Abstract
Objective
This study aimed to investigate the association between patient clinical characteristics and technique failure in peritoneal dialysis-related peritonitis (PDRP). The effect of peritonitis-associated technique failure on patient survival was also assessed.
Methods
Patients diagnosed with PDRP from January 1, 2010 to June 30, 2022 were retrospectively reviewed and analyzed. Relevant demographic, biochemical, and clinical data were collected. Univariate and multivariate logistic regression analyses were used to determine the predictors of peritonitis-associated technique failure in PD. Patients were divided into technique failure (F group) and nontechnique failure (NF group) groups. Patients were followed until death or until the date of Oct 1, 2022. Kaplan–Meier survival curves and landmark analysis were used to assess the survival of the PDRP cohort. Cox regression models were used to assess the association between potential risk factors and mortality.
Results
A total of 376 patients with 648 cases of PDRP were included in this study. Multivariate logistic regression analysis demonstrated that peritoneal dialysis (PD) duration (OR = 1.12 [1.03, 1.21], p = 0.005), dialysate WBC count on Day 3 after antibiotic therapy (OR = 1.41 [1.22, 1.64], p = 0.001), blood neutrophil-to-lymphocyte ratio (NLR) (OR = 1.83 [1.25, 2.70], p = 0.002), and serum lactate dehydrogenase (LDH) (OR = 4.13 [1.69, 10.11], p = 0.002) were independent predictors for technique failure in PDRP. Furthermore, serum high-density lipoprotein (HDL) (OR = 0.28 [0.13, 0.64], p = 0.002) was a protective factor against technique failure. According to the Kaplan–Meier analysis, patients experiencing peritonitis-associated technique failure had lower postperitonitis survival (log-rank = 4.326, p = 0.038). According to the landmark analysis, patients with a history of peritonitis-associated technical failures had a higher 8-year mortality after peritoneal dialysis. A Cox model adjusted for plausible predetermined confounders showed that technique failure was independently associated with all-cause mortality.
Conclusions
Dialysate WBC count on Day 3, PD duration, NLR, and LDH were independent risk factors for technique failure, whereas HDL was a protective factor. Peritonitis-associated technique failure had a higher risk of mortality and adverse effects on postperitonitis survival.
Introduction
Peritoneal dialysis (PD) is an important form of renal replacement therapy for patients with end-stage renal disease (ESRD), with approximately 197,000 patients worldwide utilizing this therapy, which accounts for 11% of all dialysis patients [Citation1]. Despite its benefits, PD is associated with several complications, including catheter dysfunction, poor drainage, dialysate leakage, peritoneal herniation, weakening of the abdominal wall, and peritonitis [Citation2]. PD-related peritonitis (PDRP) is the most common type of PD-related infection and can result in significant harm, including pain, transfer to hemodialysis (HD), alterations of the peritoneal membrane and peritoneal adhesions, and even death. As a result, peritonitis can make long-term treatment with PD a challenging issue [Citation2].
Technique failure, which is defined as transfer to HD for ≥30 days, death occurring within 30 days of peritonitis onset, or death during hospitalization due to peritonitis, is a common outcome of PDRP. PDRP accounts for nearly 24% of all technique failure events [Citation3]. Although the relationship between patient clinical characteristics and PDRP outcomes has been widely investigated, the risk factors associated with technique failure in PDRP have not been confirmed. Previous studies have examined some peritonitis prognosis-related factors, such as high-density lipoprotein (HDL), neutrophil-to-lymphocyte ratio (NLR), a history of at least one previous peritonitis episode, and causative organisms [Citation4–7]. However, conflicting results have been reported, with some studies demonstrating no association between these factors and treatment outcomes [Citation8].
To avoid treatment failure during PDRP episodes, continued research is needed to identify potential risk factors for technique failure. In addition, it is also worth assessing whether technical failure affects patients’ long-term survival.
Materials and methods
Study population
In this retrospective study, patients who underwent PD and who were treated for PD-related infections during hospitalization at the Hangzhou Hospital of Traditional Chinese Medicine from January 1, 2010 to June 30, 2022 were enrolled.
Peritonitis was defined as the presence of at least two of the following criteria: 1). clinical features consistent with peritonitis, including abdominal pain and/or cloudy dialysis effluent; 2). dialysis effluent white cell count > 100/μL or > 0.1*109/L (after a dwell time of at least 2 h), with > 50% polymorphonuclear leukocytes (PMN); and 3). positive dialysis effluent culture [Citation2].
