Abstract
This study was designed to examine the relative safety and efficacy of sevelamer in the treatment of chronic kidney disease (CKD) patients in comparison to placebo, calcium carbonate (CC), or lanthanum carbonate (LC). The PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI) databases were searched for articles published through 18 June 2022. The quality of relevant studies was independently analyzed by two investigators who also extracted data from these manuscripts as per Cochrane Collaboration Handbook 5.3. The safety and efficacy of sevelamer as a treatment for hyperphosphatemia in CKD patients were then examined through a meta-analysis, with the primary patient-level outcomes of interest in this analysis being all-cause mortality and the incidence of gastrointestinal adverse effects. Vascular calcification score was also examined as an intermediate outcome, while serum biochemical parameters including levels of phosphate (P), calcium (Ca), intact parathyroid hormone (iPTH), lipids, C-reactive protein (CRP), or fibroblast growth factor-23 (FGF-23) were additionally assessed. In total, this meta-analysis incorporated data from 34 randomized controlled trials (RCTs) enrolling 2802 patients. Sevelamer was associated with reduced all-cause mortality (RR 0.28, CI 0.19 − 0.41, very low certainty) and Vessel calcification score (RR −0.58, CI −1.11 to −0.04, low certainty) and induced less hypercalcemia (MD −0.28, CI 0.40 to −0.16, low certainty) and hyperphosphatemia (MD −0.22, CI −0.32 to −0.13, low certainty) when compared with Ca-based binders in CKD5D individuals. No significant differences in gastrointestinal adverse events (GAEs) incidence were observed. These data suggest that sevelamer may represent a beneficial means of protecting CKD patients against death and vessel calcification when used to treat hyperphosphatemia, while we found no clinically important benefits in decreasing gastrointestinal adverse effects.
Introduction
Chronic kidney disease (CKD) is a leading cause of chronic disease in the world, imposing a major burden on affected individuals and society as a whole such that it accounts for 2 − 3% of annual healthcare budgets in many countries [Citation1]. The 2003 National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) recommended CKD stages 3 and 4 (glomerular filtration rate [GFR] of 30 − 59 mL/min/1.73 m2 and 15 − 29 mL/min/1.73 m2, respectively), and CKD stages 5 and 5D (GFR < 15 mL/min/1.73 m2 and dialysis [Citation2]. There is a growing body of evidence that CKD 3 − 5D patients face a higher risk of developing mineral and bone disorders that entail abnormal serum calcium (Ca), phosphate (P), and intact parathyroid hormone (iPTH) concentrations. Elevated serum P levels, in particular, are linked to the incidence of vascular tissue calcification, which is closely and independently linked to overall mortality risk. A failure to adequately regulate these P levels can thus lead to a higher risk of morbidity and mortality for patients with CKD [Citation3].
Patients suffering from CKD experience the progressive deterioration of renal function, with concomitant impairment of dietary P excretion that ultimately contributes to hyperphosphatemia in individuals with advanced disease (CKD G3-G5D) [Citation4]. The use of oral P binders and the restriction of dietary orthophosphate intake can slow cardiovascular disease and vascular calcification in CKD patients, contributing to improved prognostic outcomes and survival [Citation5]. Avoiding the use of Ca-based P binders can mitigate the potential risks associated with excessive Ca load, including cardiovascular event incidence and more rapid vascular calcification, spurring interest in the development of non-Ca-based P binders including lanthanum carbonate (LC), sevelamer, and iron-based P binders [Citation6]. According to the 2017 KDIGO guidelines, excessive Ca-based P binder exposure can contribute to worse patient outcomes irrespective of GFR category, although evidence regarding the superiority of Ca-free binders as a means of preventing adverse clinical outcomes remains somewhat controversial, and the most optimal means of balancing the potential for adverse clinical outcomes against treatment-related costs and potential harm remains debatable [Citation7,Citation8].
The non-Ca-based, non-absorbable P binder sevelamer can capture P within the gastrointestinal system, thereby reducing its uptake and associated serum P levels [Citation4]. Sevelamer was approved by the US Food and Drug Administration as a treatment for the regulation of serum P levels in CKD patient populations. Many reports have also found sevelamer to be capable of reducing blood lipid and glucose concentrations, protecting against inflammatory endotoxin activity and damage to the endothelium in CKD patients [Citation9].
The present meta-analysis was conducted to systematically examine the relative safety and efficacy of sevelamer treatment for end-stage renal disease (ESRD) patients undergoing hemodialysis (HD). In total, data from 34 randomized controlled trials (RCTs) incorporating 2892 patients were analyzed with the goal of synthesizing these results to more reliably establish the optimal P binder regimens suited for clinical use in CKD patients in an effort to guide future patient treatment.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to conduct all stages of this meta-analysis [Citation10]. No ethical oversight or consent was required, as these analyses were performed solely using published data.
Search strategy
Relevant studies were identified by searching the PubMed, Embase, Cochrane Library, and CNKI databases without any language limitations for articles published as of 18 June 2021, using the following keywords: (Sevelamer) OR AND (CKD OR (chronic renal failure) OR (chronic kidney disease) OR (chronic nephropathy) OR HD OR (Peritoneal dialysis) OR (end-stage renal disease) OR ESRD). Only RCTs focused on adult patients ≥ 18 years old were eligible for inclusion.
