Abstract
Background
Heparin anticoagulation (HA) is commonly employed for membrane therapeutic plasma exchange (mTPE). However, for patients with increased bleeding risk, there were controversial opinions on the use of HA versus regional citrate anticoagulation (RCA) for mTPE. Our present study aimed to evaluate the efficacy and safety of HA vs. RCA for mTPE in patients with increased bleeding risk.
Methods
Patients with increased bleeding risk who underwent mTPE between 2014 and 2021 in our center were screened. Observations of anticoagulation efficacy and safety were used as the study endpoints.
Results
A total of 108 patients with 368 mTPE sessions were included. Of the included patients, 38 and 70 received HA and RCA mTPE, respectively. There was no significant difference in the clotting of extracorporeal circuits between the HA and RCA groups (4.1% vs. 4.4%, p = 0.605). More bleeding episodes were observed in the HA group compared to the RCA group (16.4% vs. 4.4% mTPE sessions, p < 0.001). The frequency of postoperative transfusion within 24 h (11% vs. 3.4%, p = 0.007) was significantly different in the HA and RCA group. Anticoagulation strategy (HA vs. RCA; OR 5.659, 95%CI 2.266–14.129; p < 0.001), and mean arterial pressure (prior treatment, OR 1.052, 95%CI 1.019–1.086; p = 0.002) were independent risk factors of bleeding episodes. At the end of mTPE treatment, the incidence of metabolic alkalosis (16.7% vs. 54.1%, p = 0.027) and hypocalcemia (41.7% vs. 89.2%, p = 0.001) was significantly different in the HA (n = 5, 12 sessions) and RCA (n = 22, 74 sessions) groups, respectively.
Conclusion
RCA is as effective as HA for mTPE. However, for patients with increased bleeding risk, RCA is associated with a lower risk of bleeding, compared with HA. With careful monitoring and timely adjustment, RCA most likely is a safe and effective anticoagulation option for mTPE in patients with increased bleeding risk.
Introduction
Therapeutic plasma exchange (TPE) is widely used in various fields, including renal, hematologic, neurologic, immunologic, and organ transplantation [Citation1,Citation2]. Centrifugal (cTPE) and membrane-based (mTPE) devices are available for TPE [Citation3]. Both forms are available globally, and regional economics and preferences can affect the choice of these two modes. According to reports, mTPE is most commonly used in China, Japan, and Europe, while cTPE is widely used in the United States [Citation4]. For mTPE, membrane contact reaction and biological incompatibility activated the coagulation system and led to clotting of the extracorporeal circuits, which resulted in blood loss, replacement fluid waste, and cost increase [Citation5]. Therefore, anticoagulation is important for the patency of the extracorporeal circuit.
Commonly, critically ill patients are associated with an increased bleeding risk due to recent surgery, trauma, mucosal lesions, and coagulopathy [Citation6]. For patients with increased bleeding risk, anticoagulants should be selected with consideration of superior anticoagulation efficacy while minimizing the incidence of bleeding episodes. Anticoagulation strategies for patients with a higher risk of bleeding were extremely limited before the successful implementation of regional citrate anticoagulation (RCA), and the only choice was no anticoagulation [Citation7]. However, no-anticoagulation would result in a relatively shorter filter lifespan (the incidence of clotting circuits was 6.3%–33% for mTPE treated without anticoagulation [Citation8–10]). Heparin anticoagulation (HA) is the most commonly employed anticoagulant for mTPE with the advantages of low costs, ease of administration, simple monitoring, and reversibility with protamine [Citation11,Citation12]. However, complicated pharmacokinetics and large individual differences, increased bleeding risk caused by systemic anticoagulation, heparin-induced thrombocytopenia (HIT), and heparin resistance when the low level of antithrombin III in the patient’s body is also drawbacks for HA [Citation13,Citation14]. Because of the low anticoagulant dose requirement and short treatment duration, several studies have reported HA-mTPE in patients with increased bleeding risk [Citation15]. Similarly, some patients with increased bleeding risk are treated with HA-mTPE in our center, which was a forced option before the successful use of RCA-mTPE. Compared with HA, RCA does not affect the coagulation status of the system circulation and does not increase patient bleeding risk [Citation16,Citation17]. However, citrate metabolism releases large amounts of HCO3- into the systemic blood, and the risk of alkalosis increases rapidly [Citation18,Citation19]. Citrate metabolism is mainly in the liver and is an oxygen-dependent process. For critically ill patients with impaired dysfunction and shock with muscle hypoperfusion, the risk of citrate accumulation (CA) is increased [Citation20–23]. Due to the limited clinical evidence, there are controversial opinions on the use of HA vs. RCA for mTPE for patients with increased bleeding risk, which leads to the use of different anticoagulation for mTPE in our clinical work.