Relapsing, recurrent, and repetitive peritonitis were defined as ISPD suggested. Relapsing and recurrent peritonitis are considered a single episode, whereas repetitive peritonitis is quantified based on the number of occurrences [Citation9].
Treatment and outcomes
Each patient with peritonitis was treated with standard antibiotic protocols following the latest guidelines, including the International Society for Peritoneal Dialysis (ISPD) guidelines [Citation2, Citation10–12], in which 1). empirical antibiotic therapy was initiated as soon as possible after appropriate microbiological specimens had been obtained; 2). empirical antibiotic regimens were center-specific and encompassed both gram-positive and gram-negative organisms; 3). gram-positive organisms were covered by a first-generation cephalosporin or vancomycin, and gram-negative organisms were covered by a third-generation cephalosporin or an aminoglycoside; 4). cefepime monotherapy was regarded as an acceptable alternative for empirical antibiotic regimens; and 5). catheter removal was performed in patients with refractory, relapsing, or fungal peritonitis, as recommended by the ISPD. According to KDIGO/ISPD guidelines [Citation1, Citation2], appropriate drugs were administered to treat complications such as hypertension, renal anemia, and renal bone disease. Patients with coronary heart disease, cerebrovascular disease, diabetes, hyperlipidemia, and hyperuricemia were actively treated.
Medical cure was defined as the complete resolution (culture of PD effluent was confirmed negative following appropriate treatment, with a PD effluent white cell count lower than 100/μL) together with a lack of the following complications: relapse/recurrent peritonitis, catheter removal, transfer to HD for ≥30 days, or death during the 30 days after onset of peritonitis episode [Citation1,Citation2,Citation13]. Moreover, PD technique failure was defined as the transfer to in-center HD for greater than 30 days or PDRP-associated death, which was defined as death within 30 days of the onset of PDRP [Citation1,Citation2,Citation13].
The exclusion criteria were defined as follows: 1). patients with incomplete medical records (lacking data on bacterial cultures, time of initial peritonitis episode, PD start time, and dialysate WBC count on Day 3); 2). patients who received outpatient treatment; and 3). patients who were transferred to our hospital after receiving effective treatment in another hospital and who had normal leukocyte levels in their dialysis fluid.
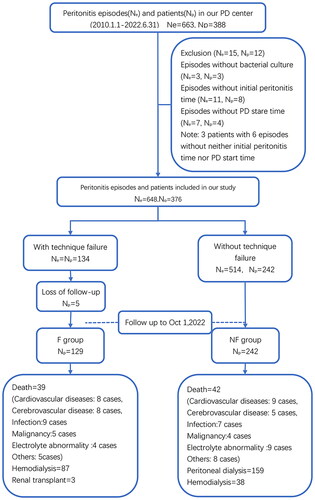
According to peritonitis outcomes, 376 patients were divided into a PD technique failure group (F group, 134 patients) and a group of patients without technique failure (NF group, 242 patients). Patients transferred to HD received regular dialysis 3 to 4 times a week and standardized management by dialysis centers to ensure adequate dialysis. Patients were followed-up until death or until the end of the study.
All of the patients provided written informed consent, and the study was approved by the Ethics Committee of Hangzhou Hospital of Traditional Chinese Medicine (Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University) with approval number: 2017KY026, 2022KY157.
Data collection
Data were obtained from the medical records of eligible patients. Basic demographic characteristics were recorded, including sex, age, comorbidities, etiology of end-stage renal disease (ESRD), number of previous PDRP episodes, elevated body mass index (BMI), and PD duration (years). Laboratory data were recorded, including body temperature, blood pressure, serum albumin, serum potassium, hemoglobin (Hb), peripheral white blood cell (WBC) count, blood neutrophil-to-lymphocyte ratio (NLR), serum C-reactive protein level (CRP), serum high-density lipoprotein (HDL), serum lactate dehydrogenase (LDH), and serum alkaline phosphatase (ALP). In addition, causative organisms were obtained within 24 h of peritonitis diagnosis. Dialysate WBC count on Day 3 was recorded on the third day after antibiotic therapy. Furthermore, urea distribution volume (Kt/V [urea]) and the peritoneal equilibration test (PET) of the latest assessment before the episode were also obtained.