Study selection
Eligible studies were those RCTs in which: (1) participants were CKD patients diagnosed with hyperphosphatemia, including patients undergoing peritoneal dialysis or HD; (2) patients in the experimental group were treated using sevelamer; (3) control patients were treated using calcium carbonate (CC), LC, placebo (P binders can be divided into two main categories: 1) Ca-containing, and 2) non-Ca-containing PBs, the latter including sevelamer, LC, sucroferric oxyhydroxide, aluminum hydroxide, and magnesium-containing PBs. CC and LC were used in this study as representatives of Ca-binding and non-Ca-based binders, respectively, and sevelamer was compared with them and placebo); and (4) sufficient information pertaining to safety and efficacy outcomes was provided. Including mortality, iPTH levels, P levels, and adverse reaction incidence. Studies were excluded if: (1) data were incomplete; (2) they were reviews, letters, or other non-original studies; or (3) they were duplicate analyses. Any discrepancies in study selection were resolved by a third investigator.
Data extraction and quality assessment
Eligible studies were reviewed by two investigators (QZ and YLZ) independent of one another, with title and abstract reviews being followed by full-text reviews when appropriate. Any discrepancies were resolved by a third investigator. The following data were extracted from all studies: first author, year, sample size, patient age, therapeutic interventions, follow-up duration, and patient outcome data.
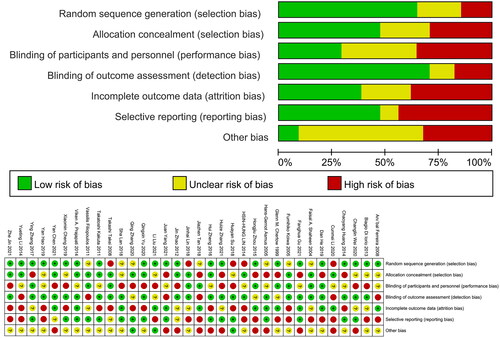
Included RCT quality was independently assessed by two investigators (QZ and YLZ), with the risk of bias being examined based on the Cochrane Collaboration tool that entailed analyses of participant blinding, personnel blinding, allocation concealment, sequence generation, outcome assessor blinding, selective outcome reporting, incomplete outcome data, and other relevant forms of bias. Each of these items was assigned a high, low, or unclear risk of bias (). Discrepancies were resolved by a third investigator (XQY).
‘Summary of findings’ table
The main results are listed in the ‘Summary of findings’ table (). The table presents the major information on the quality of the evidence, the impact of the interventions studied, and the sum of the available data on the primary outcomes. The table also includes the overall grading of the relevant evidence for each primary outcome using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) scales [Citation11,Citation12]. The GRADE approach defines the quality of a set of evidence as the degree to which one can be sure that the estimate of the effect or association is close to the true amount of a particular benefit. The risk of bias (methodological quality) within the trial, the directness of the evidence, heterogeneity, accuracy of the effect estimate, and the risk of publication bias all contribute to the quality of a set of evidence [Citation13].
Death (all causes)
Vessel calcification
Serum phosphate levels (mg/dL)
Serum calcium levels (mg/dL)
Ca × P product (mg2/dL2)
Serum iPTH levels (pg/mL)
Total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-c) (mmol/L)
C-reactive protein (CRP) (mg/dL)
Fibroblast growth factor 23 (FGF23) (pg/mL)
Treatment-related GAEs
Statistical analysis
Review Manager version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark; 2014) was used to analyze these data. Dichotomous outcome variables were reported using risk ratio (RR) values and 95% confidence intervals (CIs). Weighted mean difference (WMD) values were utilized for analyses of the effect size for continuous outcomes, whereas in cases in which different scales were used, the results were examined based on standardized mean difference (SMD) values. All outcomes were with random effects models. Heterogeneity was analyzed using the chi-square test-based Cochran’s Q test and I2 statistic. Significant heterogeneity was defined by p < 0.05 or/and I2 >50%, and p < 0.05 was the significance threshold in all analyses.
Results
Study characteristics
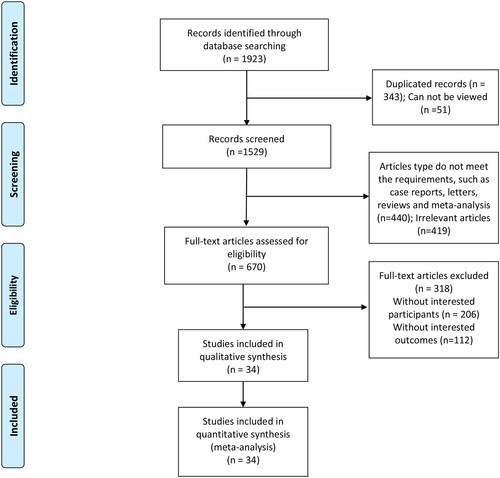
In total, 1923 potentially relevant studies were screened for meta-analysis inclusion, of which 1571 were excluded due to repeated results, failure to include patients or outcomes of interest, or because they were meta-analyses or review articles. The remaining 34 RCTs [Citation14–47] enrolling 2802 patients were included in this meta-analysis without any language restrictions. The literature search and study selection processes are outlined in .
The characteristics of included studies are summarized in . In total, three different control groups were included based on the P binders administered to patients, including 34 studies utilizing CC, two utilizing LC, and one in which patients received placebo control. No significant differences in the baseline characteristics of control and sevelamer-treated groups were observed. Included studies were from countries including Japan, South Korea, China, the UK, India, Australia, and the USA, and included follow-up durations ranging from 4 weeks to 2 years.
Table 1. Characteristics of the included randomized controlled trials.
Quality of the evidence
Based on the Cochrane Collaboration tool, the risk of bias of all included RCTs was examined (). Overall, the quality of the evidence for most studies was unsatisfactory. No high-quality evidence was found: three were of low quality, and four were of very low quality. Study limitations led to almost all projects being downgraded by one level. Most studies failed to report the methods of random sequence generation and allocation concealment adequately and therefore could not determine the risk of bias for these parameters. This was followed by imprecision, heterogeneity, and publication bias resulting in lower quality of evidence. See for more details.
Table 2. Summary of findings.
