Abstract
The role of facility-level serum potassium (sK+) variability (FL-SPV) in dialysis patients has not been extensively studied. This study aimed to evaluate the association between FL-SPV and clinical outcomes in hemodialysis patients using data from the China Dialysis Outcomes and Practice Patterns Study (DOPPS) 5. FL-SPV was defined as the standard deviation (SD) of baseline sK+ of all patients in each dialysis center. The mean and SD values of FL-SPV of all participants were calculated, and patients were divided into the high FL-SPV (>the mean value) and low FL-SPV (≤the mean value) groups. Totally, 1339 patients were included, with a mean FL-SPV of 0.800 mmol/L. Twenty-three centers with 656 patients were in the low FL-SPV group, and 22 centers with 683 patients were in the high FL-SPV group. Multivariate logistic regression analysis showed that liver cirrhosis (OR = 4.682, 95% CI: 1.246–17.593), baseline sK+ (<3.5 vs. 3.5 ≤ sK+ < 5.5 mmol/L, OR = 2.394, 95% CI: 1.095–5.234; ≥5.5 vs. 3.5 ≤ sK+ < 5.5 mmol/L, OR = 1.451, 95% CI: 1.087–1.939), dialysis <3 times/week (OR = 1.472, 95% CI: 1.073–2.020), facility patients’ number (OR = 1.088, 95% CI: 1.058–1.119), serum HCO3– level (OR = 0.952, 95% CI: 0.921–0.984), dialysis vintage (OR = 0.919, 95% CI: 0.888–0.950), other cardiovascular disease (OR = 0.508, 95% CI: 0.369–0.700), and using high-flux dialyzer (OR = 0.425, 95% CI: 0.250–0.724) were independently associated with high FL-SPV (all p < .05). After adjusting potential confounders, high FL-SPV was an independent risk factor for all-cause death (HR = 1.420, 95% CI: 1.044–1.933) and cardiovascular death (HR = 1.827, 95% CI: 1.188–2.810). Enhancing the management of sK+ of hemodialysis patients and reducing FL-SPV may improve patient survival.
Introduction
Chronic kidney disease (CKD) is a prevalent condition affecting over 10% of the adult population in China and poses a significant burden on the healthcare system [Citation1]. CKD patients accounted for 4.86% of the 20 million hospitalized patients in China in 2016, with a mortality rate of 2.56% reported among in-hospital CKD patients [Citation2]. According to data from the Chinese National Renal Data System (CNRDS), as of December 2021, there were 749,573 and 126,372 patients receiving hemodialysis and peritoneal dialysis, respectively. There is a much higher mortality rate in patients receiving hemodialysis (approximately 14%) [Citation3–5] than in healthy individuals.
According to the US Renal Data System 2018 Annual Data Report, sudden cardiac death is a leading cause of death in patients with end-stage renal disease [Citation6]. Serum potassium (sK+) level plays a critical role in dialysis patient management and is closely linked to sudden cardiac death [Citation7]. Previous studies have shown that both hyperkalemia and hypokalemia are associated with higher mortality rates in maintenance hemodialysis patients [Citation8–11]. Besides, although the concentrations of dialysate potassium (DK) were not consistent among different countries [Citation12], DK concentrations used are usually lower than those in sK+, leading to a rapid drop in sK+ levels after hemodialysis [Citation13,Citation14]. Patient-level evidence showed that lasting hypokalemia after dialysis caused higher mortality rate in patients undergoing chronic hemodialysis or peritoneal dialysis [Citation15,Citation16].
The role of sK+ levels and patient-level sK+ variability have been well documented before [Citation8,Citation10,Citation15,Citation17]. Not only the sK+ level, but also the sK+ variability was associated with clinical outcomes of dialysis patients. A previous study in peritoneal dialysis patients has shown that higher sK+ variability had significantly increased all-cause and cardiovascular death [Citation17]. Another study involved 34,167 patients in the United States demonstrated that higher sK+ variability was associated with higher all-cause death after adjusting potential confounders [Citation18]. However, previous studies have focused mainly on patient-level sK+ variability. The management practices of different dialysis centers may significantly impact patients. Facility-level variability of laboratory parameters can reflect not only the variability among different patients but also the management practices of different centers. Pisoni et al. reported a strong and positive association between facility-level variability of hemoglobin and patient mortality based on the Dialysis Outcomes and Practice Patterns Study (DOPPS) [Citation19]. Therefore, adopting facility practices that ensure tighter control of anemia may enhance the survival rates of patients undergoing hemodialysis.
