Abstract
Objective
To investigate the associations of dietary inflammation index (DII) with bone density and osteoporosis in different femoral areas.
Methods
The study population was selected from the National Health and Nutrition Examination Survey (NHANES) with the exclusion criteria of age 18, pregnancy, or missing information on DII, femoral bone marrow density (BMD), estimated glomerular filtration rate (eGFR), and urine albumin-to-creatinine ratio (UACR), or had diseases which may influence systemic inflammation. DII was calculated based on the questionnaire interview of dietary recall within 24 h. Subjects’ baseline characteristics were collected. The associations between DII and different femoral areas were analyzed.
Results
After applying exclusion criteria, 10,312 participants were included in the study. Significant differences among DII tertiles were found in BMD or T scores (p < .001) of the femoral neck, the trochanter, the intertrochanter, and the total femur. High DII was associated with low BMDs and T scores in all the femoral areas (all p < .01). Compared to low DII (tertile1, DII < 0.380 as reference), in the femoral neck, the intertrochanter, and the total femur, increased DII is independently associated with increased the possibility of the presence of osteoporosis (OR, 95% CI: 1.88, 1.11–3.20; 2.10, 1.05–4.20; 1.94, 1.02–3.69, respectively). However, this positive association was only observed in the trochanteric area of the non-Hispanic White population after full adjustment (OR, 95% CI: 3.22 (1.18, 8.79)). No significant difference in the association of DII and the presence of osteoporosis were found in subjects with or without impaired kidney function (eGFR < 60 ml/min/1.73 m2).
Conclusion
High DII is independently related to declined femoral BMD of femoral areas.
Introduction
Osteoporosis, with the characteristics of declined bone mass and degraded bone microstructure, is considered a common senile disease that could lead to an increased risk of bone fragility and fracture, and contribute to an increased mortality in patients [Citation1]. With an estimated 200 million people affected, osteoporosis has been a major global public health concern [Citation2]. As an essential determinant of bone health, the measurement of bone mineral density (BMD) is a common operational tool for the diagnosis of osteoporosis [Citation3].
Chronic systemic inflammation was found not only to contribute to an elevated risk of osteoporosis and fragility fractures but also has a close correlation with some of the critical factors in bone physiology [Citation4–6]. Studies have shown that elevated inflammatory markers in circulation may predict bone loss and resorption in the elderly [Citation7]. Others have demonstrated that higher serum inflammatory markers may be a potential risk factor for fractures in older women and men [Citation5]. These results all support the conclusion that between inflammation and osteoporosis, there should be a close relationship.
Currently, in addition to pharmacological approaches, non-pharmacological approaches such as suitable diet patterns, and adopting a physically active lifestyle, are also major inventions that may help with osteoporosis treatments and prevention [Citation8]. Nutrients from daily diets, such as unsaturated fatty acids, vitamins, fibers, and proteins, can regulate bone metabolism and help with bone health [Citation8–10].
However, dietary components have been considered to have both pro-inflammatory and anti-inflammatory potential which may influence systemic inflammation [Citation11–13]. Growing evidence has shown that dietary patterns are closely associated with BMD and fracture risk in older adults, especially in women [Citation14–16]. Therefore, a proper diet with appropriate inflammatory potential is essential for maintaining bone mass.
The dietary inflammatory index (DII), a scoring system derived from ∼ 2000 literature and based on 11 food consumption data sets worldwide, is considered a good strategy for the qualification of a diet’s inflammatory potential [Citation17]. A positive DII value indicates pro-inflammatory potential while a negative value represents anti-inflammatory potential. The higher the absolute value of DII, the stronger the effect of diet on inflammation. DII has been reported to strongly correlate with many chronic diseases, such as coronary heart disease [Citation18], metabolic syndrome [Citation19], cancer [Citation20], and osteoarthritis [Citation21]. Studies demonstrated that a low DII diet was related to improved BMD in postmenopausal women of different races [Citation22–24]. A meta-analysis based on a large population from multiple studies also indicated that a diet pattern with high pro-inflammatory potential significantly correlates with decreased lumbar and hip BMD and is a risk factor of osteoporosis and fractures in these areas [Citation25]. However, such an association between higher DII and worse BMD was rarely found in men [Citation24,Citation26,Citation27], only one study showed that a pro-inflammatory diet was linked with an increased possibility of hip osteoporosis and fracture in both Chinese men and women [Citation28]. Nevertheless, the associations between DII and osteoporosis in require further exploration.