Patient outcomes
All-cause mortality
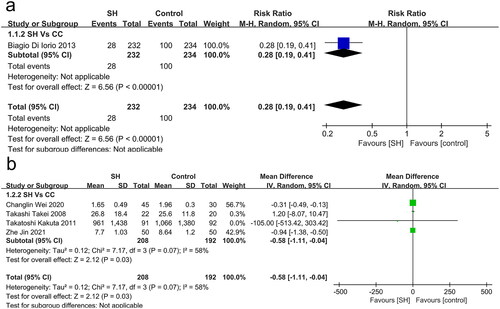
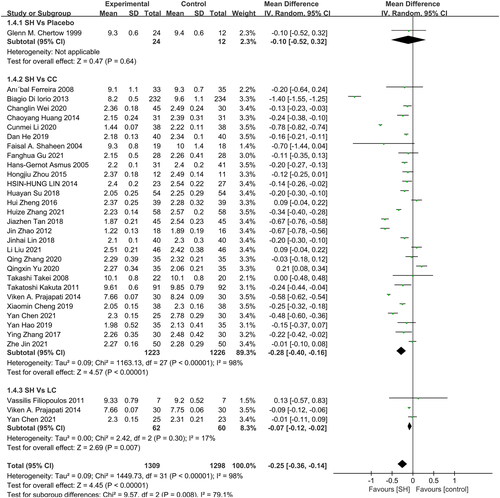
One study compared all-cause mortality in patients undergoing sevelamer and CC treatment (), revealing sevelamer to be associated with a survival benefit (RR: 0.28, 95% CI: 0.19 − 0.41, I2: NA, p < 0.001, very low certainty).
Vessel calcification
Coronary artery calcification was reported in two studies, while two other studies reported on aortic vascular calcification (). When compared to patients that underwent CC treatment, those randomized into the sevelamer treatment group exhibited reduced odds of vascular calcification (RR: −0.58, 95% CI: −1.11 to −0.04, I2 = 58%, p = 0.03, low certainty).
Biochemical outcomes
Serum phosphate levels
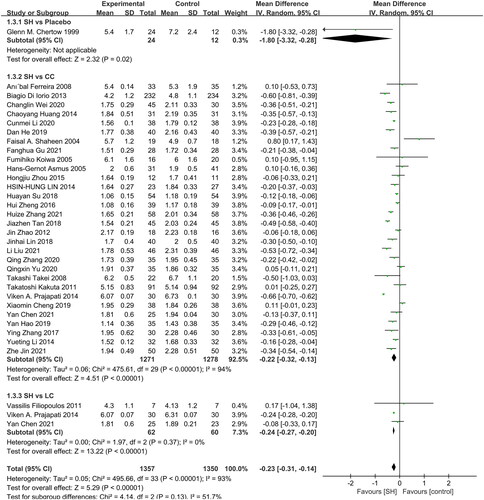
In total, 30, 3, and 1 studies reported comparisons of serum P levels in patients that underwent sevelamer treatment to those of patients treated with CC, LC, and placebo, respectively (). Overall, serum P levels were significantly lower in patients treated with sevelamer relative to those in patients treated with CC (MD: −0.22 mg/dL, 95% CI: −0.32 to −0.13, I2 = 94%, p < 0.001), LC (MD: −0.24 mg/dL, 95% CI: −0.27 to −0.20, I2 = 0%, p < 0.001), and placebo (MD: −1.80 mg/dL, 95% CI: −3.32 to −0.28, p = 0.02).
Serum calcium levels
Of the included RCTs, 28, 3, and 1, respectively, provided information pertaining to serum Ca levels in patients treated with sevelamer as compared to patients treated with CC, SH, and placebo (). Sevelamer treatment was associated with significant reductions in serum Ca relative to patients treated with CC (MD: −0.28 mg/dL, 95% CI: −0.40 to −0.16, I2= 98%, p < 0.001), LC (MD: −0.07 mg/dL, 95% CI: −0.12 to −0.02, I2= 17%, p = 0.007), and exhibited a non-significant trend toward better Ca levels relative to placebo-treated patients (MD: −0.10 mg/dL, 95% CI: −0.52 to 0.32, p = 0.64).
Ca × P product
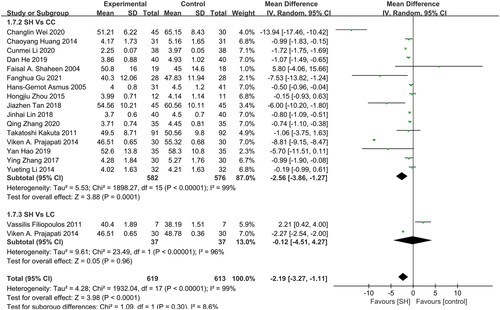
In total, 16 and 2 studies reported the Ca × P Product levels for patients treated with sevelamer as compared to patients treated using CC and LC (), respectively, revealing sevelamer treatment to be associated with lower Ca × P levels relative to CC treatment (MD: −2.56 mg2/dL2, 95% CI: −3.86 to −1.27, I2 = 99%, p = 0.0001). However, no difference between sevelamer and LC-treated patients was observed (MD: −0.12 mg2/dL2, 95% CI: −4.51 to 4.27, I2= 96%, p = 0.96), and no studies compared sevelamer treatment to placebo for this endpoint.