Currently, no study has evaluated the effect of facility-level sK+ variability (FL-SPV) on mortality in hemodialysis patients. Therefore, this study utilized the data of DOPPS phase 5, and aimed to investigate the associations between FL-SPV and patients’ outcomes, as well as the factors influencing the FL-SPV. Our results will uncover the role of FL-SPV, which may improve practice patterns related to sK+ management.
Methods
Study design
The DOPPS is an international prospective cohort study of adult patients undergoing hemodialysis. China participated in DOPPS phase 5 (2012–2015). Forty-five dialysis centers were randomly selected in Beijing, Shanghai, and Guangzhou (15 centers in each city), each with 20–40 patients (an average of 30 patients). Eligible patients were 18 years or older and had undergone regular hemodialysis for over three months. Detailed study design and patients’ baseline characteristics have been published before [Citation20].
Calculation of FL-SPV and grouping
The sK+ level was determined before dialysis sessions in accordance with the study protocol. The mean and standard deviation (SD) of baseline sK+ were calculated for patients in each of the 45 hemodialysis centers, and the SD value was defined as the FL-SPV value of each center and corresponding patients. Therefore, patients within the same hemodialysis center had the same FL-SPV values. The mean value of FL-SPV of all the participants was calculated as the cutoff value for grouping. Patients with FL-SPV values above the mean value were categorized into the high FL-SPV group, while those with FL-SPV values equal to or below the mean value were categorized into the low FL-SPV group. Mortality rates between the two groups were compared to elucidate the effect of FL-SPV on outcomes.
Data collection and outcomes
Information including demographics (age, sex, dialysis vintage, and body mass index (BMI)), primary kidney diseases, pre-dialysis laboratory values (hemoglobin, albumin, white blood cells, creatine, sK+, and serum HCO3–), dialysis prescription, and comorbidities (hypertension, diabetes, coronary artery diseases, congestive heart failure, other cardiovascular diseases, cerebrovascular diseases, hepatitis, peripheral vascular diseases, lung diseases, cancer (non-skin), gastrointestinal bleeding, and liver cirrhosis) were collected. The primary outcome of this study was all-cause death, and the secondary outcome was cardiovascular death. The date, place, and the primary and secondary causes of death were recorded. Causes of death were categorized as cardiovascular, infectious, metabolic, hepatic, gastrointestinal, other, or unknown. Death resulting from any reason was defined as all-cause death. Death with the following primary causes were defined as cardiovascular death: atherosclerotic heart disease, cardiac arrest, arrhythmia, cardiomyopathy, cerebrovascular accident (including intracranial hemorrhage), congestive heart failure, hemorrhage from ruptured vascular aneurysm, ischemic brain damage or anoxic encephalopathy, acute myocardial infarction, pulmonary embolism, stroke, and heart valve diseases. Survival/cardiovascular survival was defined as time from study entry to all-cause death/cardiovascular death or censored at the end of study. Patients lost to follow-up were censored at the date of last follow-up.
Statistical analysis
After conducting the Shapiro–Wilk test, continuous variables with normal distributions were expressed as mean and SD. For continuous variables with skewed distributions, median (interquartile range (IQR)) was used. Categorical variables were presented as numbers and percentages, and compared using the chi-square test or Fisher’s exact test. For variables with missing data of >5%, multiple imputation was used (variables imputed in this study including standardized Kt/V [stdKtV], albumin, BMI, and intradialytic weight loss). Univariate and multivariate logistic regression models were utilized to determine the factors associated with high FL-SPV. The variables analyzed included demographics, primary kidney diseases, pre-dialysis laboratory values, dialysis prescription, and comorbidities. Multivariate logistic regression analyses were performed using forward regression, and included variables with p < .10 in the univariate analysis. Survival analysis was conducted using the Kaplan–Meier method, and the log-rank test was used to compare the two groups. One unadjusted (model 1) and five adjusted Cox regression models were used to analyze factors associated with all-cause death and cardiovascular death. All Cox models accounted for facility clustering effects using the robust sandwich covariance estimate. The adjusted covariates included in model 2 were age, sex, BMI, and dialysis vintage; model 3: model 2 plus diabetes, coronary artery disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, hepatitis, cancer (non-skin), peripheral vascular disease, lung disease, hypertension, psychiatric disorder, gastrointestinal bleeding, recurrent cellulitis, fracture, and neurologic disease; model 4: model 3 plus hemoglobin, albumin, white blood cells, and serum creatinine; model 5: model 4 plus intradialytic weight loss, fistula use, primary kidney disease, standardized Kt/V, and urine output > 200 mL/day; model 6: model 5 plus mean facility-level sK+, the percentage of patients’ treatment time <240 min, the percentage of patients using high-flux dialyzer, the percentage of patients using prescribed DK of <2.5 mmol/L, and facility patient number. The hazard ratios (HRs) and 95% confidence intervals (CIs) of high FL-SPV in the six Cox models were calculated and plotted. Two-sided p < .05 was considered statistically significant. The statistical analyses were conducted using SPSS 22.0 (IBM Corp., Armonk, NY) and SAS 9.4 (SAS Institute, Cary, NC), and the figures were generated using GraphPad Prism 8 (GraphPad Prism Software Inc., San Diego, CA) and R 4.1.2 (RStudio Inc., Boston, MA).