Among the fractures caused by osteoporosis, hip fracture is the most severe one [Citation29], which is more often observed and results in more severe consequences in patients [Citation30,Citation31]. For the present study, the hip area was chosen for BMD measurement. Using a large multiracial cohort from the National Health and Nutrition Examination Survey (NHANES), we conducted this cross-sectional study to explore the association between the DII and the BMD of the femoral neck, the trochanter, the intertrochanter, and the total femur, as well as the possibility of diagnosed osteoporosis in these areas, which may bring future benefits to prevention and treatment of osteoporosis.
Materials and methods
Study design, setting, and subjects
The NHANES program is a series of continuous national surveys conducted by the National Center for Health Statistics (NCHS, Hyattsville, MD, USA), which selected a nationally representative sample to examine the nutritional and health conditions of the non-institutionalized civilian population of the United States using a complex, stratified, multistage probability sampling design [Citation32]. The NCHS authorized the survey procedure, and all subjects provided signed informed consent. NHANES provides detailed information over the website, https://www.cdc.gov/nchs/nhanes/index.htm.
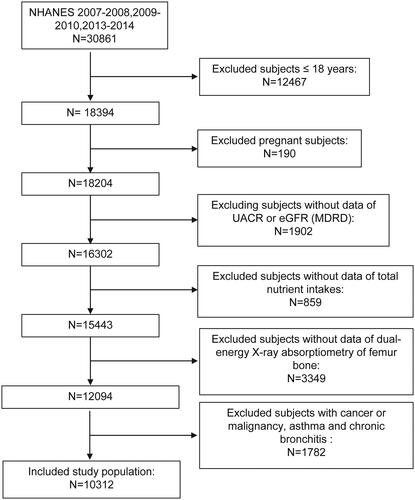
To investigate the relationship between DII and the femoral BMD and the presence of osteoporosis, we performed this study. Measurement conduction and data record were all finished by staff from the NHANES. All the data were collected from the NHANES 2007–2010 and 2013–2014, in total 3 cycles. As shown in , participants who are younger than or equal to18 years old, pregnant at the screen, or had incomplete data of total nutrition intakes, dual-energy X-ray absorptiometry of femur bone, estimated glomerular filtration rate (eGFR), and urine albumin-to-creatinine ratio (UACR), or had cancer or malignancy, asthma, and chronic bronchitis which may influence systemic inflammation were excluded. after applying exclusion criteria, 10,312 participants were included in the study.
Calculation of DII
DII score was calculated using the daily dietary information of every participant and was the exposure variable in this study. Based on robust literature, the complete DII score system contains 45 different food parameters that have pro- or anti-inflammatory properties to calculate the overall influence of a diet on systemic inflammation [Citation17]. Though only 27 or 28 of the 45 food parameters were recorded in the NHANES database, previous studies confirmed that DII scores based on <30 nutrients are still reliable [Citation17,Citation33,Citation34]. The parameters used for DII calculation in this study included energy, carbohydrate; protein; total fat; dietary fiber; cholesterol; saturated, monounsaturated, and polyunsaturated fatty acids; ω-3 and ω-6 polyunsaturated fatty acids; vitamin A, B1, B2, B3, B6, B12, C, D, and E; folic acid; alcohol; β-carotene; caffeine; iron; magnesium; zinc; and selenium. A positive or negative DII value represents a pro-inflammatory or anti-inflammatory diet, respectively.
BMD measurement and osteoporosis diagnosis
BMD was measured by dual-energy X-ray absorptiometry (DXA), data of the femoral neck, the trochanter, the intertrochanter, and the total femur were collected. The detailed DXA measurement agreement is publicly available at http://www.cdc.gov/nchs/nhanes/. Under the guidance of the |World Health Organization criteria, all the BMDs were converted into T-scores using the formula: T-score = (BMD respondent - mean BMD reference group)/SD reference group [Citation35,Citation36]. Osteopenia is diagnosed as a subject whose T score is less than −1.0 but more than −2.5, while osteoporosis is diagnosed as a subject whose T score is less than or equal to −2.5 [Citation27,Citation37].