In conclusion, we intended to explore the efficacy and safety of HA vs. RCA in mTPE for patients with increased bleeding risk by a retrospective observational study.
Materials and methods
Patients selection
Our present study was a single-center, retrospective, cohort study focused on the anticoagulation for mTPE between January 2014 and October 2021 in patients with increased bleeding risk. Diagnosis of increased bleeding risk based on five criteria: PLT < 40 × 109/L, APTT > 60 s, INR > 1.5, recent (within 7 days) active bleeding, and recent (within 7 days) trauma or surgery. Patients with any of the following conditions were excluded: (1) mTPE performed with HA + RCA; (2) mTPE performed without anticoagulation. The eligible patients were divided into the HA and RCA group by the anticoagulants they had received. Our present study was performed in accordance with the declaration of Helsinki and approved by the ethics committee of our hospital (KY20213022-1). The requirement for patient consent was waived due to the retrospective nature of the study.
Membrane therapeutic plasmapheresis protocol
Procedures were performed using the Haemoselect@ L 0.3/0.5 filter with the Diapact® continuous renal replacement therapy (B Braun; Avitum AG, 34212, Melsungen, Germany) system. Fresh frozen plasma (FFP) was used as a replacement solution. The estimated plasma volume was computed as follows: estimated plasma volume = 0.065 x (1 - hematocrit) x body weight. The amount of plasma required for each treatment is 1.5 to 2.0 times the patient’s plasma volume (kg). Blood flow was initiated at 120 mL/min, and the maximum plasma flow was controlled at 30% of the effective flow. The selection of HA and RCA is based on the time of mTPE and the physician’s experience. Before the RCA-mTPE protocol was successfully used in our center, HA-mTPE was the main anticoagulant strategy. For the HA-mTPE protocol, heparin was administered systemically through intravenous lines with an initial dose of 5–10 mg. During the mTPE treatment, typical protocols target the APTT in the extracorporeal circuit between 1.2 and 1.5 times normal or 45–60 s. Those with suspected procoagulant tendencies received additional heparin sodium with a dose of 5 mg. After 2017, more and more patients with increased bleeding risk accepted RCA-mTPE. For the RCA-mTPE protocol, a 4% trisodium citrate solution (8 g/200 mL, sodium citrate is 136 mmol per 1 liter) was infused into the arterial line, initially at a rate controlled to 110–150 mL/h. During the mTPE treatment, the blood flow and citrate dose were adjusted to maintain post-filter ionized calcium levels of 0.25–0.45 mmol/L. Dexamethasone (5 mg) and 10% calcium gluconate (10 mL) were used to prevent allergies and hypocalcemia prior to and post-treatment.
Data collection
All data were retrieved from the electronic medical records of our hospital. The following data were collected: demographic data, etiologies, mTPE-related parameters, biochemical data, vital signs, and the treatment-related complications (clotting of extracorporeal circuits, bleeding episodes, and allergy or hypocalcemia-related manifestations) during the mTPE treatment. The illness severity was assessed using the Sequential Organ Failure Assessment (SOFA).