Results
Baseline characteristics
A total of 1427 hemodialysis patients were included in the China’s DOPPS phase 5. Among them, 88 patients without sK+ data were excluded, and finally 1339 patients were included in this study. The median age of patients was 60 (range, 50–71) years old, and 54.5% of them were male. The majority (960/1339, 71.7%) of the patients used dialysate with potassium concentrations of <2.5 mmol/L.
The FL-SPV of the 45 centers was 0.800 ± 0.145 mmol/L. Using 0.800 mmol/L as the cutoff value, 23 dialysis centers with 656 patients were placed in the low FL-SPV group, and 22 dialysis centers with 683 patients were in the high FL-SPV group. Patients in the high FL-SPV group showed shorter dialysis vintage (median, 2.14 vs. 3.30 years, p < .001), more patients with urine output >200 mL/day (35.6% vs. 29.8%, p = .026), fewer patients with primary kidney disease of glomerulonephritis (34.4% vs. 43.1%, p < .001), fewer patients with hemoglobin <9 g/dL (74.8% vs. 81.6%, p = .003), fewer patients with normal sK+ (3.5 ≤ sK+ < 5.5 mmol/L, 65.4% vs. 76.7%, p < .001), lower serum HCO3– level (median, 21.00 vs. 21.90 mmol/L, p < .001), fewer patients with stdKtV ≥ 2.0 (56.8% vs. 67.0%, p = .007), more patients received dialysis less than 3 times per week (28.0% vs. 14.1%, p < .001), fewer patients with coronary artery disease (23.8% vs. 28.6%, p = .047) or other cardiovascular disease (17.2% vs. 26.2%, p < .001), and more patients with hypertension (89.4% vs. 84.5%, p = .007) or liver cirrhosis (1.9% vs. 0.6%, p = .035) than the low FL-SPV group. Detailed baseline characteristics are presented in .
Table 1. Baseline characteristics of hemodialysis patients.
Primary and secondary outcomes
A total of 200 (14.9%) patients died during the study. The most common cause of death was cardiovascular diseases (52.5%), followed by infections (19.5%) (). Seventy-six (11.6%) patients in the low FL-SPV group and 124 (18.2%) in the high FL-SPV group died (p = .001). Besides, 38 (5.8%) patients in the low FL-SPV group and 67 (9.8%) in the high FL-SPV group experienced cardiovascular death, respectively (). Patients in the high FL-SPV group had a higher risk of all-cause death (log-rank p < .001) and cardiovascular death (log-rank p = .003) ().
Figure 1. Kaplan–Meier’s curves of survival data (A) all-cause mortality; (B) cardiovascular mortality. FL-SPV: facility-level serum potassium variability. High FL-SPV: >0.800 mmol/L; low FL-SPV: ≤0.800 mmol/L.
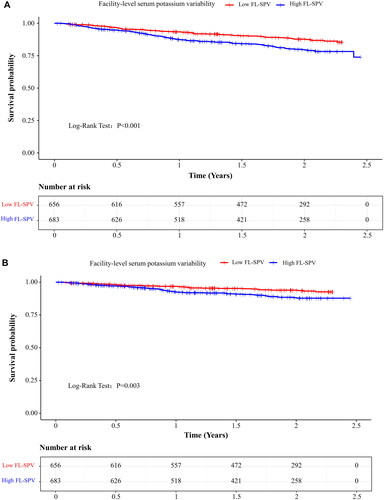
Table 2. Causes of death.