Covariate ascertainment
Categorical variables were stated as follows: gender (male, female); race/ethnicity (Mexican American, non-Hispanic White, non-Hispanic Black, other race/ethnicity); Education (less than high school, high school diploma, more than high school); Income was evaluated by the poverty income ratio (PIR, PIR <1, poor; PIR 1–3, near-poor; PIR ≥3, not poor) [Citation38]. Smokers were defined as subjects who reported smoking more than 100 cigarettes during their lifetime [Citation39]; Cardiovascular disease (CVD) was defined as “the doctor told you had congestive heart failure, coronary heart disease, angina/angina pectoris, heart attack, or a stroke”; hypertension was defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg or now taking prescribed medicine for high blood pressure. Diabetes mellitus (DM) was defined as “the doctor told you had diabetes” or taking diabetic pills to lower blood sugar now or taking insulin now or fasting plasma glucose ≥ 126 mg/dL or 75-g oral glucose tolerance test of at least 200 mg/dL or glycohemoglobin ≥ 6.5%; Liver condition was defined as “the doctor told you had a liver condition”; Hypercholesterolemia was defined as “the doctor told you had hypercholesterolemia”; Arthritis was defined as “a doctor told you had arthritis”. The use of medication was categorized as “yes” or “no”.
Continuous variables were stated as follows: Age was an age in years at screening. Dietary calcium intake was collected from the dietary intake information by 24 h dietary recalls. Blood pressure data were defined as the average values of all available systolic blood pressure (SBP) and diastolic blood pressure (DBP) values collected when the subjects visit the examination center. The measurements of white blood cell (WBC) count, neutrophil count, lymphocyte count, serum albumin, serum fast hemoglobin, serum glycohemoglobin, serum calcium, serum phosphorus, and serum c-reactive protein were used. Body mass index (BMI) information was collected from the body measurement data at the screen; For the estimated glomerular filtration rate (eGFR) calculation, serum creatinine across different time periods was calibrated as the NHANES recommended [Citation40] and the Modification of Diet in Renal Disease Study Equation [Citation41]were used. Relevant laboratory methods and detailed descriptions can be found on this website, http://www.cdc.gov/nchs/nhanes/.
It is noteworthy that dietary alcohol and vitamin D intake were included in the DII score and were not used as covariates again.
Statistical analysis
Statistical analysis was performed under the guidance of the Centers for Disease Control and Prevention (CDC) (https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx). p < .05 was considered significant. 95% confidence intervals (CI) were also shown.
To describe the baseline characteristics of subjects by tertiles of DII (< 0.380, 0.380 − 2.286, and > 2.286). Normally distributed continuous variables were represented as means ± standard deviation (SD) and the differences among tertiles were calculated by ANOVA, non-normally distributed continuous variables were represented as Median (interquartile range), and the differences among tertiles were calculated by Kruskal–Wallis H Test, while categorical variables were represented as a number with percentages and the differences among tertiles were calculated by the chi-square test. Fisher’s exact test was introduced to compute the significance of the difference if the count variable has a theoretical value of < 10.
Since there may be some possible confounding factors, the weighted linear regression models with adjustments were introduced to compare the BMD levels, T score levels, and osteoporosis incidence among different tertiles of DII. Model 1 was adjusted for age, gender, race/ethnicity; Model 2 was adjusted for age, gender, race/ethnicity, smoker, BMI, eGFR, UACR, serum C-reactive protein, WBC count, NLR, serum calcium, arthritis, aspirin use, calcitonin use, biphosphonate use, DMARDs use, calcium intake and estrogen use. Data presented as β or odds ratio (OR) with 95% CI. Subgroup analyses were also conducted.
All analyses and graphic programs were performed with the statistical software package R-3.4.3 (https://www.R-project.org, The R Foundation) and Empower-Stats (https://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA).