Serum iPTH levels
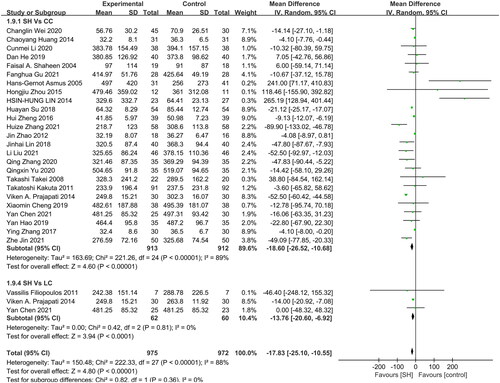
Of the included RCTs, 25 and 3 reported on comparisons of serum iPTH levels in patients treated with sevelamer relative to those of patients treated with CC and LC, respectively (). Significantly reduced serum iPTH levels were observed in sevelamer-treated patients relative to those treated with CC (MD: −18.6 pg/mL, 95% CI: −26.52 to −10.68, I2 = 89%, p < 0.001) or LC (MD: −13.76 pg/mL, 95% CI: −20.60 to −6.92, I2 = 0%, p < 0.001). No studies compared sevelamer treatment to placebo for this endpoint.
Total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-c)
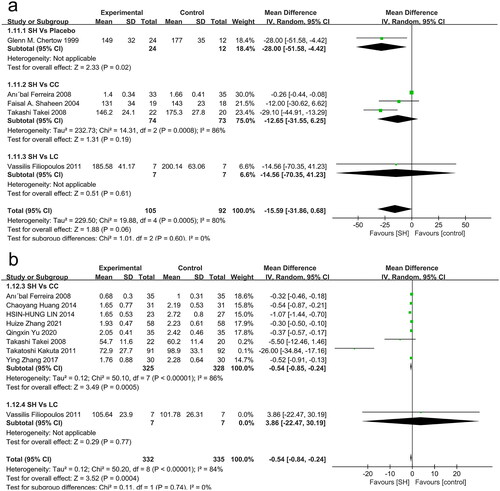
Just 3, 1, and 1 studies, respectively, compared TC levels in sevelamer-treated patients to those in patients treated with CC, LC, and placebo (). Overall, lower TC levels were observed in individuals treated with sevelamer relative to those treated with placebo (MD: −28.00 mmol/L, 95% CI: −51.58 to −4.42, p = 0.02), but no differences were observed when comparing sevelamer treatment to the CC or LC groups.
Overall, 8, 1, and 1 studies, respectively, compared LDL-c levels in sevelamer-treated patients to those of patients treated with CC, LC, and placebo (). These LDL-c levels were significantly lower in sevelamer-treated patients relative to those treated using CC (MD: −0.54 mmol/L, 95% CI: −0.85 to −0.24, I2= 86%, p = 0.0005), while no differences were observed when comparing sevelamer-treated patients to patients treated with LC (MD: 3.86 mmol/L, 95% CI: −22.47 to 30.19, p = 0.77).
C-reactive protein (CRP)
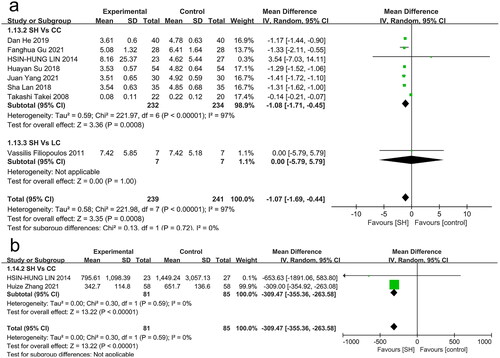
CRP levels were reported in seven studies, revealing them to be significantly lower in sevelamer-treated patients relative to CC-treated patients (MD: −1.08 mg/dL, 95% CI: −1.71 to −0.45, I2 = 97%, p = 0.0008, low certainty) (), while no corresponding differences were observed for LC.
Fibroblast growth factor-23 (FGF-23)
Relative to patients treated with CC, sevelamer treatment was associated with significantly lower FGF-23 levels (MD: −309.47 pg/mL, 95% CI: −355.36 to −263.58, I2= 0%, p < 0.001, very low certainty) (), while no comparisons between sevelamer and LC or placebo were made for this endpoint.
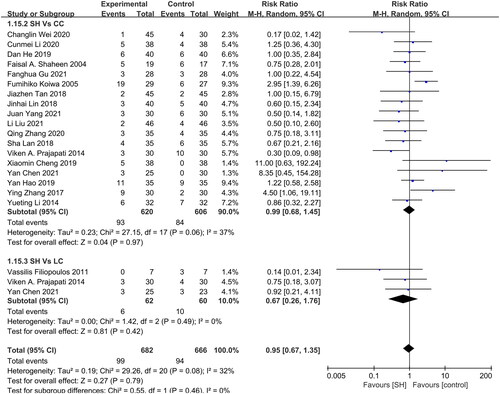
Treatment-related GAEs
No significant difference in GAE incidence was observed when comparing patients treated with CC (RR: 0.99, 95% CI: 0.68–1.45, I2= 37%, p = 0.97) and LC (RR: 0.67, 95% CI: 0.26–1.76, I2 = 0%, p = 0.42) (). No placebo-related GAEs were reported in any studies.
Assessment of publication bias
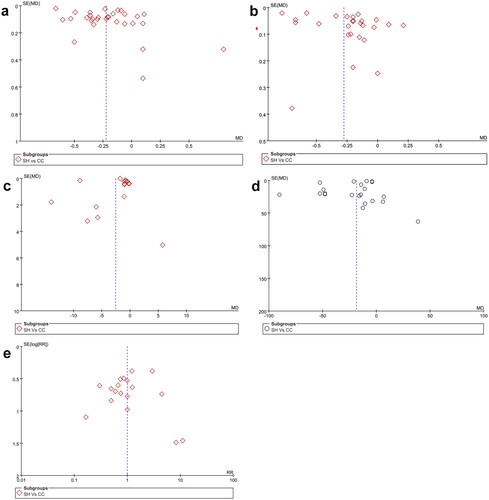
Given the limited number of trials included for many endpoints, potential publication bias pertaining to laboratory outcomes (serum phosphorus, Ca, Ca × P product, iPTH) and GAEs was assessed for the sevelamer vs. CC comparison, funnel plots were drawn. The result is shown in . It was found that all studies are located roughly on the central axis and symmetrical distribution, revealing no detected evidence of publication bias for these outcomes.