Association between baseline characteristics and high FL-SPV
The multivariate logistic regression analyses revealed that liver cirrhosis (odds ratio (OR) = 4.682, 95% CI: 1.246–17.593, p = .022), baseline sK+ (<3.5 vs. 3.5 ≤ sK+ < 5.5 mmol/L, OR = 2.394, 95% CI: 1.095–5.234, p = .029; ≥5.5 vs. 3.5 ≤ sK+ < 5.5 mmol/L, OR = 1.451, 95% CI: 1.087–1.939, p = .012), dialysis less than 3 times per week (OR = 1.472, 95% CI: 1.073–2.020, p = .017), number of patients in the dialysis center (OR = 1.088, 95% CI: 1.058–1.119, p < .001), serum HCO3– level (OR = 0.952, 95% CI: 0.921–0.984, p = .003), dialysis vintage (OR = 0.919, 95% CI: 0.888–0.950, p < .001), other cardiovascular disease (OR = 0.508, 95% CI: 0.369–0.700, p < .001), and using high-flux dialyzer (OR = 0.425, 95% CI: 0.250–0.724, p = .002) were independently associated with high FL-SPV ().
Table 3. Univariate and multivariate analyses for factors of high FL-SPV (>0.800 mmol/L).
Association between FL-SPV and survival
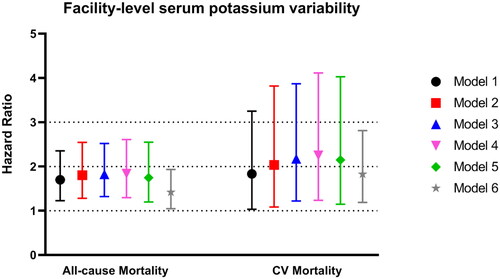
High FL-SPV was associated with unadjusted all-cause death (HR = 1.698, 95% CI: 1.225–2.353) and cardiovascular death (HR = 1.833, 95% CI: 1.031–3.250), as shown in Cox regression model 1. After adjusting for potential confounders, all the lower limits of the 95% CIs of the HRs of high FL-SPV in models 2–6 were above 1, indicating that high FL-SPV was an independently factor associated with all-cause death and cardiovascular death (). Specifically, after adjusting for all analyzed confounding factors in model 6, high FL-SPV was associated with higher risks of all-cause death (HR = 1.420, 95% CI: 1.044–1.933) and cardiovascular death (HR = 1.827, 95% CI: 1.188–2.810).
Figure 2. Associations between high facility-level serum potassium variability (>0.800 mmol/L) and all-cause mortality and cardiovascular mortality in one unadjusted and five adjusted Cox regression models. The points represent the hazard ratio values, and the bars represent the upper and lower limits of the 95% confidence intervals. Factors adjusted in the models: model 1: unadjusted; model 2: age, sex, body mass index, and dialysis vintage; model 3: model 2 plus diabetes, coronary artery disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, hepatitis, cancer (non-skin), peripheral vascular disease, lung disease, hypertension, psychiatric disorder, gastrointestinal bleeding, recurrent cellulitis, fracture, and neurologic disease; model 4: model 3 plus hemoglobin, albumin, white blood cells, and serum creatinine; model 5: model 4 plus intradialytic weight loss, fistula use, primary kidney disease, standardized Kt/V, and urine output > 200 mL/day; model 6: model 5 plus mean facility-level sK+ concentration, the percentage of patients’ treatment time <240 min, the percentage of patients using high-flux dialyzer, the percentage of patients using prescribed dialysate potassium < 2.5 mmol/L, and facility patients’ number.
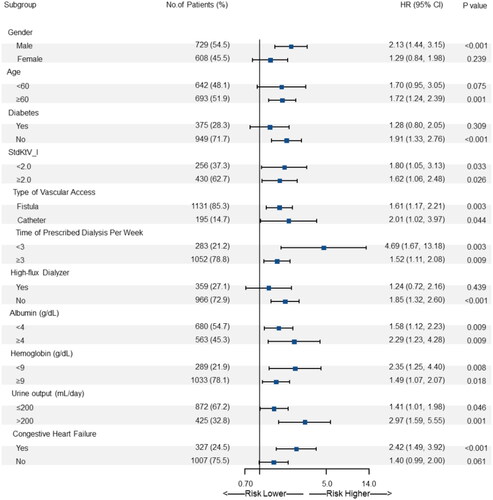
Subgroup analysis revealed that high FL-SPV was associated with a higher risk of all-cause death across all the subgroups defined according to baseline patient characteristics ().