Study endpoints
In our present study, clotting of the extracorporeal circuits was used as an efficacy endpoint for anticoagulation. Dialysis and coagulation circuit grade (Class 0: No clotting in the dialyzer; Class 1: Dialyzer fiber coagulation < 10%; Class 2: Dialyzer fiber coagulation 10% − 50%; Class 3: Dialyzer fiber coagulation >50% or significant elevation of venous pressure in the extracorporeal circuits) [Citation24,Citation25] and semi-quantitative scale (Grade 0: no detectable clotting; Grade 1: minimal clot formation; Grade 2: moderate clot formation; Grade 3: major clot formation but dialysis still possible; Grade 4: complete occlusion of dialysis or an air trap, rendering dialysis impossible) [Citation26,Citation27] was used to assess clotting of membrane plasma separator and artery or vein ampulla. Anticoagulation safety endpoints included observation of bleeding episodes, allergy or hypocalcemia-related manifestations, coagulation parameters, acid-base and electrolytes, and vital signs during the mTPE treatment. Bleeding episodes were defined as bleeding-related symptoms within 24 h after each mTPE treatment. CA was defined as reduced ionized calcium (iCa) (< 1.1 mmol/L), calcium ratio (TCa, total calcium/iCa) > 2.5, and metabolic acidosis (pH < 7.2 and/or BE < −5 mmol/L) with or without an elevated anion gap (> 11 mmol/L) [Citation28].
Statistical analyses
The categorical variable was described by constituent ratio or rate and compared by using the Chi-square test or the Fisher exact probability test. For the continuous variable, the Shapiro-Wilk test was employed for the evaluation of variable distribution. Normally distributed data were described as Mean ± SD, and paired sample t-test was used for comparison. The non-normally distributed data were described as median, 25th, and 75th quantile, and Mann–Whitney U-test was used for comparison. Difference values between groups were calculated as follows: Δ= Post-treatment - Prior treatment, and Independent-samples T-test were used for comparison. Independent predictors of bleeding episodes were assessed using the binary logistic regression analysis. p < 0.05 was considered statistically significant. All of the statistical calculations were performed by using SPSS software (IBM spss. 25.0, SPSS Inc).
Results
Baseline characteristics
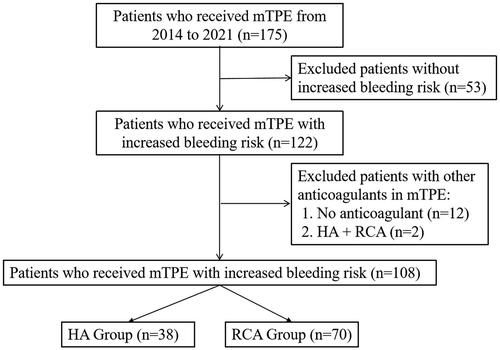
Between 2014 and 2021, 175 patients with mTPE were screened. Then, 53 patients with no increased bleeding risk, 12 patients without anticoagulation, and 2 patients with combined HA + RCA use during the mTPE treatment were excluded. Finally, 108 patients were eligible for the study, 38 for heparin and 70 for citrate. ()
Figure 1. Patient inclusion flow chart.
mTPE: membrane therapeutic plasma exchange. HA: heparin anticoagulation. RCA: regional citrate anticoagulation.
The baseline characteristics of the included patients are shown in . Demographic data were not significantly different between the HA and RCA groups (p > 0.05). There were significant differences in vascular access (p = 0.032), PT (p = 0.039), MAP (p = 0.007), and GCS (p = 0.004) between the two groups. The etiologies of the included patients are reported in .
Table 1. Patients baseline characteristics.
Table 2. Distribution of etiologies.
Efficacy
In our present study, a total of 16 (4.3%) sessions were interrupted by clotting of extracorporeal circuits. In the HA group, 3 (4.1%) of sessions were interrupted by III-level clotting of the membrane plasma separator (the dialyzer fiber clotting portion is over 50% and the average effective treatment duration is 1.62 h). In the RCA group, 12 (4.1%) and 1 (0.3%) sessions were interrupted by III-level clotting of the membrane plasma separator (the dialyzer fiber clotting portion is over 50% and the average effective treatment duration is 1.435 h) or IV-level clotting of the vein ampulla (complete occlusion of vein ampulla and the effective treatment duration is 1.3 h), respectively. For patients whose mTPE treatment was interrupted, the nursing staff replaced the circuit and restarted the process. In the comparison of the two groups, the incidence of extracorporeal circuits coagulation was not significantly different (p = 0.605).
