Abstract
In light chain amyloidosis (LA), the massive glomerular and vascular amyloid deposition leading to interstitial fibrosis/tubular atrophy (IFTA) is thought to be responsible for renal failure. The amyloid deposition in the interstitium and the tubular basement membrane (TBM) has received less attention in the study of LA. We, therefore, collected clinical and laboratory data on patients diagnosed with LA in our Nephrology Department and studied amyloid deposition in the TBM. Twelve LA patients were diagnosed by renal biopsy during a seven-year period. In 4 of the 12, amyloid deposition could also be detected in the TBM. In our first case of a patient with diabetes mellitus, non-amyloid fibrils resembling ‘diabetic fibrillosis’ were also seen by electron microscopy. Despite the double damage, IFTA was negligible, blood vessels were unaffected, and the glomerular deposition was segmental. In the other three cases, significant (>50%) IFTA and a severely reduced estimated glomerular filtration rate were already detected at the time of diagnosis and amyloid deposition was also observed in the blood vessels. These findings indicate the importance of TBM amyloid deposition in the progression of renal disease. This may represent a late-stage presentation of the disease with a heavy LC burden.
Introduction
In light chain amyloidosis (LA), abnormal insoluble proteins are formed mainly in the serum and then deposited in predilected organs. In the kidney, amyloidosis usually begins in the mesangium and later diffusely affects the other renal structures. Dominant glomerular deposition is the most common, resulting in significant nephrotic proteinuria [Citation1]. When amyloid is loaded predominantly within arteries and arterioles, proteinuria is lower, and patients usually present with renal insufficiency [Citation2]. Massive glomerular and vascular amyloid deposition leading to interstitial fibrosis/tubular atrophy is thought to be responsible for renal failure.
However, tubulointerstitial changes, alterations in the tubular epithelium, and interstitial fibrosis may also be consequences of interstitial amyloid deposition. Amyloid deposition surrounding the tubules, peritubular capillaries, and along the tubular basement membrane (TBM) has received less attention in LA research. However, the recently proposed standardized histopathological scoring system does take interstitial amyloid deposition into account [Citation3]. In fact the amyloid score correlated well with renal outcome in an independent cohort [Citation4]. Recently, a large prospective study confirmed that an elevated urinary retinol-binding protein level, indicating proximal tubule cell dysfunction, is a good predictor of renal outcome in LA, independent of estimated glomerular filtration rate (eGFR), urinary protein-to-creatinine ratio (uPCR), and clonal response [Citation5].
Light chains can cause more than one type of kidney disease. For example, patients with LA may also have light chain deposition disease (LCDD) with or without light-chain cast nephropathy [Citation6]. Non-organized, granular deposition along the TBM is well known in LCDD; however, little is known about the frequency, clinical and prognostic importance of amyloid fibril deposition along the TBM in systemic LA. The possible role of TBM amyloid in disease progression has not been investigated. Therefore, we collected clinical and laboratory data of patients diagnosed with LA in our Nephrology department between 2015 and 2022 and studied amyloid deposition in the TBM.
Case reports
Twelve LA patients were diagnosed by renal biopsy in the 7-year period. shows the essential clinical and histological data of patients. In 4 of the 12 cases, amyloid deposition in the TBM was also present (cases 1–4). It was verified in all cases with Congo red stain positivity and apple green birefringence under polarized microscopy, light chain restriction with immunofluorescence microscopy and amyloid fibrils (diameter 8–12 nm) on electron microscopy.
Table 1. Clinical and histological data of patients with light chain amyloidosis.
Case 1
The medical history of a 61-year-old female patient included osteomyelitis of the right tibia in 1975, splenectomy due to immune thrombocytopenia, hypertension, treatment for type-2 diabetes mellitus since 2012, neoadjuvant chemotherapy for rectal cancer in 2013, followed by a Hartmann’s procedure, colostomy closure, and left knee replacement in 2018. In September 2020, she was referred to a nephrologist because of increasing proteinuria (2 g/day), which began in 2018. She had diabetes that was well controlled with metformin and semiglutide. The kidney size and function were normal, as were routine blood tests and urine sediment. Serum protein electrophoresis (SPEP) confirmed an IgG lambda monoclonal component (6.3 g/l), which was also present in urine (7 mg/l), and the serum free light chain kappa/lambda ratio (sFLCk/l) was 0.13. Bone marrow flow cytometry revealed 0.45% plasma cells, of which 55% were pathological, showing lambda monoclonality.