Discussion
To the best of our knowledge, this is the first study to investigate the association between FL-SPV and clinical outcomes in hemodialysis patients. Our findings show that the all-cause and cardiovascular mortality rates among Chinese hemodialysis patients were 14.9% and 7.8%, respectively, with cardiovascular disease being the leading cause of death. High FL-SPV was found to be an independent risk factor for all-cause and cardiovascular mortality, with the high FL-SPV group having higher mortality rates than the low FL-SPV group in both the total population and most subgroups. These results suggest that optimizing the management of sK+ levels and reducing FL-SPV could potentially improve the prognosis of hemodialysis patients.
Baseline sK+ level, either higher (OR = 1.688, p = .003) or lower (OR = 2.176, p = .029) than the normal range, was a risk factor for higher FL-SPV. In other words, optimizing patient-level sK+ within the normal range can reduce FL-SPV. Moreover, serum HCO3– level was independently associated with FL-SPV. A lower serum HCO3– level indicates metabolic acidosis, which is highly connected to hyperkalemia. Hence, physicians are suggested to pay attention to the metabolic status of the patients so as to control FL-SPV. A previous study has demonstrated that time-averaged sK+ level was able to serve as a biomarker for evaluating nutritional status [Citation21]. The univariate analysis showed that hemoglobin had a protective effect on FL-SPV (OR = 0.910, p = .001), but it was not statistically significant in multivariate analysis. In fact, the association between hemoglobin and sK+ remains controversial. A recent cross-sectional study reported a positive correlation between sK+ and hemoglobin in 1652 patients on hemodialysis [Citation22]. In contrast, a prospective study showed that CKD patients with higher sK+ had lower hemoglobin levels [Citation23]. It is possible that low hemoglobin levels are indicative of overall poor patient condition such as malnutrition, and blood potassium levels may not be in the optimal range. Therefore, future studies with a larger sample size are needed to determine the correlation between hemoglobin and FL-SPV. Additionally, liver cirrhosis and primary kidney disease of hypertensive nephropathy were found to be closely related to FL-SPV. There is evidence that liver cirrhosis may result in low platelet counts, and hemodialysis patients with thrombocytopenia have an increased risk of dying from any cause [Citation4]. However, the reason why liver cirrhosis was associated with high sK+ variability remains unclear. All these above-mentioned factors may lead to poor control of patient sK+ levels in the individual-level, and thus resulting in higher inter-patient sK+ variability. Furthermore, patients in the same center often use the same dialysis prescription, such as dialysate with the same concentration of potassium. The majority (71.6%) of participants in this study used dialysate with potassium concentrations of <2.5 mmol/L, and the proportion was similar between high FL-SPV and low FL-SPV groups. Thus, this was not included in analysis of factors associated with FL-SPV. In recent years, researchers have been considering using two or three dialysate formula or individualized dialysate to improve patient outcomes [Citation24,Citation25]. Therefore, future studies could explore the association between DK concentration and FL-SPV. Finally, using high-flux dialyzer, is an independent protective factor for FL-SPV (OR = 0.425, p = .002), probably due to higher dialysis adequacy for the patients.
Thrice-weekly hemodialysis has been the standard renal replacement therapy for decades. Nevertheless, due to economic burden, the prevalence of twice-weekly hemodialysis is high in developing countries, including China [Citation26–28]. In this study, 21.2% of the patients received dialysis less than three times per week. A previous study conducted in China showed that hemodialysis performed twice weekly may increase the risk of mortality compared to thrice-weekly hemodialysis, with an HR of 4.26 [Citation28]. In contrast, most observational studies have demonstrated no significant differences in survival and hospitalization between twice- and thrice-weekly dialysis [Citation26,Citation29,Citation30]. The inconsistency across studies could be attributed to patient selection. Still, inter-dialytic intervals may be related to certain clinical or laboratory indicators. An observational study demonstrated that in patients receiving twice-weekly hemodialysis, volume status was worse than for those receiving thrice-weekly hemodialysis, especially in those with long-term dialysis experience [Citation27]. According to a recent system review, hyperkalemia is closely related to long inter-dialysis intervals [Citation31]. These results uncovered the relationship between sK+ levels and intervals of hemodialysis. In our study, we found that dialysis less than three times per week is dependently associated with high FL-SPV, which suggested that the prescription of hemodialysis in different centers may impact the FL-SPV. Patients receiving twice-weekly hemodialysis may need more intensive monitoring of sK+ levels, indicating that dialysis facilities should strengthen patient management.