Discussion
In summary, this study consisted of a systematic survey of published RCTs examining the safety and efficacy of sevelamer treatment in CKD patients undergoing maintenance dialysis. In total, 34 RCTs enrolling 2802 patients were identified for inclusion, offering comparisons of sevelamer-treated patients to patients treated using CC, LC, and/or placebo for a range of clinical and biochemical outcome variables. Pooled analyses of these results suggested that sevelamer treatment can lead to significantly improved laboratory findings in treated patients, thereby contributing to a better prognosis and reduced mortality as compared to other treatments.
In CKD5D patients, hyperphosphatemia is associated with a range of potentially severe clinical consequences, including metastatic calcification, renal bone disease, and secondary hyperparathyroidism, with higher rates of both morbidity and mortality evident in patients undergoing HD who exhibit elevated serum P levels [Citation48]. Efforts to combat this condition in ESRD patients have thus centered on a combination of restricting dietary P intake, the use of P binder drugs to mitigate its absorption through the gastrointestinal tract, and the use of dialysis to remove circulating Ps from patients [Citation49]. Sevelamer is a Ca-free P binder that has been the subject of intensive clinical interest owing to its avoidance of potential increases in the Ca load of patients undergoing dialysis [Citation50]. The efficacy of sevelamer treatment in the context of disease progression has thus been examined using a range of surrogate biomarkers to date.
The results of this study suggest that sevelamer can readily reduce serum levels of phosphorus, Ca, iPTH, and Ca × P, doing so more effectively in CKD5D patients undergoing dialysis than CC treatments, with similar superiority to LC treatment albeit with comparable efficacy in the overall reduction of Ca × P levels. Just one of the included studies compared sevelamer-associated changes in serum biomarkers to those of patients treated with a placebo and showed that the sevelamer group had lower blood phosphorus levels. A French study in patients with CKD stages 3D and 4 also tested the effect of placebo versus sevelamer carbonate (4.8 g daily) [Citation51]. After 12 weeks of treatment with sevelamer, urinary phosphorus was reduced but serum FGF23, α-klotho, or P levels were essentially unchanged.
In some observational reports, increases in serum Ca, phosphorus, and Ca × P levels in dialysis patients have been linked to an independent increase in the odds of arterial calcification and cardiovascular death [Citation52–54]. Serum phosphorus levels are considered an unfavorable biomarker associated with residual phosphorus [Citation55]. The onset of hyperphosphatemia is often preceded by the dysregulation of inflammatory mediators and mineral regulators, including FGF-23, 1,25-dihydroxyvitamin D, and iPTH, and these changes have also been shown to be independently related to higher odds of mortality [Citation56]. Hyperphosphatemia can also drive increased FGF secretion by bone cells, counteracting increases in the renal excretion of P [Citation57]. Increased levels of FGF23 directly contribute to left ventricular hypertrophy and congestive heart failure [Citation58,Citation59]. Indeed, these FGF-23 levels rise as renal function is reduced and have been linked to the progression of disease in mild-to-moderate CKD patients [Citation60]. CKD patients may have already experienced prolonged high FGF23 levels when starting dialysis and 36% of CKD patients already have heart failure at the time of dialysis initiation [Citation61]. With CKD progression, the impairment of homeostatic responses contributes to higher rates of hyperphosphatemia and hypocalcemia, leading to elevated PTH release from parathyroid cells through Ca-sensing receptor activity [Citation62]. Elevated PTH is associated with pro-inflammatory effects, elevated interleukin 6, impaired myocardial energy production, and myocardial fibrosis [Citation63,Citation64]. High P levels have been confirmed to impair the reabsorption of P by the renal tubules, exacerbating hyperparathyroidism and influencing the maintenance of normal serum P concentrations in line with previously reported analyses [Citation65,Citation66].
Sevelamer exhibits beneficial effects beyond protecting against hyperphosphatemia, including its ability to lower TC, LDL-c, and apolipoprotein B levels linked to cardiovascular disease risk while also decreasing inflammatory protein levels, including CRP and β2-microglobulin [Citation67]. Consistently, relative to CC treatment, sevelamer reduced patient LDL-c levels while also reducing TC levels relative to those in placebo-treated patients. This is consistent with the results of two prior studies [Citation68,Citation69]. Sevelamer exhibits similar physicochemical properties to those of bile separators, enabling it to complex with bile acids such that they are excreted in the stool, resulting in impaired fat absorption. Cholesterol is then converted into bile acids to counteract this change, resulting in lower LDL-c and TC levels in treated patients [Citation67]. CRP is an acute phase protein that is often upregulated in CKD patients owing to the fact that hyperphosphatemia is often considered a ‘microinflammatory’ condition [Citation70]. Inflammatory mediators such as IL-6, IL-10, TNF-α, and CRP are often linked to the incidence of complications in CKD patients, and serum CRP and apolipoprotein B levels have been reported to drop following sevelamer treatment, corresponding to the beneficial effects of this drug [Citation67,Citation71]. Sevelamer functions through its bile acid complexing characteristics, which are related to the absorption of factors that can promote systemic inflammation via the intestines [Citation4].