A renal biopsy was performed, revealing only one sclerotic glomerulus among the 23 glomeruli, and negligible IFTA (less than 10%). Immunofluorescence revealed segmental linear IgG and lambda deposition along the glomerular basement membrane and the TBM (). The non-immunological trapping of linear IgG and albumin in thickened abnormal basement membranes is common, leading to nonspecific staining in patients with diabetes. Congo red staining was verified in the periodic acid-Schiff (PAS) negative mesangium, peritubula, and TBM, but not in the blood vessels. Among the non-organized diabetic fibrils in the expanded mesangial area and the TBM, electron microscopy (EM) confirmed immunoglobulin-related organized, 8–12 nm wide amyloid fibrils in the TBM also. The patient was diagnosed with diabetic kidney disease and lambda light-chain amyloidosis. There was no restrictive cardiomyopathy and the NT-proBNP level was normal; however, carpal tunnel syndrome was verified by electroneurography. Chemotherapy was postponed because inflammation in the left knee necessitated the removal of the patient’s prosthesis and the reinsertion of the prosthesis a year later. In August 2022, double-J stenting was performed for ureterolithiasis. The patient is on conservative treatment, eGFR is 88 ml/min/1.73 m2, proteinuria was unchanged, and urinary protein-to-creatinine ratio (uPCR) is 225 mg/mmol now.
Figure 1. (case 1): (A) Segmental lambda positivity of the tubular and glomerular basement membrane (100×) (B) Congo red stain - apple green birefringence under polarized microscopy - segmental glomerular and tubular basement membrane positivity (200×) (C) Electron microscopy, peripheral glomerular capillary segment, low magnification (4000×). Nodular mesangial expansion with diabetic fibrillosis (white triangle) and thickened glomerular basement membrane measuring 1200 nm (white arrow). The urine space is narrow with extensive podocyte foot process effacement (white star) (D) Electron microscopy, tubular basement membrane. Three different types of partially organized fibrillary structures in and around the tubular basement membrane. Collagen fibers’ diameter ∼30 nm (black star), amyloid fibers’ diameter ∼10–12nm (white arrow) and diabetic fibrillosis like structures’ diameter ∼10 nm (black arrow).
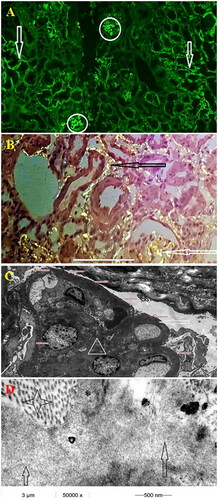
Case 2
A 67-year-old man had a history of regular alcohol consumption and anemia requiring transfusion due to a bleeding duodenal ulcer and paroxysmal atrial fibrillation. In January 2022, he was referred to the nephrology department because of a rapidly decreasing eGFR of 17 ml/min/1.73 m2 and increased proteinuria with a uPCR of 596 mg/mmol. Both kidneys were normal in size, but a CT scan verified the presence of a solid tumor in the liver. SPEP revealed monoclonal gammopathy (IgA lambda 2.4 g/l) with a normal sFLCk/l of 0.743 and no detectable monoclonal component in the urine. Renal histology showed 29 subtotally sclerotic and 4 normal glomeruli, with an IFTA of 60%. Immunofluorescence staining showed monoclonal lambda light-chain restriction primarily in the mesangial region, blood vessels, and tubules. In these localizations, a PAS-negative, Congo red positive, apple-green birefringent material was detected under polarized microscopy. The EM examination confirmed 8–12 nm wide amyloid fibrillar structures. A subsequent liver biopsy confirmed cholangiocellular carcinoma; therefore, a liver segment resection was performed. During the long postoperative recovery period, the kidney function decreased, and hemodialysis was initiated. Chemotherapy was not initiated for AL amyloidosis, and both oncologists and hematologists recommended conservative treatment. Multiple liver metastases were observed, and the patient died in December 2022.