Safety
Comparison of treatment-related complications
In our present study, there were no significantly different in the incidence of allergy or hypocalcemia-related manifestations in the HA and RCA group, respectively (p > 0.05). More bleeding episodes were observed in the HA group compared to the RCA group (16.4% vs. 4.4%, p < 0.001). The frequency of postoperative transfusion within 24 h was also significantly different in the HA and RCA group (11% vs. 3.4%, p = 0.007). ()
Table 3. Comparison of the treatment-related complications with different methods of anticoagulation.
Comparison of coagulation parameters
Compared with pretreatment values, changes in RBC, HB, HCT, PLT, PT, APTT, and INR were no significant differences after treatment in the two groups (p > 0.05). In the comparison of the two groups, Δ the value of the above parameters is also not significantly different (p > 0.05). ()
Table 4. Comparison of laboratory index, SOFA scores, and vital signs before and after HA or RCA-mTPE treatment.
Comparison of acid-base and electrolytes
In our present study, 13.2% (5/38) and 31.4% (22/70) of patients (12 and 74 sessions) had the data of electrolyte analysis in the HA and RCA group, respectively. At the end of mTPE, the incidence of metabolic alkalosis (PH > 7.45 and HCO3- > 27 mmol/L) and hypocalcemia [Citation29,Citation30] (iCa < 1.1 mmol/L) was significantly different in the HA and RCA groups, respectively (16.7% vs. 54.1%, p = 0.027; 41.7% vs. 89.2%, p = 0.001). Meanwhile, ΔiCa, ΔTCa/iCa, and ΔHCO3- were also significantly different between the two groups (p < 0.05). Instead, the incidence of hypernatremia and acidosis were no significant difference in the HA and RCA groups, respectively (p > 0.05). Finally, our results show that no sessions developed CA during the mTPE treatment between the two groups. ()
Table 5. Comparison of the incidence of electrolyte and acid-base disturbances before and after HA or RCA-mTPE treatment.
Table 6. Comparison of changes in acid-base and electrolyte before and after HA or RCA-mTPE treatment.
Comparison of vital signs
In our present study, there were no significant differences in MAP and HR between the two groups before and after treatment (p > 0.05). ()
Predictors of bleeding episodes in patients with increased bleeding risk underwent mTPE
A total of 368 mTPE sessions were included in both univariate and multivariate analyses. Age, gender, BMI, vascular access, anticoagulation strategy, vasopressors, mechanical ventilation, inclusion criteria of increased bleeding risk, biochemical parameters (prior treatment, including blood cell analysis, coagulation function tests, total bilirubin, and creatinine), SOFA scores and vital signs (prior treatment) were assessed as potential risk factors.
In the univariate analysis, active bleeding within 7d (p = 0.038), heparin anticoagulation (p = 0.001), and MAP (p = 0.012) were significantly related to the bleeding episodes. In the multivariate analysis model, anticoagulation strategy (HA vs. RCA; OR 5.659, 95%CI 2.266–14.129; p < 0.001), and MAP (prior treatment, OR 1.052, 95%CI 1.019–1.086; p = 0.002) were independent risk factors of bleeding episodes even after the adjustment of APTT, INR, and PLT (prior treatment). ()
Table 7. Predictors of bleeding episodes in patients with increased bleeding risk underwent mTPE treatment.
Discussion
To the best of our knowledge, our present study is the first cohort study to evaluate the efficacy and safety of HA vs. RCA for mTPE in patients with increased bleeding risk. Our present study has several findings: 1) in patients with increased bleeding risk, RCA was as effective as HA for mTPE to prevent clotting of extracorporeal circuits; 2) the use of RCA for mTPE could significantly reduce the risk of bleeding episodes compared with HA.
