Abstract
Background
Idiopathic membranous nephropathy (IMN) is the leading cause of nephrotic syndrome in the elderly. The treatment of idiopathic membranous nephropathy is quite challenging due to the particularity of elderly patients. This study intends to investigate the clinicopathological characteristics and initial therapeutic effect of idiopathic membranous nephropathy among elderly patients.
Methods
A retrospective study of 67 elderly patients (58.2% male, median age 69.0 years, range, 65–83 years) with biopsy-proven membranous nephropathy was conducted at Guangdong Provincial People’s Hospital from 2016 to 2020. Data on clinicopathological characteristics and initial therapeutic effects were analyzed.
Results
Of the 67 patients, the mean eGFR of overall patients was 66.49 mL/min/1.73m2 while the median urine protein-to-creatinine ratio (uPCR) and urine albumin-to-creatinine ratio (uACR) was 5676.73 mg/g and 2951.56 mg/g, respectively. Pathological data revealed that the membranous Churg’s stage II was the most frequent (71.64%). Moreover, glomerular PLA2R antigen fluorescence intensity of (+) and IgG4 antigen fluorescence intensity of (++) were detected in 63.6% and 86.4% of all patients, respectively. Overall, 44 patients, accounting for 65.7%, achieved remission including complete remission and partial remission within 1 year after renal biopsy. Compared with a non-remission group, the levels of uPCR (6274.6 vs. 3235.6 mg/g, p = 0.007) and uACR (3433.6 vs. 1773.2 mg/g, p = 0.017) were significantly higher in remission group. The proportion of immunosuppressive therapy in the remission group was also higher (86.4% vs. 30.4%, p < 0.01). Compared with conservative treatment, patients with combined treatment with glucocorticoid and cyclophosphamide (CTX) or glucocorticoid and calcineurin inhibitor (CNIs) achieved higher remission rate (glucocorticoid plus cyclophosphamide vs. conservative treatment, 84.6% vs. 27.3%, p = 0.001; glucocorticoid plus calcineurin inhibitor vs. conservative treatment, 88.0% vs. 27.3%, p < 0.001). Further analysis showed that compared with patients who underwent conservative treatment, the proportion of males, the levels of uPCR, uACR, BUN, Scr, CysC and PLA2R antigen-positive staining rate in kidney biopsy were higher in those who underwent combined treatment with glucocorticoid and CTX, while the levels of eGFR, TP and ALB were lower (p < 0.05). In addition, patients who received combined treatment with glucocorticoid and CNIs had higher levels of uPCR, uACR, TC and lower levels of TP, ALB than those who received conservative treatment (p < 0.05). Moreover, there were no statistically significant differences in the 1-year progression rate in eGFR between the immunosuppressive treatment group and conservative treatment group (3.3 vs. 0.2 ml/min/1.73m2, p = 0.852).
Conclusions
Most elderly patients diagnosed with IMN had multiple comorbidities, and the membranous Churg’s stage II was most common. The glomerular PLA2R and IgG4 antigen deposition were frequently detected accompanied by glomerulosclerosis and severe tubulointerstitial injury. For high-risk elderly patients with severe proteinuria, early initial immunosuppressive therapy could achieve a higher urinary protein remission rate. Therefore, it is crucial for clinicians to balance the risks and benefits of immunosuppressive therapy based on clinical and pathological characteristics and develop individualized treatment regimens for elderly patients with IMN.
Introduction
Membranous Nephropathy (MN) is an autoimmune glomerular disease. Approximately 75% of MN is idiopathic membranous Nephropathy (IMN), and 20–25% of MN is secondary MN caused by systemic diseases such as virus infections, malignant tumors, systemic autoimmune diseases and drugs [Citation1]. With the improvement of economic development level, general health awareness and medical technologies, more and more elderly people receive kidney biopsy. A number of clinical studies in China have shown that primary glomerular disease is most common in the elderly, of which the most common type is idiopathic membranous nephropathy with an increasing trend in the incidence year by year [Citation2–9]. With the progressively increasing older population, ageing has gradually become a prominent public health problem around the world [Citation10,Citation11]. The 2017 Global Burden of Disease Study showed that the growing burden of chronic kidney disease (CKD) is driven mainly by population aging [Citation12].