Results
Baseline characteristics
A total of 10,312 subjects were included in this study after the exclusion of those with age ≤18 years old, pregnant, or loss information of eGFR, UACR, nutrient intake or bone density, or disease that may influence systemic inflammation including cancer or malignancy, asthma, and chronic bronchitis. after (). The baseline characteristics of the whole study population and subjects by tertiles of DII are given in . The medium age of the population is 50.0 and 50.81% were men. Most of the subjects were obese (median (interquartile range) BMI: 27.59 (24.15–31.47) kg/mCitation2]. Regarding the metabolic syndrome, though the blood pressure was not high (120.67 mm Hg of medium SBP, and 69.33 mm Hg of medium DBP when measured at participation), nearly half of the subjects has a hypertension history (40.14%). What’s more, over half of the population has DM (58.57%) or hypercholesterolemia (58.68%), while CVD (7.79%), and liver condition (3.10%) were not very common.
Table 1. Baseline characteristics of the study population by tertiles of dietary inflammatory index.
People who had higher DII were more female, less educated, lower in income, higher in BMI, and smokers (all p < .01). They also had a higher prevalence of hypertension, DM, and arthritis, and a higher systolic and diastolic blood pressure, serum C-reactive protein, and WBC count, but has lower serum albumin and calcium intake (all p < .05). No significant difference in serum phosphorus was observed among different levels of DII. Across the tertiles of the DII, we found statistically significant associations with BMDs or T scores of all femoral regions (p < .001). For all the BMDs and T scores of all femoral areas, higher DII was associated with lower BMD and T scores (all p < .001).
Association of DII with femoral BMD and T score
We further analyzed the BMDs () and T scores () of different femur regions including the femoral neck, the trochanter, the intertrochanter, and the total femur based on the tertiles of DII by weighted linear regression models with different adjustment. A higher DII score was found to have a significant association with more BMD loss in all the studied femoral areas (all p < .0001) (). This association still exists after adjustment for age, gender, race/ethnicity, smoker, BMI, eGFR, UACR, serum C-reactive protein, WBC count, NLR, serum calcium, arthritis, aspirin use, calcitonin use, biphosphonate use, DMARDs use, calcium intake and estrogen use. Similarly, there was a positive correlation between higher DII and lower T scores in all the femoral areas (all p < .0001) ().
Table 2. The association betwwen DII and femoral BMD.
Table 3. The association between DII and femoral T score.
Association of DII with femoral osteoporosis
We further investigated whether the DII score is associated with the possibility of the presence of osteoporosis in the femoral neck, the trochanteric, the intertrochanteric, and the total femoral areas (). Compared to low DII (tertile1, DII < 0.380 as reference), in the femoral neck, the intertrochanter, and the total femur, increased DII is independently associated with an increased possibility of the presence of osteoporosis (OR, 95% CI: 1.88,1.11–3.20; 2.10, 1.05–4.20; 1.94,1.02–3.69, respectively). However, this positive association was not observed in the trochanteric area exists after full adjustment (OR, 95% CI: 1.62, 0.85–3.09).
Table 4. The association between DII and the possibility of the presence of osteoporosis.
Intriguingly, subgroup analysis () demonstrated a positive correlation between higher DII and increased possibility of the presence of osteoporosis in the trochanteric area of the non-Hispanic White population, indicating that they may pay more attention to the BMD of this area than the other races (, Supplemental Figure 2). Other results of the subgroup analysis were consistent with the preliminary results. Though we have observed that the declined eGFR was related to a decreased BMD (Supplemental Figure 1) and increased chance of the presence of osteoporosis (Supplemental Table 2), no significant difference in the association between DII and the presence of osteoporosis was found when we compare the subjects with impaired kidney function (eGFR < 60 mL/min/1.73 m2) with those who have better kidney function (eGFR ≥ 60 mL/min/1.73 m2). We also made a subgroup analysis by vitamin D intake for the association between the DII without vitamin D and the presence of femoral osteoporosis (Supplemental Table 1), due to that vitamin D is closely related to bone health. No significant results were found in the trochanter, intertrochanter, and total femur, but an increased risk of the presence of osteoporosis in the femoral neck was found in subjects with high DII without vitamin D when vitamin D intake ≥3.2 µg/d.