Case 3
A 69-year-old male patient had a myocardial infarction in 2002, and a history of kidney stones, Lyme disease, prostate hyperplasia, fatty liver, and diabetes mellitus. In February 2016, he was admitted to the nephrology department due to a decrease to 19 ml/min/1.73 m2 in eGFR and nephrotic syndrome with a uPCR level of 1332 mg/mmol. An increased sFLCk/l value of 7 was verified without detectable monoclonal components in the SPEP. Renal histology revealed one sclerotic glomerulus and 18 glomeruli with extensive kappa restriction with PAS-negative, Congo red positive and apple green birefringent material in the mesangium, efferent, afferent, and smaller arterioles. IFTA was 50%. EM confirmed the presence of 8–12 nm wide amyloid fibrillar structure in the TBM also. Bone marrow biopsy revealed a 10% plasma cell infiltration; FISH was negative, confirming monoclonal gammopathy of renal significance as a cause of kappa LA. The patient had no extrarenal involvement. Chemotherapy was initiated; however, unstable angina developed before the second cycle. Urgent coronarography and coronary artery bypass surgery were performed; however, the patient died three days later.
Case 4
In October 2019, 55-year-old female patient presented with a history of kidney stones and cholecystectomy. Proteinuria and microscopic hematuria were detected, with a slight decrease in kidney function. One year later, urgent hemodialysis was initiated due to an abrupt deterioration of kidney function (eGFR of 9 ml/min/1.73 m2), and she was referred to our nephrology department. Both kidneys were normal in size, microscopic hematuria and nephrotic proteinuria (uPCR:967 mg/mmol) were present. Both ANCA and a-GBM tests were negative. A renal biopsy was performed. Lambda LA involving the glomeruli, interstitium, and blood vessels was verified with advanced glomerulosclerosis and an IFTA >60%. EM confirmed amyloid 8–12 nm fibrillar structure in the aforementioned localization and TBM. SPEP detected free lambda light chains using only immunofixation. SFLCk/l was 0.078. Bone marrow biopsy revealed a 20% plasma cell infiltration; therefore, the final diagnosis was AL amyloidosis due to multiple myeloma. Echocardiography showed restrictive cardiomyopathy, serum NT-proBNP level was 19,831 ng/l; and there were no other signs of extrarenal amyloid deposition. Chemotherapy was then initiated, but sudden cardiac death occurred after the second cycle.
Discussion
We retrospectively reviewed patients with LA who had been diagnosed by renal biopsy in the last 7 years. Among the 12 LA patients, interstitial amyloid deposition was present in 8, including 4 with amyloid deposition involving the TBM. Interstitial involvement appeared to be similar to that observed in large cohorts. Fun et al. reported interstitial amyloid deposits in 63.7% (165/259) of LA cases [Citation7]. In a Mayo Clinic cohort of systemic (but 85.9% immunoglobulin amyloid type) amyloidosis, the interstitium was involved in 58% of the cases, whereas TBM deposition was found in only 8% (38/470) of the cases [Citation8]. Compared to patients without TBM involvement, 3 of our patients with TBM amyloid deposition were at a later stage of the disease, presenting with lower eGFR (9–19 ml/min/1.73 m2), and higher rate of both sclerotic glomeruli and IFTA. Late diagnosis probably contributed to the higher frequency of TBM involvement because the main serum creatinine was only 1.3 mg% in the Mayo Clinic cohort [Citation8].
In 3 of 4 patients with TBM amyloid deposition (case 2–4), advanced glomerulosclerosis and tubulointerstitial damage were present at the time of diagnosis, and renal failure progressed despite chemotherapy. However, in case 1, sclerosis and fibrosis were negligible, and kidney function remained normal for 2.5 years without chemotherapy, even though the TBM was affected by both amyloid fibrils and non-amyloid fibrillosis. This indicates that amyloid deposition in the TBM alone does not play a significant role in tubulointerstitial damage. In contrast, there are opposing observations. In a case report of a patient with rheumatoid arthritis, TBM amyloid deposition with negligible glomerular and absent vascular involvement was proposed to be responsible for renal failure [Citation9]. Kuruda found a similar frequency of circumferential TBM amyloid deposition in rheumatoid arthritis and LA; however, its influence on renal function remains unclear [Citation10].
In cases 2–4, there was significant amyloid deposition in the arterioles as well as in the smaller arteries, which was absent in case 1. Among patients without TBM involvement, those with blood vessel amyloid deposition (cases 7–12), IFTA varied between 10 and 50%, but in those who did not have vascular involvement (cases 5–6), IFTA was less than 10%. The narrowing and occlusion of vessels by amyloid deposition may contribute to advanced tubulointerstitial fibrosis. In a report from the Mayo Clinic, 12 of 234 patients with AL amyloidosis had vascular-limited renal amyloidosis. Compared with patients with diffuse renal involvement, patients with vascular-limited amyloidosis have higher serum creatinine levels at the time of diagnosis and significantly shorter survival times [Citation2]. Besides localization, amyloid load is also an independent predictor of AL amyloidosis prognosis. Computer-assisted software is currently used to estimate the amyloid area involved [Citation7,Citation11]. The missing vascular and only focal and segmental glomerular amyloid involvement indicated a limited amyloid load in case 1 which could also explain the favorable prognosis.