A blood pressure measurement lower than 90/60 mmHg was defined as hypotension. A body temperature higher than 37.5 °C was defined as fever. In addition, a serum albumin level lower than 30 g/L was defined as hypoalbuminemia. A serum potassium level lower than 3.5 mmol/L was defined as hypokalemia. Moreover, BMI ≥ 25 kg/m2 was defined as being overweight. Comorbid conditions were scored into three groups according to the Charlson Comorbidity Index (CCI) [Citation14]: low (2 points, due to the fact that patients with kidney failure by definition already have 2 points), intermediate (3–4 points), and severe comorbidity (≥5 points).
Post-peritonitis survival time was calculated from the date of PDRP onset to the date of death or to the end of the study. The survival analysis model utilized data from all of the patients (except for those patients who lacked further information during the 30-day follow-up period).
Statistical analyses
Statistical analyses were performed by using Statistical Package for Social Science Software version 26.0. The results are expressed as the mean ± standard deviation (SD) for continuous normally distributed data or as the median and interquartile range (IQR) for continuous nonnormally distributed data. Frequencies and percentages were used for the categorical data. To normalize the distribution, some clinical characteristics were transformed to natural logarithms (Ln), including dialysate WBC count on Day 3 (third day after antibiotic therapy), WBC, HB, NLR, CRP, HDL, LDH, and ALP. Univariate logistic regression analysis was used to determine the predictors of treatment failure. Significant univariate analysis variables with p-values < 0.05 were incorporated into the multivariate logistic regression analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Moreover, a two-tailed p-value < 0.05 was considered to be statistically significant. Kaplan–Meier survival analysis was performed and compared by using log-rank tests. A multivariable Cox model was used to analyze the mortality risk of technique failure by adjusting for sex, age, CCI, and other factors. Furthermore, forest plots for risk factors, Kaplan–Meier survival curves, and landmark curves were plotted by using R software (Version 4.0.5, https://CRAN.R-project.org/mirrors. html).
Results
Patient characteristics
A total of 648 episodes experienced by 376 patients were included in the final analysis (). Patient characteristics and comorbidities are presented in . A total of 186 (49.5%) patients were male. The overall median age was 60 years (IQR: 49–69), and the median PD duration was 4 years (IQR: 2–7). A total of 134 (20.7%) episodes were identified as treatment failures over the 30-day follow-up.
Table 1. Demographic and clinical characteristics of 648 episodes of PDRP associated peritonitis.
Glomerulonephritis (82.1%) and diabetic nephropathy (8.6%) were the major primary renal diseases that caused ESRD; in addition, 446 (68.8%) patients had hypertension, 137 (21.1%) patients had diabetes mellitus, and 116 (17.9%) patients had coronary artery disease.
The causative organisms of peritonitis episodes are summarized as follows: gram-positive bacteria (315 episodes, 48.6%), gram-negative bacteria (152 episodes, 23.5%), polymicrobial (pure gram-positive; 31 episodes, 4.8%), mixed gram-positive and gram-negative organisms (17 episodes, 2.6%), fungi (31 episodes, 4.8%), and tuberculosis (2 episodes, 0.3%). Negative culture results were observed in 110 (17.0%) patients.
Hypokalemia rates, PD duration, number of previous episodes, dialysate WBC count on Day 3, and peripheral WBC, NLR, CRP, LDH, and ALP levels were higher in the F group than in the NF group. Moreover, hypertension complication rates and HDL levels in the F group were significantly lower than those in the NF group (p < 0.05).