Table 5. Subgroup analysis of the association between DII and the possibility of the presence of osteoporosis.
Discussion
Our results showed that higher DII, indicating diet patterns with more pro-inflammatory potential, is independently associated with BMD loss in all the femoral areas and is associated with the presence of osteoporosis in the femoral neck, the intertrochanter, and the total femur. Though this positive association was not observed in the trochanteric area in the whole population after full adjustment, subgroup analysis revealed its existence in the non-Hispanic White population, indicating that they may pay more attention on the BMD of this area than the other races. Subjects with or without kidney function impairment (eGFR < 60 mL/min/1.73 m2) showed no significant difference on the relationship between DII and femoral BMD or the presence of femoral osteoporosis.
Accumulating evidence has demonstrated that dietary continents could influence inflammatory cytokines secretion [Citation12,Citation42]. DII was developed by calculating a score for 45 food parameters reported to regulate the levels of 6 specific inflammatory biomarkers (IL-1β, IL-4, IL-6, IL-10, tumor necrosis factor-α (TNF-α), and CRP) to quantify the actual effect of diet on inflammation [Citation17], and has been found to closely associated with several inflammatory cytokines including CRP, IL-6, and homocysteine [Citation34,Citation43,Citation44]. There may be several mechanisms by which a pro-inflammatory diet leads to poor musculoskeletal health [Citation45]. A pro-inflammatory diet may lead to enhanced oxidative stress and immune disorders which induced elevated circulating inflammatory cytokines [Citation18]. Meanwhile, it could synergize with aging, which is also an important trigger of immune dysregulation, to induce increased production of proinflammatory cytokines that result in prolonged inflammation and adverse musculoskeletal health [Citation45]. Elevated serum proinflammatory markers, such as TNF-α, CRP, and IL-6, are associated with various musculoskeletal conditions such as fractures [Citation5,Citation6], declined muscular mass and strength [Citation46–48], and frailty [Citation5]. Existing studies demonstrated that inflammatory cytokines, by promoting the production of receptor activators of NF-κB ligand (RANKL), can directly stimulate differentiation and overactivation of the osteoclast [Citation49]. IL-1 and IL-6 were found to be able to interfere with bone remodeling by enhancing bone resorption and suppressing bone formation [Citation50,Citation51]. Another study also showed that higher CRP levels were associated with lower trabecular bone scores and bone-quality index [Citation52].
Our result that higher DII scores were strongly related to BMD loss is consistent with many previous studies [Citation20,Citation23,Citation27,Citation53–55]. Though there is research reported that a diet with high pro-inflammatory potential was associated with an elevated risk of osteoporotic hip fracture in both men and women of an elderly Chinese population [Citation28], most evidence has been observed that a pro-inflammatory diet pattern was significantly associated with more chance of being diagnosed as osteoporosis [Citation27,Citation56] and fractures [Citation26] in women, but not in men. These data supported our finding that DII or osteoporosis diagnoses had a positive association in the femoral neck, the intertrochanter, and the total femur of the general population and, intriguingly, in the trochanteric area of the non-Hispanic White population. Chronic kidney disease (CKD) was revealed as an individual risk factor of osteoporosis, patients with CKD often suffer chronic inflammation and bone metabolism disorder [Citation57]. Studies have revealed that CKD could negatively affect bone strength, and impaired eGFR was closely associated with an increased risk of hip fracture [Citation58] and tibial microstructural impairment [Citation59]. Consistently, our results have observed that the declined eGFR was related to a decreased BMD and increased chance of the presence of osteoporosis in femoral areas. However, no significant influence on the association between DII and the presence of osteoporosis by the declined eGFR was found.
As a chronic disease whose pathology is closely related to systemic inflammation, osteoporosis may be affected by various nutrition intakes [Citation4–6]; meanwhile, the loss of bone density becomes more and more severe with time pass by. So, it is an important strategy for osteoporosis prevention to have a properly controlled overall intake of various nutrition after middle age [Citation60]. Given the rising incidence of fractures worldwide [Citation61], more efforts should be made to construct and implement a dietary pattern with better overall nutritional quality and anti-inflammatory properties to improve BMD loss and prevent osteoporosis.