Case 1 had long-standing diabetes mellitus with histologically verified diabetic nephropathy; therefore, ‘diabetic fibrillosis’ emerged in the background as non-amyloid TBM fiber. These fibrils generally have a similar diameter (10 nm) as immunoglobulin-related amyloid fibrils (8–12 nm), especially in the expanded diabetic mesangium [Citation12,Citation13]. They may represent a type of fibrillary, perhaps glycosylated, collagen cross-linked to other proteins, making it impossible for them to be properly catabolized. The N-glycosylation process also plays a major role in LC amyloidogenesis [Citation14]; therefore, the collagen fibrils may have helped in the precipitation of LC in our case. This can also explain the patient’s carpal tunnel syndrome, which is common in both diabetes mellitus and LA, but its frequency is higher in LA, and can be the first warning sign of the disease.
Apart from the uncertain effect on renal function, amyloid deposition in the TBM was not associated with other clinical consequences. Glucosuria was observed only in one patient without TBM amyloid involvement. No other symptoms were suggestive of Fanconi syndrome. This is typically observed in light chain proximal tubulopathy when the excess free light chain injures proximal tubular epithelial cells, sometimes with crystal formation in the cytoplasm [Citation15]. Very rarely, intracellular amyloid, termed amyloid proximal tubulopathy, has been detected in such cases [Citation16]. One of our patients experiencing LA without TBM amyloid involvement (case 11) had LC cast nephropathy, but no amyloid casts were formed, which is not a rare phenomenon in such cases [Citation6,Citation17]. None of the patients had polyuria or diabetes insipidus, which have been reported in cases with predominant tubulointerstitial amyloid involvement [Citation18]. This is mostly observed when the medullary region is involved. In cases with non-significant glomerular deposition (resulting in less proteinuria), the clinical presentation could suggest acute interstitial nephritis, especially in patients with a predisposition, such as in Sjogren syndrome [Citation19]. Rarely, giant cell reactions provoked by interstitial amyloid deposits are observed, typically in AA amyloidosis, but they also occur in LA and LCDD [Citation20,Citation21].
Amyloid formation does not depend on the amount of light chains but on mutations. The lambda light chain is significantly more amyloidogenic than the kappa light chain. The variable region of the lambda light chain sequences has the most extensive non-conservative and total number of mutations compared to all other sequence groups [Citation22]. Lambda LC was also overrepresented in our LA cohort; however, with respect to TBM involvement, the type of LC was not different (kappa/lambda ratio of 1/3 vs. 2/6).
There is substantial information about renal amyloidogenesis in LA; however, amyloid deposition within the TBM is not well established. Amyloid formation begins in the mesangium. The normal smooth muscle phenotype of mesangial cells changes to a macrophage phenotype upon recognition of precursor light chains. Amyloidogenic light chains are internalized in mesangial cells and transported to lysosomes for digestion to form amyloid fibrils [Citation13]. This process is the same in the proximal tubular cells. They are able to reabsorb the immunoglobulin light chain through the cubilin-megalin receptors and by clathrin-dependent endocytosis, and amyloid may form in tubular cells. Accordingly, during the EM examination of the TBM, it was established that in cases 1 and 4, amyloid accumulated more on the cell side of the TBM; however, in case 2, it was dominant on the interstitial side. This may suggest that amyloid deposition can also occur from the interstitium; although peritubular capillaries have a fenestrated endothelium, their basement membrane, like the glomerular basement membrane, is impermeable to amyloid.
Based on this small cohort study, we would like to raise awareness and emphasize the importance of finding amyloid deposition in the TBM. This usually represents a late-stage presentation of the disease with a heavy LC burden. However, it can occur in cases with a smaller amyloid load and does not in and of itself lead to the deterioration of kidney function or tubular dysfunction. Amyloid deposition in the TBM depends not only on the concentration and properties of folding immunoglobulins, but also on the local environment. In the present case (case 1), ‘diabetic fibrillosis’ may have served as a precipitating factor.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hopfer H, Wiech T, Mihatsch MJ. Renal amyloidosis revisited: amyloid distribution, dynamics and biochemical type. Nephrol Dial Transplant. 2011;26(9):1–6.