Univariate logistic regression analysis
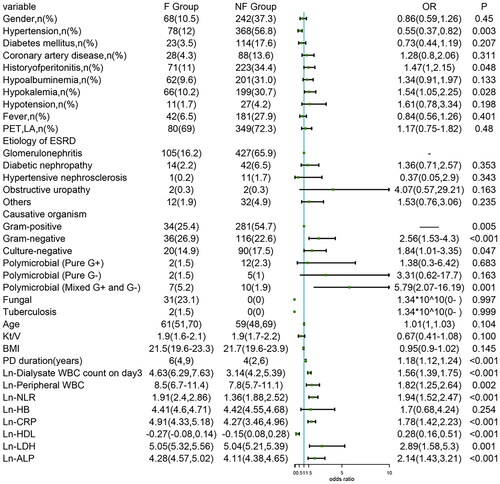
ISPD Peritonitis Recommendations [Citation2] recommend immediate catheter removal when fungi are identified in PD effluent; therefore, all of the fungal peritonitis cases were regarded as technique failures. Furthermore, our study observed only 2 cases of tuberculous peritonitis, which resulted in technique failure. Fungal and tuberculous peritonitis cases were excluded when the effect of causative organisms on treatment outcomes was analyzed, as they have high poor outcome rates. Eleven variables were closely associated with technique failure. In addition, the duration of PD (years) (OR = 1.18 [1.12, 1.24], p < 0.001), number of previous episodes (OR = 1.26 [1.10, 1.44], p = 0.001), dialysate WBC count on Day 3 (OR = 1.56 [1.39, 1.75], p < 0.001), peripheral WBC (OR = 1.82 [1.25, 2.64], p = 0.002), NLR (OR = 1.94 [1.52, 2.47], p < 0.001), CRP (OR = 1.78 [1.42, 2.23], p < 0.001), HDL (OR = 0.28 [0.16, 0.51], p < 0.001), LDH (OR = 2.89 [1.58, 5.3], p = 0.001), ALP (OR = 2.14 [1.43, 3.21], p < 0.001), hypertension complications (OR = 0.55 [0.37, 0.82], p = 0.003), hypokalemia (OR = 1.54 [1.05, 2.25], p = 0.028), and causative organisms (gram-negative [OR = 2.56, 95% CI=∼1.53–4.3, p < 0.001], culture-negative [OR = 1.84, 95% CI=∼1.01–3.35, p = 0.047, mixed gram-positive and gram-negative [OR = 5.79, 95% CI=∼2.07–16.19, p = 0.001] vs. gram-positive) were significantly associated with technique failure ().
Multivariate logistic regression analysis
The significant univariate analysis variables were included in the multivariate logistic regression analysis. Long PD duration (years), dialysate WBC count on Day 3, NLR, HDL, and LDH remained significantly associated with technique failure after the multivariate analysis ().
Table 2. Multivariate analysis of risk factors associated with technique failure in 648 episodes of PDRP.
We observed that PD duration (OR = 1.12 [1.03, 1.21], p = 0.005), dialysate WBC count on Day 3 (OR = 1.41 [1.22, 1.64], p < 0.001), NLR (OR = 1.83 [1.25, 2.70], p = 0.002), HDL (OR = 0.28 [0.13, 0.64], p = 0.002), and LDH (OR = 4.13 [1.69, 10.11], p = 0.002) were independent predictors of technique failure in PDRP.
Prognosis and follow-up
Of the initial 376 patients who were included in the study, 5 patients in the F group were lost to follow-up, thus resulting in 371 patients being included in the survival analysis. During the follow-up period, 39 patients in the F group died due to reasons other than technique failure, whereas 87 patients switched to HD, and 3 patients received a renal transplant. In the NF group, 42 patients died due to reasons other than technique failure, whereas 159 patients continued on peritoneal dialysis, and 38 patients switched to HD due to various reasons (such as catheter malfunction, inadequate dialysis, and fluid overload). Additionally, 3 patients in the NF group received renal transplants.
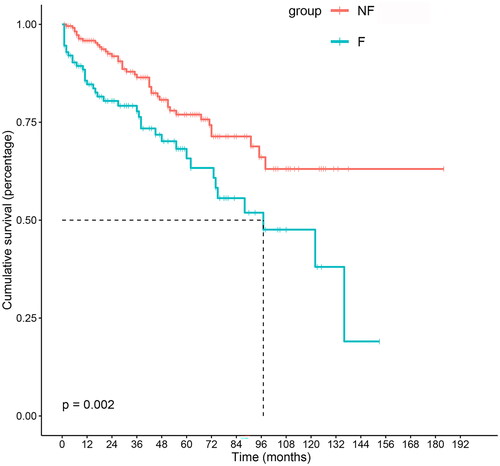
The overall median follow-up time after peritonitis was 44 months (IQR: 18–75). For the F group, it was 42 months (IQR: 16–79), and for the NF group, it was 44 months (IQR: 19–74). The 1-, 3-, 5-, and 10-year survival rates of the F group were 94.6%, 88.4%, 80.4%, and 47.6%, respectively. For the NF group, these rates were 99.6%, 97.3%, 95.8%, and 87.9%, respectively. Kaplan–Meier analysis of postperitonitis survival demonstrated that patients in the NF group had better survival than those in the F group (log-rank = 9.372, p = 0.002), as shown in and . To investigate the association between possible risk factors and patient survival, a Cox regression model was created. Each potentially modifiable risk factor was adjusted for plausible predetermined confounders (age, sex, BMI, CCI, and hypotension). The results showed that peritonitis-associated technique failure was independently associated with all-cause mortality (HR = 1.65 [1.06–2.59], p = 0.027), as shown in .