There are several limitations that should be discussed. First, only correlation conclusions but no causal conclusions could be drawn since it is a cross-sectional study, more prospective studies are needed for further investigation. Second, most of the data for subjects from NHANES were just collected at one time point which keeps us from determining the association over time and may also induce bias in the classification of the specific population such as subjects with or without impairment of kidney function. Third, the dietary information came from the self-reported questionnaires, which might result in a recall or misclassification bias. Fourth, data from other bone areas were not collected for BMD analysis, and only the data of bone density but not femoral fractures were collected as the outcome for bone health. Fifth, the findings may not generalize to individuals under 18 years and pregnant females. Sixth, residual confounding is always a concern even after multivariable adjustment. Despite these limitations, our study observed the associations between DII and femoral bone density and bring further understanding of osteoporosis prevention.
Conclusion
In this study, we used a large multiracial cohort from NHANES to explore the association between the DII and the BMD or the presence of osteoporosis in the femoral areas, we found a higher DII, which indicates diet patterns with more pro-inflammatory potential, is independently associated with declined femoral BMD and with the presence of trochanteric osteoporosis in the non-Hispanic White population. However, no significant difference in the relationship between DII and the presence of femoral osteoporosis was observed between subjects with or without kidney function impairment (eGFR < 60 mL/min/1.73 m2).
Author contributions
SL and MZ designed the study. SL analyzed and interpreted the data. SL drafted the manuscript. SL and MZ revised the manuscript. All authors read and approved the final manuscript.
Ethical approval
The studies involving human participants were reviewed and approved by The National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Supplemental Material
Download TIFF Image (4.4 MB)Supplemental Material
Download TIFF Image (5.5 MB)Supplemental Material
Download PDF (59.1 KB)Supplemental Material
Download PDF (113 KB)Acknowledgments
We thank the participants and staff of the NHANES.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data used in the study are publicly available online. (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx and https://www.cdc.gov/nchs/data-linkage/mortality-public.htm).
Additional information
Funding
References
- Kanis JA, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):1–11.
- Kushchayeva Y, Pestun I, Kushchayev S, et al. Advancement in the treatment of osteoporosis and the effects on bone healing. JCM. 2022;11(24):7477.
- Xue S, Kemal O, Lu M, et al. Age at attainment of peak bone mineral density and its associated factors: the national health and nutrition examination survey 2005-2014. Bone. 2020;131:115163.
- Lorenzo J. Interactions between immune and bone cells: new insights with many remaining questions. J Clin Invest. 2000;106(6):749–752.
- Cauley JA, Danielson ME, Boudreau RM, et al. Inflammatory markers and incident fracture risk in older men and women: the health aging and body composition study. J Bone Miner Res. 2007;22(7):1088–1095.
- Barbour KE, Boudreau R, Danielson ME, et al. Inflammatory markers and the risk of hip fracture: the women’s health initiative. J Bone Miner Res. 2012;27(5):1167–1176.
- Ding C, Parameswaran V, Udayan R, et al. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab. 2008;93(5):1952–1958.
- Guo D, Zhao M, Xu W, et al. Dietary interventions for better management of osteoporosis: an overview. Crit Rev Food Sci Nutr. 2023;63(1):125–144.
- Ilesanmi-Oyelere BL, Kruger MC. B vitamins and homocysteine as determinants of bone health: a literature review of human studies. J Hum Nutr Diet. 2022 [cited 2022 Sep 2]. DOI:10.1111/jhn.13080
- Wilson-Barnes SL, Lanham-New SA, Lambert H. Modifiable risk factors for bone health & fragility fractures. Best Pract Res Clin Rheumatol. 2022;36(3):101758.
- Ma Y, Hébert JR, Li W, et al. Association between dietary fiber and markers of systemic inflammation in the women’s health initiative observational study. Nutrition. 2008;24(10):941–949.
- Esmaillzadeh A, Kimiagar M, Mehrabi Y, et al. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137(4):992–998.
- Zhao G, Etherton TD, Martin KR, et al. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134(11):2991–2997.