Most elderly patients with IMN begin with nephrotic syndrome presenting a large amount of proteinuria, edema, hyperlipidemia, hypertension and hypoalbuminemia. Treatment for the elderly with IMN is very difficult because most of them have a variety of underlying diseases, lower immunity, high prevalence of infection and poor medical compliance.
However, there have been few reports about the treatment of elderly patients with IMN [Citation13–19]. This study aimed to better understand clinical and pathological characteristics in elderly Chinese patients and focus on treatment and initial outcomes, which might provide a certain theoretical basis for diagnosis and treatment for elderly patients with IMN.
Materials and methods
Study participations
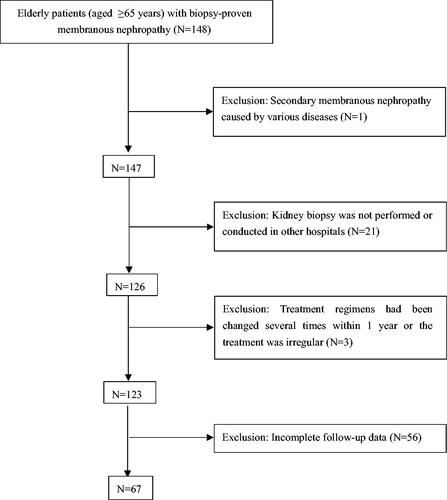
This retrospective study enrolled 67 elderly patients (aged ≥65 years) with biopsy-proven membranous nephropathy in the Department of Nephrology and Geriatric Nephrology of Guangdong Provincial People’s Hospital from 2016 to 2020. Exclusion criteria were as follows:(1) secondary membranous nephropathy caused by hepatitis infection, autoimmune diseases (such as systemic lupus erythematosus, rheumatoid arthritis and Sjögren’s syndrome), malignant tumors, heavy metal poisoning and other diseases; (2) kidney biopsy was not performed or conducted in other hospitals; (3) treatment regimens had been changed several times within 1 year or the treatment was irregular; (4) incomplete follow-up data. displays the entire screening process. This study was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital, registration number KY-Q-2022-193.
Clinical and laboratory data
General information (sex and age) and clinical records (systolic blood pressure [SBP], diastolic blood pressure [DBP]) and multiple comorbidities (including hypertension, diabetes, chronic bronchitis/emphysema, coronary heart disease [CHD], arteriosclerosis, and cerebrovascular disease, etc.) were collected. Laboratory data included urine protein-to-creatinine ratio (uPCR), urinary albumin-to-creatinine ratio (uACR), blood urea nitrogen (BUN), serum creatinine (Scr), total protein (TP), cystatin C (CysC), albumin (ALB), hemoglobin (Hgb), and low-density lipoprotein (LDL), total triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), and uric acid (UA). The estimated glomerular filtration rate (eGFR) was calculated based on the CKD Epidemiological Collaboration (CKD-EPI) equation.
Pathological data
All renal tissue samples were examined by light microscopy, immunofluorescence and electron microscopy examination. On light microscopy, each kidney sample was observed for the total number of glomeruli, glomerular sclerosis, mesangial proliferation, basement membrane thickening, tubular degeneration and atrophy, interstitial fibrosis, interstitial inflammation, etc. Immunofluorescence examination included the type, intensity and site of immunoglobulin, complement and phospholipase A2 receptor (PLA2R) antigen deposition. Electron microscopic detection was used to evaluate the degree of basement membrane thickening and electron-dense deposits.