Efficacy
The basic principle of RCA is the infusion of citrate into the extracorporeal circuits. Subsequently, the anticoagulation effect of sodium citrate relies on the formation of a complex with ionized calcium, which is one of the essential components of the coagulation cascade [Citation31]. The superiority of RCA to mTPE filter lifespan has been confirmed by many studies [Citation10,Citation32,Citation33]. In our present study, the incidence of clotting in the extracorporeal circuits is also very low (4.4%) in the RCA group. Heparin provides anticoagulation by activating antithrombin III and inhibiting various active clotting factors, and the plasma half-life is approximately 90 min but can increase up to 3 h in the presence of renal insufficiency. According to previous studies, the incidence of extracorporeal circuit clotting has been reported to be 1.8% − 7.5% in HA-mTPE [Citation8,Citation10,Citation15,Citation32]. In our present study, 4.1% of circuit coagulation was recorded in the HA group, which is the average reported in previous studies. Our current findings demonstrate that HA and RCA are comparable in preventing the mTPE coagulation circuits in patients with increased bleeding risk.
Safety
Bleeding episodes
Bleeding episodes are the most common adverse event for anticoagulation. HA is a systemic anticoagulant commonly used in mTPE treatment. Prolonged APTT indicates the efficacy of HA but also increased bleeding risk [Citation34,Citation35]. In our present study, we failed to observe the prolongation of APTT after mTPE treatment in the HA group, but there were more bleeding episodes (16.4%) recorded in the HA group compared with the RCA group. Due to the retrospective nature of this study, the collection of coagulation parameters is more affected by the time of blood collection. The coagulation components of the replacement plasma will improve the coagulation status of the patients and mask this trend as well. Compared with HA, RCA limits anticoagulation to the extracorporeal circuit. Multiple studies have demonstrated that RCA significantly reduces bleeding episodes (0%) in mTPE therapy [Citation10,Citation32,Citation33]. In our present study, 4.1% of bleeding episodes were recorded in the RCA group, which most likely is due to the progress of patient disease instead of RCA.
Patients with liver failure are known to be associated with an increased risk of bleeding [Citation36]. Yuan et al. [Citation15] reported that the incidence of bleeding episodes in HA-mTPE patients with liver failure was 13.7%, and 1.3% of treatments were interrupted due to bleeding episodes. In our present study, 32 patients with liver failure were treated with 57 mTPE (Appendix). Among the included patients, the incidence of bleeding episodes was 26.9% in HA-mTPE. Although the dose of heparin in our center was lower than that reported by Yuan et al. (the first dose was 2500UI, and the additional dose was 50UI/h), the incidence of bleeding episodes was about twice as high. Due to the small sample size, our results may be biased. Furthermore, the former excluded patients with active bleeding before starting the study, which is also a crucial reason.
In our present study, the incidence of bleeding episodes was significantly different between the two anticoagulation methods. And, our results suggest that RCA-mTPE could reduce more than 2.266 times the risk of bleeding episodes in patients with increased bleeding risk compared with HA-mTPE. Also, transfusion requirements for patients in the HA group were significantly higher than in the RCA group. And, an increase in bleeding episodes is most likely one of the causes of the significantly increased need for blood transfusions. Finally, HA was identified as one of the independent risk factors for bleeding episodes in the multivariate analysis of our study. In the multivariate analysis model, MAP (prior treatment) was identified as another independent risk factor for bleeding episodes. It is known that low MAP often accompanies severe disease conditions, including trauma, shock, and multiple organ failure, and is associated with increased bleeding risk in clinical practice.