- Langsetmo L, Hanley DA, Prior JC, et al. Dietary patterns and incident low-trauma fractures in postmenopausal women and men aged >/= 50 y: a population-based cohort study. Am J Clin Nutr. 2011;93(1):192–199.
- Pedone C, Napoli N, Pozzilli P, et al. Dietary pattern and bone density changes in elderly women: a longitudinal study. J Am Coll Nutr. 2011;30(2):149–154.
- Hardcastle AC, Aucott L, Fraser WD, et al. Dietary patterns, bone resorption and bone mineral density in early post-menopausal Scottish women. Eur J Clin Nutr. 2011;65(3):378–385.
- Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696.
- Wu L, Shi Y, Kong C, et al. Dietary inflammatory index and its association with the prevalence of coronary heart disease among 45,306 US adults. Nutrients. 2022;14(21):4553.
- Zhang X, Guo Y, Yao N, et al. Association between dietary inflammatory index and metabolic syndrome: analysis of the NHANES 2005-2016. Front Nutr. 2022;9:991907.
- Jayedi A, Emadi A, Shab-Bidar S. Dietary inflammatory index and site-specific cancer risk: a systematic review and dose-response meta-analysis. Adv Nutr. 2018;9(4):388–403.
- Wang H, Liao R, Tang W, et al. Dietary inflammation index and osteoarthritis in the elderly: is there a mediating role of physical activity? Br J Nutr. 2022;128(11):2258–2266.
- Orchard T, Yildiz V, Steck SE, et al. Dietary inflammatory index, bone mineral density, and risk of fracture in postmenopausal women: results from the women’s health initiative. J Bone Miner Res. 2017;32(5):1136–1146.
- Na , Park Shivappa. Association between inflammatory potential of diet and bone-mineral density in Korean postmenopausal women: data from fourth and fifth Korea national health and nutrition examination surveys. Nutrients. 2019;11(4):885.
- Chen Y, Chen FH, Chen YQ, et al. Higher modified dietary inflammatory index is associated with increased risk of osteoporosis in US adults: data from NHANES. Front Nutr. 2022;9:891995.
- Fang Y, Zhu J, Fan J, et al. Dietary inflammatory index in relation to bone mineral density, osteoporosis risk and fracture risk: a systematic review and meta-analysis. Osteoporos Int. 2021;32(4):633–643.
- Veronese N, Stubbs B, Koyanagi A, et al. Pro-inflammatory dietary pattern is associated with fractures in women: an eight-year longitudinal cohort study. Osteoporos Int. 2018;29(1):143–151.
- Zhao S, Gao W, Li J, et al. Dietary inflammatory index and osteoporosis: the national health and nutrition examination survey, 2017-2018. Endocrine. 2022;78(3):587–596.
- Zhang Z-Q, Cao W-T, Shivappa N, et al. Association between diet inflammatory index and osteoporotic hip fracture in elderly Chinese population. J Am Med Dir Assoc. 2017;18(8):671–677.
- Cooper C, Cole ZA, Holroyd CR, et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int. 2011;22(5):1277–1288.
- Robertson L, Black C, Fluck N, et al. Hip fracture incidence and mortality in chronic kidney disease: the GLOMMS-II record linkage cohort study. BMJ Open. 2018;8(4):e020312.
- Lee CL, Tsai SF. The impact of protein diet on bone density in people with/without chronic kidney disease: an analysis of the national health and nutrition examination survey database. Clin Nutr. 2020;39(11):3497–3503.
- Chen TC, et al. National health and nutrition examination survey: estimation procedures, 2011-2014. Vital Health Stat. 2018;2:1–26.
- Li A, Chen Y, Schuller AA, et al. Dietary inflammatory potential is associated with poor periodontal health: a population-based study. J Clin Periodontol. 2021;48(7):907–918.
- Shivappa N, Steck SE, Hurley TG, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. 2014;17(8):1825–1833.
- Dimai HP. Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, T- and Z-score, and reference databases. Bone. 2017;104:39–43.
- Looker AC, Orwoll ES, Johnston CC, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12(11):1761–1768.
- Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–489.