The 2016 annual data report of China kidney disease network revealed that about half of patients receiving dialysis have cardiovascular disease. Inpatients with CKD were most likely to suffer from coronary heart disease, followed by heart failure, stroke, and atrial fibrillation [Citation2]. Cardiovascular death in dialysis patients accounted for more than half of deaths in this study. The DIET-HD study showed that among 2163 deaths of patients from hemodialysis, 826 (38%) died from cardiovascular disease [Citation32]. In another prospective cohort study, 958 of 2087 deaths were caused by cardiovascular causes [Citation33]. These studies reported that cardiovascular death was the most common cause of death in dialysis patients, consistent with our results. The sK+ level is closely associated with sudden cardiac arrest and cardiovascular death, which requires special attention in management of dialysis patients. The second most common cause of death was infection. Bloodstream infections are significantly associated with morbidity, hospitalization, and death in hemodialysis patients [Citation34]. Therefore, more attention should be paid to the prevention and treatment of all kinds of infection.
The relationship between intra-patient sK+ variability and clinical outcomes in dialysis patients has been reported previously. Dashputre et al. found that high sK+ variability was associated with increased all-cause mortality (HR = 1.14, 95% CI: 1.03–1.25) [Citation18]. Another prospective study involving 886 Chinese patients who received peritoneal dialysis demonstrated a close relationship between intra-patient sK+ variability and all-cause mortality as well as cardiovascular death [Citation17]. Li et al. revealed that higher sK+ fluctuation was significantly associated with an increase in all-cause and cardiovascular mortality [Citation21]. A large-scale cohort study of 81,013 hemodialysis patients reported that an sK+ level of 4.6–5.3 mEq/L was associated with the lowest risk of death [Citation8]. The importance of hyperkalemia as an individual risk factor that can lead to life-threatening conditions has been studied. However, managing potassium variability to a low level is equally crucial in improving the survival of the general patient population in a dialysis center. Several studies have demonstrated that high potassium variability is associated with an increased risk of cardiovascular events and mortality in HD patients [Citation17,Citation18,Citation21]. Therefore, it is imperative for the medical staff in a dialysis center to manage potassium variability to a low level, and keep potassium levels within the optimal range, ensuring improved overall patient outcomes.
FL-SPV may not only reflect the sK+ variability among patients, but also among hospitals with different management practices [Citation19]. FL-SPV is expected to be less affected by individual patient health status than a measure based solely on patient variability, thus reducing selection bias [Citation17]. In this study, the total and cardiovascular death rates of the low FL-SPV group were lower than those of high FL-SPV group, and multivariate analysis also indicated that FL-SPV was an independent factor of all-cause mortality and cardiovascular mortality. Furthermore, results from subgroup analysis were consistent with those from the total population. Based on the results, we suggested that manage the sK+ variability at the facility-level in hemodialysis patients can help reduce cardiovascular accidents and prolong survival. As reported by He et al., individualized dialysate reduces the risk of post-dialysis hypokalemia compared to dialysate with a fixed concentration [Citation27]. Further study is needed to determine whether individualized dialysate can improve long-term clinical outcomes. Notably, multiple factors regarding practice pattern, including mean facility-level sK+ concentration, treatment time, the use of high-flux dialyzer, DK concentration (whether <2.5 mmol/L), and facility patient number, were adjusted to analyze the associations between high FL-SPV and patient survival in this study. Hence, we could obtain relatively comprehensive analysis results.