Cardiovascular disease has emerged as a cause of death for over half of all patients undergoing dialysis in recent decades, and moderate-to-severe CKD patient increases in serum P levels have been linked to increased rates of VC. As such, therapies focused on lowering circulating P levels have the potential to reduce the incidence of VC and thereby lower patient mortality rates [Citation72–74]. When patients exhibit a positive Ca balance, this can impact VC onset and progression and may protect against subendocardial perfusion, aggravating transmission abnormalities, while increasing cardiovascular disease-associated risk [Citation75]. Elemental Ca can bind to Ps derived from dietary sources, resulting in the formation of insoluble Ca P when then undergoes fecal excretion. Ca salts, however, can also be internalized through the gastrointestinal tract, increasing the potential for serum Ca overload [Citation49]. Multiple clinical reports have consistently identified a higher risk of arterial stiffness and VC in patients undergoing Ca-based P binder treatment relative to sevelamer treatment [Citation55]. One recent meta-analysis found that VC progression and associated mortality were less pronounced or less rapid in patients undergoing treatment with sevelamer relative to patients treated with Ca-based binders, but that these differences were not significant nor did patients treated with LC exhibit significantly lower rates of VC progression or mortality [Citation54,Citation76]. The calcification of arterial vessels and soft tissues is thought to be a primary driver of patient mortality [Citation62], and coronary artery calcification has been linked to higher rates of ventricular arrhythmias. However, the data necessary to examine the link between Ca-based P binders and cardiovascular event incidence were not available for this study. In one prior RCT, however, researchers did not detect any significant change in cardiovascular event incidence in comparisons of sevelamer, LC, iron-based P binders, bixalomer, and control patients [Citation77].
To date, studies comparing P binders (sevelamer, lanthanum, Ca, and ferric citrate) with placebo or usual care without binders have been limited primarily to adult patients with CKD G2 to G5 who do not require dialysis, and few studies have involved patients with CKD G5D. In this study, the superiority of non-Ca-based- over Ca-based binders and placebo in ESRD patients with GFR < 15 was confirmed. In summary, the results of the present meta-analysis are in line with those of other analyses demonstrating a survival benefit in patients undergoing sevelamer treatment, emphasizing its potential utility as a pharmacological agent that can improve the prognosis of CKD. In other studies, these benefits have been attributed to changes in PTH, LDL-c, and other parameters. Here, sevelamer treatment was associated with improvements in patient mortality through the beneficial regulation of lipid metabolism and a negative Ca balance. This provides a more credible basis for future treatment options.
There are some limitations to this meta-analysis. For one, CC was considered equivalent to other forms of Ca-based P binders including Ca acetate such that only CC and sevelamer were compared in these analyses. Second, the follow-up duration of many of these RCTs was less than 1 year, which is relatively short and precludes the reliable analysis of long-term outcomes. Third, the majority of these trials employed a treat-to-target approach such that the administered P binder dose was regularly adjusted in many patients as appropriate. It is also important to note that the different brands or generics used in each study also had an impact on the study results. There was also significant heterogeneity in our study. We believe that part of this stems from the fact that subgroup analyses based on age, duration, or risk of follow-up or study bias were not performed. We have not maximized the inclusion of gray literature in this review; this may have biased our review and been a source of publication bias. The KDIGO 2017 guidelines suggest that sufficient evidence pertaining to the safety and efficacy of P binders in non-dialyzed CKD G3a-G5 patients is lacking, and this controversy has been further driven by recent evidence that individuals with normal renal function experienced higher mortality rates and increased CVS when using Ca-based P binders [Citation7]. As such, this study only enrolled CKD5D patients undergoing dialysis with the aim of mitigating heterogeneity among patient subpopulations. Unfortunately, only patients who had HD were ultimately included in the study, while all patients with peritoneal dialysis were not included because they did not meet the inclusion criteria or met the exclusion criteria.
Rather than solely assessing serum P levels as the target readout, studies of P binders should examine potential beneficial impacts on mineral homeostasis, bone histomorphometry, vascular biology, and mortality. Due to the lack of available data, no further analyses of bone tissue were performed. Together, these data highlight the need for further RCTs focused on exploring the efficacy and durability of sevelamer treatment in the context of long-term CKD patient outcomes, including cardiovascular events, fracture, calciphylaxis, hyperchloremic acidosis, and overall health-related quality of life.
Consent for publication
Written informed consent for publication was obtained from all participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of the article are available in the manuscript.
Additional information
Funding
References
- Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96(6):1–17.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201.
- Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19(2):213–216.
- Basutkar RS, Varghese R, Mathew NK, et al. Systematic review and meta-analysis of potential pleiotropic effects of sevelamer in chronic kidney disease: beyond phosphate control. Nephrology (Carlton). 2022;27(4):337–354.
- Lopes AA, Tong L, Thumma J, et al. Phosphate binder use and mortality among hemodialysis patients in the dialysis outcomes and practice patterns study (DOPPS): evaluation of possible confounding by nutritional status. Am J Kidney Dis. 2012;60(1):90–101.
- Floege J. Phosphate binders in chronic kidney disease: a systematic review of recent data. J Nephrol. 2016;29(3):329–340.
- St Peter WL, Fan Q, Weinhandl E, et al. Economic evaluation of sevelamer versus calcium-based phosphate binders in hemodialysis patients: a secondary analysis using centers for medicare & medicaid services data. Clin J Am Soc Nephrol. 2009;4(12):1954–1961.
- Erratum: kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO. Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) (2017). Kidney Int Suppl. 2017;7:1–59.
- Ikee R, Tsunoda M, Sasaki N, et al. Emerging effects of sevelamer in chronic kidney disease. Kidney Blood Press Res. 2013;37(1):24–32.
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394.
- Schünemann HJ, Oxman AD, Higgins JP, et al. Chapter 12: interpreting results and drawing conclusions. In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. London: The Cochrane Collaboration; 2011.
- Ferreira A, Frazão JM, Monier-Faugere MC, et al. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol. 2008;19(2):405–412.