Table 3. Kaplan–Meier survival analysis and Log Rank test of postperitonitis overall mortality on two groups.
Table 4. Risk factors associated with mortality in a Cox regression modelTable Footnotea.
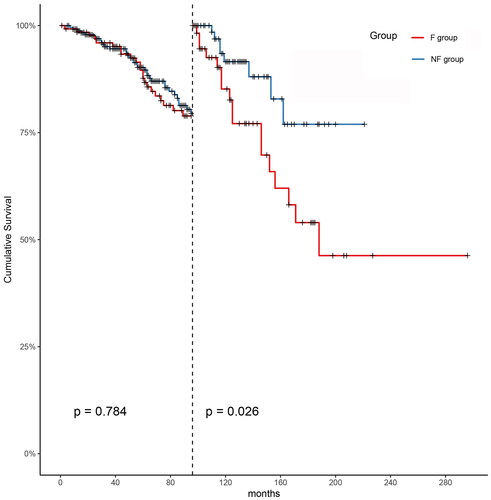
The overall median follow-up time post-PD was 92 months (IQR: 57–127). For the F and NF groups, it was 103 months (IQR: 63–166) and 83 months (IQR: 55–121), respectively. The 1-, 3-, 5-, and 10-year survival rates of the F group were 96.8%, 87.7%, 81.3%, and 42.6%; for the NF group, these rates were 98.7%, 96.5%, 94.6%, and 87.0%, respectively. There was no difference in survival rates between the two groups for the first 8 years post-PD (p = 0.784). After the first 8 years, landmark analysis demonstrated that patients in the NF group had better survival than those in the F group (p = 0.026), as shown in and .
Table 5. Landmark analysis and Log Rank test of post-PD overall mortality on two groups.
Discussion
The prevention of technique failure remains a challenge for peritoneal dialysis. There are several causes for technique failure, including catheter-related problems, clearance or ultrafiltration (UF) problems, peritoneal leakage, and the risk or diagnosis of encapsulating peritoneal sclerosis (EPS), among other factors [Citation15].
Recent studies have found that the APD modality is associated with a reduced risk of technique failure compared to CAPD [Citation13, Citation15, Citation16]. Therefore, elevated BMI and reduced serum albumin levels are also regarded as being predictors of peritoneal dialysis withdrawal and all-cause mortality [Citation17,Citation18], and diabetes, cardiovascular disease, and other complications appear to be risk factors for death or switching to HD [Citation13,Citation19]. Employment status and educational level may be different in people with and without technique failure [Citation13, Citation20].
Therefore, PDRP is the most common cause of technique failure. The risk indicators for technique failure in PDRP need further discussion. It is our aim to explore the potential etiology of peritonitis-associated technical failure in the PDRP population in our center.
Our study investigated patients who underwent PD and who experienced PDRP in our center from 2010 to 2022. The findings are discussed in two parts, including the factors influencing technique failure and survival analysis of peritonitis-associated technique failure.
Risk factors
The significant association between PD duration and technique failure in PDRP supports the findings of previous investigations [Citation4]. One possible explanation for this effect is that long-term PD may affect the peritoneum, as Tara et al. [Citation21] suggested. Alterations in tissue-resident macrophages could render long-term PD patients more susceptible to developing peritonitis. This correspondingly puts the patients at a higher risk of EPS, which is an urgent indication to discontinue PD. With improving worldwide survival rates, the number of patients undergoing long-term PD is increasing, thus making it even more important to prevent PDRP episodes during PD therapy.
It has also been concluded from earlier studies [Citation22] that a dialysate effluent WBC count > 1,000/µL on Days 3–4 is associated with a substantially higher likelihood of technique failure. ISPD peritonitis guideline recommendations [Citation2] have also suggested it as an independent prognostic marker for treatment failure. Our study confirms that a high level of dialysis WBC count on Day 3 is related to a higher risk of technique failure. Furthermore, the higher count may be indicative of a poor outcome resulting from early treatment with empirical antibiotic therapy.
The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count of the peripheral blood. The NLR is a useful indicator of the systemic inflammatory response, which can be used to evaluate acute or chronic inflammation [Citation23]. Our study showed that an elevated NLR was a significant predictor of peritonitis-associated technique failure. This association was also reported by He et al. [Citation5], which potentially indicates a novel predictor for peritonitis-associated technique failure.