Our study had some limitations. Although this was a prospective study, it was initiated between 2012 and 2015 and certain data were not collected, for instance, post-hemodialysis sK+. Besides, due to incomplete data collection, other factors possibly affecting sK+ level, such as diet and medications (including the use of renin angiotensin aldosterone system inhibitors), were not included in the analysis. Although we determined an association between sK+ variability in the facility-level and clinical outcomes of hemodialysis patients, we were unable to collect data on all management practices for sK+ variability in different centers, so specific measures to reduce FL-SPV require further exploration. In addition, this is a relatively small sample-sized study in this field. Nevertheless, our study provides preliminary insights into the association of FL-SPV and mortality in hemodialysis patients and may impact real-world clinical practice. Finally, future prospective studies may treat the practice patterns of each dialysis center as an explanatory variable, and analyze its impact on patient outcomes.
In conclusion, FL-SPV in hemodialysis patients is associated with all-cause mortality and cardiovascular mortality in hemodialysis patients, and reduce the FL-SPV may improve the prognosis of hemodialysis patients. More attention should be paid to managing sK+ level in dialysis patients and preventing hyperkalemia or hypokalemia. Further research and implementation projects aimed at reducing FL-SPV in hemodialysis centers are needed.
Ethical approval
The study was approved by the Ethics Committee of Peking University People’s Hospital (ethical approval number: 2018PHB028-01). Other participating sub-centers also obtain ethics committee approval documents prior to the start of clinical trials.
Consent form
All patients signed the written informed consent.
Author contributions
Conception and design of research: Xinju Zhao and Li Zuo; analyzed data: Xinju Zhao; interpreted results of experiments: Xinju Zhao and Li Zuo; prepared figures: Xinju Zhao; drafted manuscript: Xinju Zhao; edited and revised manuscript: Liangying Gan, Fan Fan Hou, Xinling Liang, Zhaohui Ni, Xiaonong Chen, Yuqing Chen, and Li Zuo; approved final version of manuscript: Liangying Gan and Li Zuo.
Acknowledgements
The Dialysis Outcomes and Practice Patterns Study (DOPPS) Program in China is supported by Vifor Fresenius Renal Pharma, Sanofi Renal, Nipro Trading (Shanghai) Co., Ltd., 3SBio Inc., B. Braun, and CEMMA MEDICAL.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used of this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):1–10.
- Yang C, Gao B, Zhao X, et al. Executive summary for China Kidney Disease Network (CK-NET) 2016 annual data report. Kidney Int. 2020;98(6):1419–1423.
- Zhao X, Niu Q, Gan L, et al. Blood flow rate: an independent risk factor of mortality in Chinese hemodialysis patients. Semin Dial. 2022;35(3):251–257.
- Zhao X, Niu Q, Gan L, et al. Thrombocytopenia predicts mortality in Chinese hemodialysis patients–an analysis of the China DOPPS. BMC Nephrol. 2022;23(1):11.
- Niu Q, Zhao X, Gan L, et al. Physical function and clinical outcomes in hemodialysis patients: China Dialysis Outcomes and Practice Patterns Study. Kidney Dis. 2021;7(4):315–322.
- Genovesi S, Boriani G, Covic A, et al. Sudden cardiac death in dialysis patients: different causes and management strategies. Nephrol Dial Transplant. 2021;36(3):396–405.
- United States Renal Data System. USRDS annual data report: epidemiology of kidney disease in the United States; 2018. Available from: https://www.usrds.org/adr.aspx
- Kovesdy CP, Regidor DL, Mehrotra R, et al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2(5):999–1007.
- Yusuf AA, Hu Y, Singh B, et al. Serum potassium levels and mortality in hemodialysis patients: a retrospective cohort study. Am J Nephrol. 2016;44(3):179–186.
- Lee S, Kang E, Yoo KD, et al. Lower serum potassium associated with increased mortality in dialysis patients: a nationwide prospective observational cohort study in Korea. PLOS One. 2017;12(3):e0171842.
- Hwang JC, Wang CT, Chen CA, et al. Hypokalemia is associated with increased mortality rate in chronic hemodialysis patients. Blood Purif. 2011;32(4):254–261.
- Karaboyas A, Zee J, Brunelli SM, et al. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2017;69(2):266–277.
- Agar BU, Culleton BF, Fluck R, et al. Potassium kinetics during hemodialysis. Hemodial Int. 2015;19(1):23–32.
- Brunelli SM, Spiegel DM, Du Mond C, et al. Serum-to-dialysate potassium gradient and its association with short-term outcomes in hemodialysis patients. Nephrol Dial Transplant. 2018;33(7):1207–1214.