- Di Iorio B, Molony D, Bell C, et al. Sevelamer versus calcium carbonate in incident hemodialysis patients: results of an open-label 24-month randomized clinical trial. Am J Kidney Dis. 2013;62(4):771–778.
- Wei C, Yan H, Cai X, et al. Effects of low-dose calcitriol combined with sevelamer carbonate in delaying vascular calcification in patients undergoing maintenance hemodialysis. Clin Misdiagn Misther. 2020;33:45–50.
- Huang C. Effect of sevelamer hydrochloride on hyperphosphatemia in maintenance hemodialysis patients with nephropathy. Contemp Med. 2014;20:68–69.
- Li C, Zhang J, Bai Y, et al. Clinical observation of sevelamer in treatment of hyperphosphatemia in maintenance hemodialysis. Drug Clin. 2020;35:1200–1203.
- He D, Lu Z, Lu J. Effect of sevelamer carbonate on microinflammation and prognosis in patients with chronic renal failure complicated with hyperphosphatemia. Hebei Med. 2019;25:1850–1854.
- Shaheen FA, Akeel NM, Badawi LS, et al. Efficacy and safety of sevelamer. Comparison with calcium carbonate in the treatment of hyperphosphatemia in hemodialysis patients. Saudi Med J. 2004;25(6):785–791.
- Fanghua G. Comparison of the clinical efficacy and safety of sevelamer carbonate and calcium carbonate in the treatment of chronic kidney disease hyperphosphatemia. Chin J Clin Rational Drug Use. 2021;14:92–94.
- Koiwa F, Onoda N, Kato H, et al. Prospective randomized multicenter trial of sevelamer hydrochloride and calcium carbonate for the treatment of hyperphosphatemia in hemodialysis patients in Japan. Ther Apher Dial. 2005;9(4):340–346.
- Chertow GM, Burke SK, Lazarus JM, et al. Poly[allylamine hydrochloride] (RenaGel): a noncalcemic phosphate binder for the treatment of hyperphosphatemia in chronic renal failure. Am J Kidney Dis. 1997;29(1):66–71.
- Asmus HG, Braun J, Krause R, et al. Two year comparison of sevelamer and calcium carbonate effects on cardiovascular calcification and bone density. Nephrol Dial Transplant. 2005;20(8):1653–1661.
- Zhou H, Feng Y, Jia X, et al. The effect of sevelamer hydrochloride on hyperphosphatemia in chronic renal failure patients on maintenance hemodialysis. Chin J Blood Purif. 2015;14:608–611.
- Lin HH, Liou HH, Wu MS, et al. Long-term sevelamer treatment lowers serum fibroblast growth factor 23 accompanied with increasing serum klotho levels in chronic haemodialysis patients. Nephrology (Carlton). 2014;19(11):672–678.
- Su H, Zeng L, Wang L. Effect of sevelamer carbonate on serum phosphorus, calcium and inflammatory reaction in patients with chronic renal insufficiency and hyperphosphatemia. Med Innov Chin. 2018;15:38–41.
- Zhang H, Mao Y, Xu H, et al. Clinical trial of sevelamer carbonate in the treatment of patients with maintenance hemodialysis. Chin J Clin Pharmacol. 2021;56:70–71.
- Zheng H, Liu Z. The use of sevelamer carbonate in the treatment of chronic kidney disease patients with hyperphosphatemia. Shandong Med. 2016;56:70–71.
- Jia Zhen T. Comparative study of sevelamer hydrochloride and calcium carbonate in the treatment of end-stage renal disease with maintenance hemodialysis hyperphosphatemia. J Clin Med. 2018;5:114–117.
- Lin J, Zeng T, Wang R. Effect of svilam carbonate tablets on the level of calcium and phosphorus in maintenance hemodialysis patients with chronic renal failure. Chin J Dial Artif Organs. 2018;29:3–4–7.
- Zhao J, Chen X, Zhang J, et al. Effect of sevelamer hydrochloride on serum phosphorus in uremia patients with secondary hyperparathyroidism using calcitriol pulse therapy. Hainan Med J. 2012;23:37–38.
- Yang J. Efficacy of sevelamer carbonate in the treatment of hyperphosphatemia in patients with chronic renal failure undergoing maintenance hemodialysis. Strait Pharm J. 2021;33:150–152.
- Liu L, Qiu H. Effect of sevelamer carbonate on parameters of chronic kidney disease mineral bone disorder in dialysis patients. Chin Prescript Drugs. 2021;19:86–87.
- Yu Q, Zeng J, Wu H. Clinical effect of sviram carbonate combined with calcitriol in the treatment of uremia complicated with secondary hyperparathyroidism. Forum Prim Care Med. 2020;24:4203–4204.
- Zhang Q, Peng W, Ouyang F, et al. Efficacy and safety of sevelamer and calcium carbonate D3 in regulating calcium and phosphorus levels in maintenance hemodialysis patients with chronic renal failure. Mod Chin Doct. 2020;58:50–53.
- Lan S, Zhang G. Effectiveness of sevelamer on hyperphosphatemia in chronic renal failure patients with maintenance hemodialysis and its influence on inflammatory factors. Med Innov Chin. 2018;15:142–145.
- Takei T, Otsubo S, Uchida K, et al. Effects of sevelamer on the progression of vascular calcification in patients on chronic haemodialysis. Nephron Clin Pract. 2008;108(4):c278–283.
- Kakuta T, Tanaka R, Hyodo T, et al. Effect of sevelamer and calcium-based phosphate binders on coronary artery calcification and accumulation of circulating advanced glycation end products in hemodialysis patients. Am J Kidney Dis. 2011;57(3):422–431.
- Filiopoulos V, Koutis I, Trompouki S, et al. Lanthanum carbonate versus sevelamer hydrochloride: improvement of metabolic acidosis and hyperkalemia in hemodialysis patients. Ther Apher Dial. 2011;15(1):20–27.