Citrate related complications
Metabolic alkalosis
Citrate concentrations in FFP have been measured and reported from 17 to 21 mmol/L in one case report [Citation37]. According to our RCA-mTPE protocol, an additional citrate load of 22.9 to 31.3 mmol/h was added to the patient after mTPE treatment. However, no more than 30% of pre-filter citrate is cleared through the membrane plasma filter [Citation38,Citation39]. The remaining citrate enters the body and metabolizes to large amounts of bicarbonate [Citation40]. Compared with CRRT, mTPE has no acid replacement fluid to neutralize the bases generated by citrate metabolism. Therefore, metabolic alkalosis should be considered when mTPE is combined with RCA and FFP. Currently, several studies report an alkalosis tendency during RCA-mTPE [Citation10,Citation32,Citation33]. Likewise, we also observed an increase in PH, HCO3-, and BE after RCA-mTPE treatment. Compared with the HA group, the RCA group developed more metabolic alkalosis after mTPE treatment, which was closely related to the infusion of the additional citrate load. In clinical work, physicians cannot avoid consecutive-mTPE treatments, because the efficiency of TPE is affected by pathogen production and clearance rates. And this is the main reason why we report a higher incidence of metabolic alkalosis before RCA-mTPE treatment. Indeed, metabolic alkalosis was present in only 3 (13.6%) patients before the first RCA-mTPE, whereas this increased to 16 (73%) after the end of mTPE treatments. Alkalosis is currently considered a benign complication of RCA and is a sign of excess citrate or, more commonly, low hemofilter clearance [Citation41]. However, several studies have reported progressive metabolic alkalosis during consecutive RCA-mTPE treatments [Citation38,Citation42,Citation43]. Therefore, careful monitoring of electrolyte and acid-base changes is necessary for patients treated with RCA-mTPE treatment.
Hypernatremia and hypomagnesemia
When we use a 4% trisodium citrate solution, the extra Na+ load of the human body will also increase (1 mmol trisodium citrate contains 3 mmol Na+). In our present study, we found that hypernatremia was rare after RCA-mTPE treatment. Meanwhile, there was no significant difference in Na+ levels before and after mTPE treatment in the RCA group. Citrate is also a chelate of magnesium, and if the citrate-magnesium complex is removed by an extracorporeal circuit, there is a risk of inducing hypomagnesemia [Citation33]. However, the assessment of hypomagnesemia is difficult because magnesium ions are not part of routine electrolyte testing.
Citrate accumulation
CA is a potentially lethal complication of RCA [Citation41]. Severe CA can cause severe hypocalcemia and acidosis, which can be life-threatening for critically ill patients. As we know, severely impaired liver function or shock with muscle hypoperfusion reduced the rate of citrate metabolism, thereby increasing the risk of CA [Citation44]. Initially, the ratio of total calcium to ionic calcium (TCa/iCa) ≥2.5 was used as an indirect criterion for determining whether CA occurred in patients with liver failure [Citation18]. Based on the above criteria, Ma et al. [Citation32] reported that the incidence of CA in patients with acute-on-chronic liver failure immediately after mTPE treatment, 2 h after mTPE therapy, and the next morning were 100%, 34%, and 0%, respectively. However, additional citrate infusion during the RCA-mTPE treatment increased the risk of hypocalcemia. Compared with HA-mTPE, our results showed that more patients developed hypocalcemia and more drastic changes in iCa levels after RCA-mTPE treatment. TCa/iCa will show positive results due to the reduction of ionic calcium even though CA does not actually occur. In our present study, the CA definition was based on four criteria [Citation28], which were considered more objective and stricter than the definition used in the study by Ma et al. This is the most important reason for the very low incidence of CA in our present study.
Limitations
First, the retrospective and observational nature is one of the limitations of our present study. A multivariate analysis was performed in our study to reduce the selection bias associated with retrospective studies and to provide more reliable conclusions. We believe that data from the real-world most likely could provide useful clues and supplements to prospective studies. Second, serum citrate concentration, which is supposed to be the most reliable diagnostic criterion for CA, is not routinely tested in clinical practice. However, we employed the definition of CA by Khadzhynov et al. [Citation28], which was considered to be more objective and commonly accepted in previous studies, to provide more solid results.