- Huang Y, Zeng M, Zhang L, et al. Dietary inflammatory potential is associated with sarcopenia among chronic kidney disease population. Front Nutr. 2022;9:856726.
- Jiang M, Sun J, Zou H, et al. Prognostic role of neutrophil to high-density lipoprotein cholesterol ratio for All-Cause and cardiovascular mortality in the general population. Front Cardiovasc Med. 2022;9:807339.
- Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165(7):473–481.
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254.
- Nowlin SY, Hammer MJ, D’Eramo Melkus G. Diet, inflammation, and glycemic control in type 2 diabetes: an integrative review of the literature. J Nutr Metab. 2012;2012:542698. (2012).
- Shivappa N, Hébert JR, Rietzschel ER, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios study. Br J Nutr. 2015;113(4):665–671.
- Tandorost A, Kheirouri S, Moludi J, et al. Association of dietary inflammatory index (DII) with disease activity and inflammatory cytokines in the patients with rheumatoid arthritis. Int J Clin Pract. 2021;75(11):e14792.
- Cervo MMC, Scott D, Seibel MJ, et al. Proinflammatory diet increases circulating inflammatory biomarkers and falls risk in community-dwelling older men. J Nutr. 2020;150(2):373–381.
- Kim B-J, Lee SH, Kwak MK, et al. Inverse relationship between serum hsCRP concentration and hand grip strength in older adults: a nationwide population-based study. Aging. 2018;10(8):2051–2061.
- Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the health ABC study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–M332.
- Bano G, Trevisan C, Carraro S, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017;96:10–15.
- Wagner D, Fahrleitner-Pammer A. Levels of osteoprotegerin (OPG) and receptor activator for nuclear factor kappa B ligand (RANKL) in serum: are they of any help? Wien Med Wochenschr. 2010;160(17–18):452–457.
- Loi F, Córdova LA, Pajarinen J, et al. Inflammation, fracture and bone repair. Bone. 2016;86:119–130.
- Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2:14.
- Rolland T, Boutroy S, Vilayphiou N, et al. Poor trabecular microarchitecture at the distal radius in older men with increased concentration of high-sensitivity C-reactive protein–the STRAMBO study. Calcif Tissue Int. 2012;90(6):496–506.
- Mohammadisima N, Farshbaf-Khalili A, Ostadrahimi A, et al. Positive relation between dietary inflammatory index and osteoporosis in postmenopausal women. Int J Vitam Nutr Res. 2022 [cited 2022 Dec 19]. DOI:10.1024/0300-9831/a000773
- Su Y, Elshorbagy A, Turner C, et al. The association of circulating amino acids and dietary inflammatory potential with muscle health in Chinese community-dwelling older people. Nutrients. 2022;14(12):2471.
- Song D, Kim J, Kang M, et al. Association between the dietary inflammatory index and bone markers in postmenopausal women. PLOS One. 2022;17(3):e0265630.
- Kim HS, Sohn C, Kwon M, et al. Positive association between dietary inflammatory index and the risk of osteoporosis: results from the KoGES_health examinee (HEXA) cohort study. Nutrients. 2018;10(12):1999.
- Hara T, Hijikata Y, Matsubara Y. Effectiveness of pharmacological interventions versus placebo or no treatment for osteoporosis in patients with CKD stages 3-5D: editorial summary of a cochrane review. Am J Kidney Dis. 2022;80(6):794–796.
- Kim SH, Yi SW, Yi JJ, et al. Chronic kidney disease increases the risk of hip fracture: a prospective cohort study in Korean adults. J Bone Miner Res. 2020;35(7):1313–1321.
- Tsuji K, Kitamura M, Chiba K, et al. Comparison of bone microstructures via high-resolution peripheral quantitative computed tomography in patients with different stages of chronic kidney disease before and after starting hemodialysis. Ren Fail. 2022;44(1):381–391.
- Movassagh EZ, Vatanparast H. Current evidence on the association of dietary patterns and bone health: a scoping review. Adv Nutr. 2017;8(1):1–16.
- Wong RMY, Wong PY, Liu C, et al. The imminent risk of a fracture-existing worldwide data: a systematic review and meta-analysis. Osteoporos Int. 2022;33(12):2453–2466.