- Prajapati VA, Galani VJ, Shah PR. A comparative study of phosphate binders in patients with end stage kidney disease undergoing hemodialysis. Saudi J Kidney Dis Transpl. 2014;25(3):530–538.
- Cheng X, Bai Y. Effect of sevelamer on maintenance hemodialysis patients with secondary hyperparathyroidism. Anhui Med J. 2019;40:933–935.
- Chen Y, Zhang Y, Yao X, et al. Observation on the efficacy of different medication regimens on secondary hyperparathyroidism in hemodialysis patients. Mod Chin Doct. 2021;59:39–43.
- Yan H. End-stage renal disease maintenance hemodialysis to take the carbonic acid sevelamer treatment on its effectiveness of hyperphosphatemia. J Northern Pharm. 2019;16:177–178.
- Zhang Y. Analysis of short-term efficacy of sevelame hydrochloride in the treatment of hyperphosphatemia in maintenance hemodialysis patients. J Chin Prescript Drug. 2017;15:82–83.
- Li Y, Lin Z, Cai Z. The comparative analysis of two kinds 0f phosphate binder in the treatment of maintenance hemodialysis patients with hyperphosphatemia. J Chin Prescript Drug. 2014;12:12–13.
- Zhe J, Chen J, Rong C. Effect of sevelamer carbonate on the clinical efficacy of chronic kidney disease-mineral and bone disorder in the patients with maintenance hemodialysis. For Primary Care Med. 2021;25:1806–1808.
- Ammirati AL, Dalboni MA, Cendoroglo M, et al. Coronary artery calcification, systemic inflammation markers and mineral metabolism in a peritoneal dialysis population. Nephron Clin Pract. 2006;104(1):c33–40.
- Sprague SM. A comparative review of the efficacy and safety of established phosphate binders: calcium, sevelamer, and lanthanum carbonate. Curr Med Res Opin. 2007;23(12):3167–3175.
- London GM, Marty C, Marchais SJ, et al. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15(7):1943–1951.
- Liabeuf S, Ryckelynck JP, El Esper N, et al. Randomized clinical trial of sevelamer carbonate on serum klotho and fibroblast growth factor 23 in CKD. Clin J Am Soc Nephrol. 2017;12(12):1930–1940.
- Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617.
- Young EW, Akiba T, Albert JM, et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2004;44(2):34–38.
- Stevens LA, Djurdjev O, Cardew S, et al. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol. 2004;15(3):770–779.
- Raggi P, Vukicevic S, Moysés RM, et al. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin J Am Soc Nephrol. 2010;5(1):S31–S40.
- Felsenfeld AJ, Levine BS, Rodriguez M. Pathophysiology of calcium, phosphorus, and magnesium dysregulation in chronic kidney disease. Semin Dial. 2015;28(6):564–577.
- Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215.
- Scialla JJ, Wolf M. Roles of phosphate and fi-broblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol. 2014;10(5):268–278.
- Olauson H, Vervloet MG, Cozzolino M, et al. New insights into the FGF23-Klotho axis. Semin Nephrol. 2014;34(6):586–597.
- Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the mild to moderate kidney disease (MMKD) study. J Am Soc Nephrol. 2007;18(9):2600–2608.
- Stack AG, Bloembergen WE. A cross-sectional study of the prevalence and clinical correlates of congestive heart failure among incident US dialysis patients. Am J Kidney Dis. 2001;38(5):992–1000.
- Ruospo M, Palmer SC, Natale P, et al. Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane Database Syst Rev. 2018;8(8):Cd006023.
- Bogin E, Massry SG, Harary I. Effect of Para-thyroid hormone on rat heart cells. J Clin Invest. 1981;67(4):1215–1227.
- Tastan I, Schreckenberg R, Mufti S, et al. Parathyroid hormone improves contractile performance of adult rat ventricular cardiomyocytes at low concentrations in a non-acute way. Cardiovasc Res. 2009;82(1):77–83.
- Miyamoto K, Ito M, Segawa H, et al. Secondary hyperparathyroidism and phosphate sensing in parathyroid glands. J Med Invest. 2000;47:118–122.
- Geng Y, Mosyak L, Kurinov I, et al. Structural mechanism of ligand activation in human calcium-sensing receptor. eLife. 2016;5:e13662.
- Ferramosca E, Burke S, Chasan-Taber S, et al. Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am Heart J. 2005;149(5):820–825.
- Habbous S, Przech S, Acedillo R, et al. The efficacy and safety of sevelamer and lanthanum versus calcium-containing and iron-based binders in treating hyperphosphatemia in patients with chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32(1):111–125.
- Chertow GM, Burke SK, Dillon MA, et al. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant. 1999;14(12):2907–2914.
- Lee BT, Ahmed FA, Hamm LL, et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 2015;16:77.
- Shantouf R, Budoff MJ, Ahmadi N, et al. Effects of sevelamer and calcium-based phosphate binders on lipid and inflammatory markers in hemodialysis patients. Am J Nephrol. 2008;28(2):275–279.
- Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–1483.
- Guérin AP, London GM, Marchais SJ, et al. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15(7):1014–1021.
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20(2):381–387.
- Elder GJ, Center J. The role of calcium and non calcium-based phosphate binders in chronic kidney disease. Nephrology (Carlton, Vic). 2017;22:42–46.
- Guerin AP, Blacher J, Pannier B, et al. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103(7):987–992.
- Phannajit J, Wonghakaeo N, Takkavatakarn K, et al. The impact of phosphate lowering agents on clinical and laboratory outcomes in chronic kidney disease patients: a systematic review and meta-analysis of randomized controlled trials. J Nephrol. 2022;35(2):473–491.