Conclusion
In patients with increased bleeding risk, HA and RCA were not significantly different in anticoagulation efficacy for mTPE. However, RCA-mTPE could significantly reduce the incidence of bleeding episodes, compared with HA in patients with increased bleeding risk. With careful monitoring and timely adjustment, RCA most likely is a safe and effective anticoagulation option for mTPE in patients with increased bleeding risk. Further randomized controlled trials are warranted to provide stronger evidence.
Ethical approval and consent to participate
The study was approved by the ethics committee of the Xijing hospital, the Fourth Military Medical University (KY20213022-1), and performed in accordance with the Declaration of Helsinki. The informed consent was waived because of the retrospective study design.
Authors’ contributions
All the authors have made an intellectual contribution to the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the writing committee of the American society for apheresis: the eighth special issue. J Clin Apher. 2019;34(3):1–11.
- Abe T, Matsuo H, Abe R, et al. The Japanese society for apheresis clinical practice guideline for therapeutic apheresis. Ther Apher Dial. 2021;25(6):728–876.
- Chen YY, Sun X, Huang W, et al. Therapeutic apheresis in kidney diseases: an updated review. Ren Fail. 2022;44(1):842–857.
- Ahmed S, Kaplan A. Therapeutic plasma exchange using membrane plasma separation. Clin J Am Soc Nephrol. 2020;15(9):1364–1370.
- Bowry SK, Kircelli F, Himmele R, et al. Blood-incompatibility in haemodialysis: alleviating inflammation and effects of coagulation. Clin Kidney J. 2021;14(Suppl 4):i59–i71.
- Retter A, Barrett NA. The management of abnormal haemostasis in the ICU. Anaesthesia. 2015;70 Suppl 1(Suppl 1):121–127. e40–1.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84.
- Brunetta Gavranić B, Bašić-Jukić N, Premužić V, et al. Membrane therapeutic plasma exchange with and without heparin anticoagulation. J Clin Apher. 2017;32(6):479–485.
- Gashti CN, Andreoli DC, Patel D. Membrane-based therapeutic plasma exchange (mTPE): technical and clinical experience. J Clin Apher. 2018;33(1):38–45.
- Yuan F, Li Z, Li X, et al. Application of regional citrate anticoagulation in membrane therapeutic plasma exchange. Int Urol Nephrol. 2020;52(12):2379–2384.
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120(10):1371–1383.
- Bauer PR, Ostermann M, Russell L, et al. Plasma exchange in the intensive care unit: a narrative review. Intensive Care Med. 2022;48(10):1382–1396.
- van de Wetering J, Westendorp RG, van der Hoeven JG, et al. Heparin use in continuous renal replacement procedures: the struggle between filter coagulation and patient hemorrhage. J Am Soc Nephrol. 1996;7(1):145–150.
- Syed S, Reilly RF. Heparin-induced thrombocytopenia: a renal perspective. Nat Rev Nephrol. 2009;5(9):501–511.
- Yuan S, Qian Y, Tan D, et al. Therapeutic plasma exchange: a prospective randomized trial to evaluate 2 strategies in patients with liver failure. Transfus Apher Sci. 2018;57(2):253–258.
- Bai M, Zhou M, He L, et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med. 2015;41(12):2098–2110.
- Kindgen-Milles D, Brandenburger T, Dimski T. Regional citrate anticoagulation for continuous renal replacement therapy. Curr Opin Crit Care. 2018;24(6):450–454.
- Tolwani A, Wille KM. Advances in continuous renal replacement therapy: citrate anticoagulation update. Blood Purif. 2012;34(2):88–93.
- Monchi M. Citrate pathophysiology and metabolism. Transfus Apher Sci. 2017;56(1):28–30.
- Kramer L, Bauer E, Joukhadar C, et al. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med. 2003;31(10):2450–2455.
- Bauer E, Derfler K, Joukhadar C, et al. Citrate kinetics in patients receiving long-term hemodialysis therapy. Am J Kidney Dis. 2005;46(5):903–907.
- Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649–672.
- Zhang W, Bai M, Yu Y, et al. Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: a systematic review and meta-analysis. Crit Care. 2019;23(1):22.