- Eirin A, Irazabal MV, Gertz MA, et al. Clinical features of patients with immunoglobulin light chain amyloidosis (AL) with vascular-limited deposition in the kidney. Nephrol Dial Transplant. 2012;27(3):1097–1101.
- Rubinstein S, Cornell RF, Du L, et al. Novel pathologic scoring tools predict end-stage kidney disease in light chain (AL) amyloidosis. Amyloid. 2017;24(3):205–211.
- Hoelbeek JJ, Kers J, Steenbergen EJ, et al. Renal amyloidosis: validation of a proposed histological scoring system in an independent cohort. Clin Kidney J. 2021;14(3):855–862.
- Rezk T, Salota R, Gan JJ, et al. Urinary retinol binding protein predicts renal outcome in systemic immunoglobulin light-chain (AL) amyloidosis. Br J Haematol. 2021;194(6):1016–1023.
- Said SM, Best Rocha A, Valeri AM, et al. The characteristics of patients with kidney light chain deposition disease concurrent with light chain amyloidosis. Kidney Int. 2022;101(1):152–163.
- Fan Y, Liang D, Rui H, et al. Using digital whole-slide images to evaluate renal amyloid deposition and its association with clinical features and outcomes of AL amyloidosis. J Nephrol. 2021;34(5):1747–1756.
- Said SM, Sethi S, Valeri AM, et al. Renal amyloidosis: origin and clinicopathologic correlations of 474 recent cases. Clin J Am Soc Nephrol. 2013;8(9):1515–1523.
- Hiki Y, Horii A, Kokubo T, et al. A case of rheumatoid arthritis with renal tubular amyloidosis. Nephron. 1994;68(3):394–395.
- Kuroda T, Ito Y, Imai N, et al. Significant association between renal function and area of amyloid deposition in kidney biopsy specimens from patients with AA amyloidosis associated with rheumatoid arthritis and AL amyloidosis. Amyloid. 2019;26(sup1):125–126.
- Kuroda T, Tanabe N, Kobayashi D, et al. Significant association between renal function and amyloid-positive area in renal biopsy specimens in AL amyloidosis. BMC Nephrol. 2012;13:118.
- Sohar E, Ravid M, Ben-Shaul Y, et al. Diabetic fibrillosis. Am J Med. 1970;49(1):64–69.
- Herrera GA, Turbat-Herrera EA. Renal diseases with organized deposits: an algorithmic approach to classification and clinicopathologic diagnosis. Arch Pathol Lab Med. 2010;134(4):512–531.
- Nevone A, Girelli M, Mangiacavalli S, et al. An N-glycosylation hotspot in immunoglobulin κ light chains is associated with AL amyloidosis. Leukemia. 2022;36(8):2076–2085.
- Zhu L, Wang L, Shi H, et al. Combined crystal-storing histiocytosis, light chain proximal tubulopathy, and light chain crystalline podocytopathy in a patient with multiple myeloma: a case report and literature review. Ren Fail. 2023;45(1):2145970.
- Larsen CP, Borrelli GS, Walker PD. Amyloid proximal tubulopathy: a novel form of light chain proximal tubulopathy. Clin Kidney J. 2012;5(2):130–132.
- Gibier JB, Gnemmi V, Glowacki F, et al. Intratubular amyloid in light chain cast nephropathy is a risk factor for systemic light chain amyloidosis. Mod Pathol. 2018;31(3):452–462.
- Asmundsson P, Snaedal J. Persistent water diuresis in renal amyloidosis. A case report. Scand J Urol Nephrol. 1981;15(1):77–79.
- Inoue R, Fujigaki Y, Kobayashi K, et al. Clinical presentation of tubulointerstitial nephritis caused by amyloid light-chain amyloidosis in a patient with Sjögren’s syndrome. Intern Med. 2017;56(4):419–423.
- Hill GS, Morel-Maroger L, Méry JP, et al. Renal lesions in multiple myeloma: their relationship to associated protein abnormalities. Am J Kidney Dis. 1983;2(4):423–438.
- Troxell ML, Griffiths R, Schnadig I, et al. Light chain renal amyloidosis with prominent giant cells. Am J Kidney Dis. 2013;62(6):1193–1197.
- Poshusta TL, Sikkink LA, Leung N, et al. Mutations in specific structural regions of immunoglobulin light chains are associated with free light chain levels in patients with AL amyloidosis. PLoS One. 2009;4(4):e5169.