- Ohnishi T, Kimachi M, Fukuma S, et al. Postdialysis hypokalemia and all-cause mortality in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2019;14(6):873–881.
- Szeto C-C, Chow K-M, Kwan BC-H, et al. Hypokalemia in Chinese peritoneal dialysis patients: prevalence and prognostic implication. Am J Kidney Dis. 2005;46(1):128–135.
- Xu Q, Xu F, Fan L, et al. Serum potassium levels and its variability in incident peritoneal dialysis patients: associations with mortality. PLOS One. 2014;9(1):e86750.
- Dashputre AA, Potukuchi PK, Sumida K, et al. Predialysis potassium variability and postdialysis mortality in patients with advanced CKD. Kidney Int Rep. 2021;6(2):366–380.
- Pisoni RL, Bragg-Gresham JL, Fuller DS, et al. Facility-level interpatient hemoglobin variability in hemodialysis centers participating in the Dialysis Outcomes and Practice Patterns Study (DOPPS): associations with mortality, patient characteristics, and facility practices. Am J Kidney Dis. 2011;57(2):266–275.
- Zhao X, Niu Q, Gan L, et al. Baseline data report of the China Dialysis Outcomes and Practice Patterns Study (DOPPS). Sci Rep. 2021;11(1):873.
- Li SH, Xie JT, Long HB, et al. Time-averaged serum potassium levels and its fluctuation associate with 5-year survival of peritoneal dialysis patients: two-center based study. Sci Rep. 2015;5:15743.
- Yin S, Du Y, Guo Y, et al. Multifactorial analysis of renal anemia-associated substandard hemoglobin levels and prevalence of anemia in patients on maintenance hemodialysis in Liaoning province: a cross-sectional study. Ann Palliat Med. 2022;11(12):3743–3754.
- Wang HH, Hung CC, Hwang DY, et al. Hypokalemia, its contributing factors and renal outcomes in patients with chronic kidney disease. PLOS One. 2013;8(7):e67140.
- Mercadal L, Lambert O, Couchoud C, et al. Prescription patterns of dialysate potassium and potassium binders and survival on haemodialysis—the French Renal Epidemiology and Information Network Registry. Nephrol Dial Transplant. 2021;36(1):151–159.
- He H, Wu J, Lu W, et al. A multicenter study of hemodialysis using individualized dialysate potassium concentrations. Ann Palliat Med. 2021;10(12):12218–12229.
- Lin X, Gu L, Zhu M, et al. Clinical outcome of twice-weekly hemodialysis patients with long-term dialysis vintage. Kidney Blood Press Res. 2018;43(4):1104–1112.
- Fang N, Che M, Shi L, et al. B-type natriuretic peptide levels and volume status in twice-weekly hemodialysis patients. Ren Fail. 2021;43(1):1259–1265.
- Sun Y, Wang Y, Yu W, et al. Association of dose and frequency on the survival of patients on maintenance of hemodialysis in China: a Kaplan–Meier and Cox-proportional hazard model analysis. Med Sci Monit. 2018;24:5329–5337.
- Yan Y, Wang M, Zee J, et al. Twice-weekly hemodialysis and clinical outcomes in the China Dialysis Outcomes and Practice Patterns Study. Kidney Int Rep. 2018;3(4):889–896.
- Panaput T, Thinkhamrop B, Domrongkitchaiporn S, et al. Dialysis dose and risk factors for death among ESRD patients treated with twice-weekly hemodialysis: a prospective cohort study. Blood Purif. 2014;38(3–4):253–262.
- Bem D, Sugrue D, Wilding B, et al. The effect of hyperkalemia and long inter-dialytic interval on morbidity and mortality in patients receiving hemodialysis: a systematic review. Ren Fail. 2021;43(1):241–254.
- Su G, Saglimbene V, Wong G, et al. Healthy lifestyle and mortality among adults receiving hemodialysis: the DIET-HD study. Am J Kidney Dis. 2022;79(5):688–698.e1.
- Saglimbene VM, Wong G, Teixeira-Pinto A, et al. Dietary patterns and mortality in a multinational cohort of adults receiving hemodialysis. Am J Kidney Dis. 2020;75(3):361–372.
- Fisher M, Golestaneh L, Allon M, et al. Prevention of bloodstream infections in patients undergoing hemodialysis. Clin J Am Soc Nephrol. 2020;15(1):132–151.