- Mei CL, Ye CY, Rong S. A practical handbook for dialysis. 2nd ed. Beijing: People’s Medical Publishing House; 2009. p. 53–115.
- Singer RF, Williams O, Mercado C, et al. Regional citrate anticoagulation in hemodialysis: an observational study of safety, efficacy, and effect on calcium balance during routine care. Can J Kidney Health Dis. 2016;3:22.
- Cheng YL, Yu AW, Tsang KY, et al. Anticoagulation during haemodialysis using a citrate-enriched dialysate: a feasibility study. Nephrol Dial Transplant. 2011;26(2):641–646.
- Wright S, Steinwandel U, Ferrari P. Citrate anticoagulation using ACD solution a during long-term haemodialysis. Nephrology. 2011;16(4):396–402.
- Khadzhynov D, Schelter C, Lieker I, et al. Incidence and outcome of metabolic disarrangements consistent with citrate accumulation in critically ill patients undergoing continuous venovenous hemodialysis with regional citrate anticoagulation. J Crit Care. 2014;29(2):265–271.
- Pepe J, Colangelo L, Biamonte F, et al. Diagnosis and management of hypocalcemia. Endocrine. 2020;69(3):485–495.
- Rhee H, Berenger B, Mehta RL, et al. Regional citrate anticoagulation for continuous kidney replacement therapy with calcium-containing solutions: a cohort study. Am J Kidney Dis. 2021;78(4):550–559.e1.
- Morgera S, Schneider M, Slowinski T, et al. A safe citrate anticoagulation protocol with variable treatment efficacy and excellent control of the acid-base status. Crit Care Med. 2009;37(6):2018–2024.
- Ma Y, Chen F, Xu Y, et al. Safety and efficacy of regional citrate anticoagulation during plasma adsorption plus plasma exchange therapy for patients with acute-on-chronic liver failure: a pilot study. Blood Purif. 2019;48(3):223–232.
- Peng X, Xie X, Yin J, et al. Anticoagulation effect and safety observation of regional citrate anticoagulation for double-filtration plasmapheresis in critical patients. Blood Purif. 2020;49(5):542–549.
- Betjes MG, van Oosterom D, van Agteren M, et al. Regional citrate versus heparin anticoagulation during venovenous hemofiltration in patients at low risk for bleeding: similar hemofilter survival but significantly less bleeding. J Nephrol. 2007;20(5):602–608. PMID: 17918147.
- MacEwen C, Watkinson P, Winearls C. Circuit life versus bleeding risk: the impact of achieved activated partial thromboplastin time versus achieved filtration fraction. Ther Apher Dial. 2015;19(3):259–266.
- Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369(26):2525–2534.
- van der Meulen J, Janssen MF, Oe PL. Cardiac arrest during rapid plasma exchange for haemolytic uraemic syndrome. Nephrol Dial Transplant. 1994;9:1841. PMID: 7708283.
- Pearl RG, Rosenthal MH. Metabolic alkalosis due to plasmapheresis. Am J Med. 1985;79(3):391–393.
- Cid J, Carbassé G, Gamir M, et al. Acid-base balance disturbances in plasma exchange depend on the replacement fluid used. Transfusion. 2015;55(11):2653–2658.
- Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2014;371(15):1434–1445.
- Schneider AG, Journois D, Rimmelé T. Complications of regional citrate anticoagulation: accumulation or overload? Crit Care. 2017;21(1):281.
- Choi MY, Lee JD, Lee SH, et al. Metabolic alkalosis induced by plasmapheresis in a patient with systemic lupus erythematosus. J Korean Med Sci. 1993;8(3):207–209.
- Nagai Y, Itabashi M, Mizutani M, et al. A case report of uncompensated alkalosis induced by daily plasmapheresis in a patient with thrombotic thrombocytopenic purpura. Ther Apher Dial. 2008;12(1):86–90.
- Oudemans-van Straaten HM, Ostermann M. Bench-to-bedside review: citrate for continuous renal replacement therapy, from science to practice. Crit Care. 2012;16(6):249.