The association between HDL and peritonitis-associated treatment failure is controversial. A study investigated the relationship between lipid disorders and peritoneal dialysis (PD) treatment and found that based on the univariate analysis, there was an association between HDL levels and treatment failure [Citation24]. Our study suggested that HDL was a significant protective factor against technique failure, which is similar to the results of previous studies [Citation4]. Previous studies have confirmed that HDL particles can inhibit the virulence-mediated functions of Staphylococcus aureus, including the activation, attraction, and lysis of neutrophils, both in vitro and in vivo [Citation25]. However, another study demonstrated that HDL can lose its anti-inflammatory and antioxidant functions; moreover, HDL paradoxically adopts proinflammatory characteristics and promotes systemic oxidative stress in chronic inflammatory disorders [Citation26]. This may explain the conflicting results pertaining to the relationship between HDL and technique failure in peritonitis. Furthermore, lipid disorders are present in patients with ESRD. When considering the controversial relationship between lipid disorders and PD, the effects of dyslipidaemia on the prognosis of PDRP require further investigation.
We observed that higher LDH levels were associated with an increased risk of technique failure. A previous study demonstrated an association between higher LDH concentrations and mortality in HD patients [Citation27], whereas the association of LDH levels and the prognosis of peritonitis in PD patients has never been detected in previous studies. LDH is released by inflammatory cells, which contributes to the inflammatory state [Citation28]. LDH has also been described as a prognostic marker in infectious disease states [Citation29].
In our study, cultured organisms, CRP, and ALP were risk factors in the univariate logistic regression analysis (but not in the multivariate logistic regression analysis).
Unsurprisingly, as ISPD Peritonitis Recommendations [Citation2] recommend immediate catheter removal when fungi are identified in PD effluent, fungi were a risk factor for technique failure in PDRP. We observed that episodes of gram-negative peritonitis and polymicrobial peritonitis involving mixed gram-positive and gram-negative organisms exhibited a higher risk of technique failure than those caused by gram-positive organisms alone. Similar results have been reported in previous studies [Citation7, Citation9, Citation30, Citation31]. According to Ying et al. [Citation7], gram-negative bacteria may be linked to contact contamination, outlet infection, or cross-wall migration from conditions such as constipation, colitis, or infections in the abdominal cavity. This scenario may explain the observed situation.
This controversy also exists in studies investigating the relationship between CRP and peritonitis-associated technique failure. In a prospective cohort study of patients undergoing PD, higher CRP levels were independently associated with adverse outcomes in PD-associated peritonitis [Citation32]. However, other studies have observed no relationship between CRP and outcome [Citation33]. In our study, CRP was considered to be a risk factor (but not an independent risk factor). When considering that high levels of CRP reflect a strong inflammatory immune response, we suppose that factors other than inflammation play a role during peritonitis and require further investigation.
Ye et al. found that higher serum ALP levels were associated with a higher risk of short-term adverse peritonitis outcomes [Citation34]. Campbell et al. confirmed that endogenous intestinal ALP reduced the activity of the NF-kB signaling pathway induced by LPS, which led to a reduction in proinflammatory factors [Citation35]. We also suggest that high ALP is associated with a higher risk of PD technique failure (but is not an independent risk factor). Further research is needed to determine if elevated ALP is a risk factor for technique failure following PDRP.
Another study reported that higher albumin levels indicated a higher probability of peritonitis recovery [Citation36]. However, our study did not support this conclusion. Albumin levels can be used as a nutritional status indicator in PD patients. We believe that hypoalbuminemia is a consequence of peritonitis but does not necessarily reflect the severity of peritonitis.
Hemoglobin, blood pressure, Kt/v, PET, and BMI during peritonitis episodes were also recorded. However, no effect on peritonitis outcome was detected from these parameters in this study.
Survival analysis
Our study identified that patients experiencing peritonitis-associated technique failure had higher overall mortality rates than nonfailure patients, which is supported by a study conducted in Australia [Citation37].
When considering the impact of PD duration, no significant difference was observed in the 8-year survival rates among patients with or without peritonitis-associated technique failure. Peritonitis-associated technique failure may be associated with higher overall mortality beyond 8 years after dialysis. This scenario may highlight the need for enhanced surveillance of peritonitis-associated technique failure patients, especially at 8 years after PD. To the best of our knowledge, this is the first study to investigate the effect of PDRP history on technique failure and postdialysis survival rates.
