.7–35.3 g/dL), indicating normocytic normochromic anemia. Peripheral blood analysis showed no abnormal cells. Test results for thyroid and liver function were also normal. However, serum levels of iron, total iron binding capacity, and ferritin were abnormal at 13 μg/dL (reference range 40–188 μg/dL), 153 μg/dL (reference range 270–440 μg/dL), and 523 ng/mL (reference range 4.0–87.0 ng/mL), respectively, suggesting anemia of inflammation. Unexpectedly, considering the severity of anemia, the serum erythropoietin (EPO) level (13.8 mIU/mL) remained within its reference range (4.2–23.7 mIU/mL). Similarly, her renal function showed an estimated glomerular filtration rate (eGFR) of 65.4 mL/min/1.73 m2, which was relatively normal, with normal kidney size and no glomerular microhematuria or albuminuria. Despite these, renal tubulointerstitial damage was diagnosed based on a renal biopsy and elevated renal tubular marker levels, such as urinary N-acetyl-β-D-glucosaminidase, α1-microglobulin, and β2-microglobulin. Renal pathology revealed overall normal glomeruli except one glomerulus with a cellular crescent (). However, whole-layer fibrinoid necrosis was observed in small arteries, surrounded by inflammatory cells (). Vasculitis caused inflammatory cells to infiltrate the perivascular tubules and interstitium, resulting in ATIN (). As immunofluorescence staining and electron microscopy revealed no immune complexes or immunoglobulin deposition, the patient was diagnosed with ATIN caused by MPO-ANCA-associated vasculitis (MPO-AAV). Initially, intravenous methylprednisolone 500 mg/day was administered for 3 days, which quickly improved the fever and jaw claudication, followed by oral prednisolone (25 mg/day) and intravenous rituximab (375 mg/m2/week). Her anemia improved along with the levels of urinary tubular markers and serum ferritin over the treatment course (). After treatment with rituximab, oral steroids were tapered off, and her eGFRs fluctuated between 42.9 and 45.3 mL/min/1.73 m2.
Figure 1. Representative image of renal biopsy. Severe tubulointerstitial damage with whole layer fibrinoid necrosis in the small arteries and tubulitis (200×, PAM).
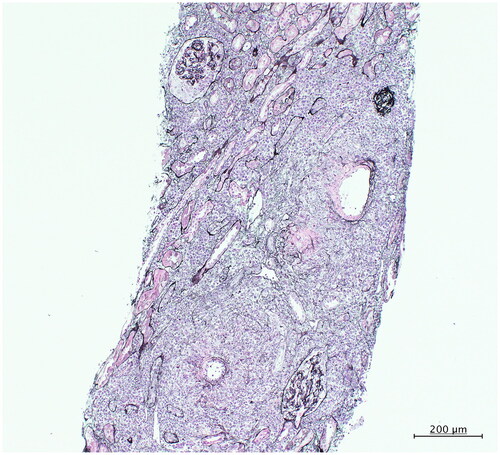
Table 1. Relevant laboratory parameters from referral to treatment.
Anemia in inflammatory states such as ATIN and acute kidney injury is typically associated with systemic inflammation, which may directly impair iron availability leading to blunted erythropoiesis, which is called the anemia of inflammation and refers to anemia associated with chronic inflammatory disease [Citation4]. Therefore, differentiating renal anemia from the anemia of inflammation is important from the viewpoints of both pathophysiology and treatment.
EPO is produced by REP cells in the renal tubulointerstitium, especially those in close association with the peritubular capillaries and proximal convoluted tubules. It has a short half-life in the blood and is not stored in the body. Thus, a lower serum EPO level in anemia is directly associated with decreased EPO production in REP cells [Citation5]. Lipkin et al. reported unusually low levels of EPO (18.2 ± 9.5 mIU/mL) for anemia (mean Hb level 10.3 g/dL) and a mean ferritin level of 705 ng/mL in patients with acute renal failure (mean serum creatinine level 8.18 mg/dL) caused by various etiologies [Citation6]. Despite a lower serum EPO level, eGFR levels were still maintained in the present case, in contrast to the report by Lipkin et al. although a feigned reduction due to oral administration of trimethoprim-sulfamethoxazole was noted during the course. In another study by Zhang et al. [Citation7], the serum EPO level was found to be as low as 4.21 ± 1.47 mIU/mL for anemia (mean Hb level 10.7 g/dL), and the mean ferritin level was 336 ng/mL in acute renal failure (mean serum creatinine level 2.33 mg/dL) caused by drug-induced ATIN. Interestingly, Jelkmann et al. have reported that proinflammatory cytokines can inhibit EPO production [Citation8]. Based on the serum ferritin levels, the inflammatory state in the present case was not considered as severe as in the above-mentioned studies with acute renal failure. Therefore, though systemic inflammation caused by MPO-AAV might directly impair EPO production, the above-mentioned differences in the EPO levels might partly result from the differences in the degrees of acute tubulointerstitial damage which could affect EPO production despite the relatively normal renal function as indicated by eGFR.
In summary, we report a case of renal anemia in a patient with vasculitis-induced ATIN and relatively normal renal function. Decreased EPO production caused by acute tubulointerstitial damage can be partly responsible for renal anemia, despite relatively normal renal function. Therefore, EPO measurement may help to establish the cause of anemia even in patients with normal renal function.
Ethics Approval
All procedures performed in the present study were in accordance with the ethical standards of the national research committee at which the studies were conducted and with the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Informed consent was obtained from the patient.
Consent for publication
We obtained verbal consent from the patient to publish this case report and have listed it in the medical records. The requirement for written consent was waived by the Institutional Review Board at St. Marianna University School of Medicine, since the patient cannot be identified.
Authors’ contributions
MO and HF: Writing draft and Data curation (These two authors equally contributed to this manuscript as the co-first authors). HS: Reviewing and Editing draft. SU: Reviewing and Editing draft. RF: Reviewing and Editing draft. NT: Conceptualization, Reviewing and Editing draft, and Supervision. All authors have read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
There is no data set associated with the present case report.
Additional information
Funding
References
- De Nicola L, Minutolo R, Chiodini P, et al. Prevalence and prognosis of mild anemia in non-dialysis chronic kidney disease: a prospective cohort study in outpatient renal clinics. Am J Nephrol. 2010;32(6):533–540.
- Suzuki N, Yamamoto M. Roles of renal erythropoietin-producing (REP) cells in the maintenance of systemic oxygen homeostasis. Pflugers Arch. 2016;468(1):3–12.
- Mercadal L, Metzger M, Casadevall N, NephroTest Study Group, et al. Timing and determinants of erythropoietin deficiency in chronic kidney disease. Clin J Am Soc Nephrol. 2012;7(1):35–42.
- Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28(4):671–681, vi.
- Zeisberg M, Kalluri R. Physiology of the renal interstitium. Clin J Am Soc Nephrol. 2015;10(10):1831–1840.
- Lipkin GW, Kendall RG, Russon LJ, et al. Erythropoietin deficiency in acute renal failure. Nephrol Dial Transplant. 1990;5(11):920–922.
- Zhang YJ, Li XM. Relationship among the characteristics of anemia, serum level of erythropoietin and the renal tubulointerstitial injury in drug-associated renal parenchymal acute renal failure patients. Beijing Da Xue Xue Bao Yi Xue Ban. 2005;37(5):471–475.
- Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18(8):555–559.