The results of the Cox regression analysis indicate that a history of peritonitis-associated technical failure is an independent risk factor for higher mortality. We speculate that this effect could be linked to extended malnutrition, intestinal dysfunction, and a state of low-grade inflammation that is elicited by peritonitis-associated technical failure.
Limitations
(1). This study was a single-center retrospective study, which indicates that the results may not be applicable to other centers. (2). There was a proportion of patients lost at the follow-up, which may have impacted the long-term prognosis comparisons between the groups. (3). Clinicians are likely to withdraw PD when patients with long-term PD develop peritonitis when considering the risk of developing EPS or other severe situations. Iatrogenic selection remains a confounding factor that cannot be eliminated.
Conclusion
A long PD duration, high NLR, high dialysate WBC count on Day 3, and high LDH were found to be independent risk factors for treatment failure in PDRP, whereas high HDL levels were a protective factor. Peritonitis-associated technique failure was associated with poorer postperitonitis survival. However, there was no difference in the mortality rates among patients with and without peritonitis-associated technique failure during the first 8 years after PD. However, beyond 8 years, peritonitis-associated technique failure patients had a higher mortality risk.
Ethical approval
The current study protocol was approved by the Ethics Committee of Hangzhou Hospital of Traditional Chinese Medicine, which is affiliated with the Zhejiang Chinese Medical University (approval number: 2017KY026, 2022KY157).
Author contributions
Jin Yu, Lingli Zhu, Mengli Tong, Jun Ni and Hua Wang wrote parts of the manuscript. Jin Yu, Lingli Zhu, Mengli Tong and Hua Wang did the literature research and prepared the data for analysis. Jin Yu and Lingli Zhu prepared . Jin Yu, Lingli Zhu and Hua Wang contributed significantly to the discussion on the interpretation of the results. All authors read and approved the final manuscript.
Acknowledgement
The preprint was published in https://www. researchsquare. com/article/rs-1852781/v1, DOI: https://doi. org/10. 21203/rs. 3.rs-1852781/v1, 22 Jul, 2022, representing an earlier, partial results from the study’s findings.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zeidan A, Bhandari S. Anemia in peritoneal dialysis patients; iron repletion, current and future therapies. Perit Dial Int. 2017;37(1):1–11.
- Li PK-T, Chow KM, Cho Y, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42(2):110–153.
- Mcdonald SCP, Hurst K, Jose M, et al. Peritoneal dialysis. ANZdata 2018 annual report. 2018.
- Liu X, Qin A, Zhou H, et al. Novel predictors and risk score of treatment failure in peritoneal Dialysis-Related peritonitis. Front Med. 2021;8:639744.
- He P, He LJ, Huang C, et al. Neutrophil-to-Lymphocyte ratio and treatment failure in peritoneal Dialysis-Associated peritonitis. Front Med. 2021;8:699502.
- Tan YL, Ooi GS, Vaithilingam I. Factors predicting clinical outcomes of continuous ambulatory peritoneal dialysis associated peritonitis - A single Centre study. Med J Malaysia. 2021;76(3):382–389.
- Zeng Y, Jiang L, Lu Y, et al. Peritoneal dialysis-related peritonitis caused by gram-negative organisms: ten-years experience in a single center. Ren Fail. 2021;43(1):993–1003.
- Ma Y, Wang Y, Wang H-Y, et al. Predictors of short-term outcomes of patients with peritoneal dialysis-associated peritonitis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40(1):13–20.
- Al Sahlawi M, Zhao J, McCullough K, et al. Variation in peritoneal Dialysis-Related peritonitis outcomes in the peritoneal dialysis outcomes and practice patterns study (PDOPPS). Am J Kidney Dis. 2022;79(1):45–55 e41.
- Piraino B, Bailie GR, Bernardini J, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005;25(2):107–131.
- Li PK-T, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393–423.
- Li PK-T, Szeto CC, Piraino B, et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36(5):481–508.
- Bonenkamp AA, van Eck van der Sluijs A, Dekker FW, et al. Technique failure in peritoneal dialysis: modifiable causes and patient-specific risk factors. Perit Dial Int. 2023;43(1):73–83.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Perl J, Davies SJ, Lambie M, et al. The peritoneal dialysis outcomes and practice patterns study (PDOPPS): unifying efforts to inform practice and improve global outcomes in peritoneal dialysis. Perit Dial Int. 2016;36(3):297–307.
- Perl J, Fuller DS, Bieber BA, et al. Peritoneal Dialysis-Related infection rates and outcomes: results from the peritoneal dialysis outcomes and practice patterns study (PDOPPS). Am J Kidney Dis. 2020;76(1):42–53.
- Hao N, Cheng B-C, Yang H-T, et al. Time-varying serum albumin levels and all-cause mortality in prevalent peritoneal dialysis patients: a 5-year observational study. BMC Nephrol. 2019;20(1):254.
- See EJ, Johnson DW, Hawley CM, et al. Risk predictors and causes of technique failure within the first year of peritoneal dialysis: an Australia and New Zealand dialysis and transplant registry (ANZDATA) study. Am J Kidney Dis. 2018;72(2):188–197.
- Kolesnyk I, Dekker FW, Boeschoten EW, et al. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Perit Dial Int. 2010;30(2):170–177.
- Kim HJ, Lee J, Park M, et al. Lower education level is a risk factor for peritonitis and technique failure but not a risk for overall mortality in peritoneal dialysis under comprehensive training system. PLoS One. 2017;12(1):e0169063.
- Sutherland TE, Shaw TN, Lennon R, et al. Ongoing exposure to peritoneal dialysis fluid alters resident peritoneal macrophage phenotype and activation propensity. Front Immunol. 2021;12:715209.
- Nochaiwong S, Ruengorn C, Koyratkoson K, et al. A clinical risk prediction tool for Peritonitis-Associated treatment failure in peritoneal dialysis patients. Sci Rep. 2018;8(1):14797.
- Li X, Wu J, Mao W. Evaluation of the neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, and red cell distribution width for the prediction of prognosis of patients with hepatitis B virus-related decompensated cirrhosis. J Clin Lab Anal. 2020;34(11):e23478.
- Huang Y-J, Jiang Z-P, Zhou J-F, et al. Hypertriglyceridemia is a risk factor for treatment failure in patients with peritoneal dialysis-related peritonitis. Int Urol Nephrol. 2022;54(7):1583–1589.
- Hommes JW, Kratofil RM, Wahlen S, et al. High density lipoproteins mediate in vivo protection against staphylococcal phenol-soluble modulins. Sci Rep. 2021;11(1):15357.
- Vaziri ND. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol. 2016;12(1):37–47.
- Ryu SY, Kleine C-E, Hsiung J-T, et al. Association of lactate dehydrogenase with mortality in incident hemodialysis patients. Nephrol Dial Transplant. 2021;36(4):704–712.
- Miyoshi N, Tanigawa T, Nishioka S, et al. Association of salivary lactate dehydrogenase level with systemic inflammation in a japanese population. J Periodontal Res. 2018;53(4):487–494.
- Su Y, Ju M-J, Ma J-F, et al. Lactate dehydrogenase as a prognostic marker of renal transplant recipients with severe community-acquired pneumonia: a 10-year retrospective study. Ann Transl Med. 2019;7(22):660.
- Dias RCB, Vieira MA, Moro AC, et al. Characterization of Escherichia coli obtained from patients undergoing peritoneal dialysis and diagnosed with peritonitis in a Brazilian Centre. J Med Microbiol. 2019;68(9):1330–1340.
- Htay H, Cho Y, Pascoe EM, et al. Multicentre registry data analysis comparing outcomes of culture-negative peritonitis and different subtypes of culture-positive peritonitis in peritoneal dialysis patients. Perit Dial Int. 2020;40(1):47–56.
- Zalunardo NY, Rose CL, Ma IW, et al. Higher serum C-reactive protein predicts short and long-term outcomes in peritoneal dialysis-associated peritonitis. Kidney Int. 2007;71(7):687–692.
- Yang C-Y, Chen T-W, Lin Y-P, et al. Determinants of catheter loss following continuous ambulatory peritoneal dialysis peritonitis. Perit Dial Int. 2008;28(4):361–370.
- Ye H, Lin X, Qiu Y, et al. Higher alkaline phosphatase was associated with the short-term adverse outcomes of peritoneal dialysis-related peritonitis. Clin Chem Lab Med. 2015;53(4):e113–116.
- Campbell EL, MacManus CF, Kominsky DJ, et al. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci U S A. 2010;107(32):14298–14303.
- Meng L, Yang L, Zhu X, et al. Development and validation of a prediction model for the cure of peritoneal Dialysis-Associated peritonitis: a multicenter observational study. Front Med (Lausanne). 2022;9:875154.
- Boudville N, Kemp A, Clayton P, et al. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol. 2012;23(8):1398–1405.