Glomerular sclerosis (including segmental sclerosis and global sclerosis) was scored semi-quantitatively using a four-grade method 0: no glomerular sclerosis; 1: glomerular sclerosis rate <25%; 2: glomerular sclerosis rate in 25% to 50%; 3: glomerular sclerosis rate >50%.
Evaluation of tubulointerstitial injury consists of four parts: tubular degeneration, tubular atrophy, interstitial fibrosis and interstitial inflammation. Each part was scored 0-1 based on the presence or absence, and the score for each item was summed for a total score. Semi-quantitative scoring of the degree of tubulointerstitial injury was performed: 0, no tubulointerstitial injury; 1-2, mild injury; 3, moderate injury; 4, severe injury.
According to the classification criteria of Ehrenreich and Churg [Citation20], the pathologic stage was classified into stage I, II, III and IV.
The degree of phospholipase A2 receptor (PLA2R) and IgG4 deposition was classified semi-quantitatively according to fluorescence intensity varying from 0 to 5 (−, +/−, +, ++, +++) respectively.
Treatment outcomes
The treatment outcomes were classified as remission (including complete remission and partial remission) and non-remission during at least 1-year follow-up from kidney biopsy. The observation period for non-remission group was at least 12 months, and the longest one was for 24 months. Referring to the 2012 KDIGO guideline [Citation21], complete remission, partial remission and no remission were defined as follows: (1) Complete remission (CR): urinary protein excretion <0.3 g/d (uPCR <300 mg/g or <30 mg/mmol), confirmed by two values at least 1 week apart, accompanied by a normal serum albumin concentration, and normal serum creatinine. (2) Partial remission (PR): urinary protein excretion <3.5 g/d (uPCR <3500 mg/g or <350 mg/mmol) and a 50% or greater reduction from peak values, confirmed by two values at least 1 week apart, accompanied by an improvement or normalization of the serum albumin concentration and stable serum creatinine. (3) No remission (NR): patients did not achieve clinical remission after treatment.
Statistical analysis
All data analysis was performed using the SPSS 26.0 statistical software. Quantitative variables with normal distributions were described as mean ± standard deviation and compared between groups by t test. Moreover, non-normally distributed data were expressed as median (lower quartile, upper quartile) and compared by non-parametric test (Mann–Whitney U test). A non-parametric test (Mann–Whitney U test) was also used for the comparison of grade data. Counting data were expressed as n (%) and were compared by chi square (χ2) test or Fisher exact probability methods. P value <0.05 was considered statistically significant.
Results
General clinical data
A total of 67 patients diagnosed with IMN were enrolled and analyzed in this study. There were 44 patients (65.7%) in the remission group and 23 patients (34.3%) in the non-remission group. A comparison of baseline data between the remission group and non-remission group is shown in . As shown in , of 67 elderly patients, the proportion of males was 58.2% and the median age was 69.0 years. Also, the mean eGFR was 66.49 mL/min/1.73m2 and the median uPCR and uACR were 5676.7 and 2951.6 mg/g, respectively. Compared with non-remission group, the levels of uPCR (6274.6 vs. 3235.6 mg/g, p = 0.007) and uACR (3433.6 vs. 1773.2 mg/g, p = 0.017) were significantly higher in remission group. The proportion of immunosuppressive therapy in the remission group was also higher (86.4% vs. 30.4%, p < 0.01). There were no significant differences in sex, age, the levels of SBP, DBP, eGFR, BUN, Scr, TP, ALB, CysC, TG, TC, LDL, HDL, Hgb and UA between the two groups (p > 0.05). Meanwhile, the differences in the incidence of various comorbidities were not significant between the two groups (p > 0.05).
Table 1. Comparison of baseline data between the remission group and non-remission group.
Pathological data
The remaining pathological data of 67 patients with IMN were retrospectively analyzed for the partial pathological data of 3 patients were missing. In this study, the membranous Churg’s stage II was most common whether in the remission group or non-remission group. Moreover, glomerular PLA2R antigen fluorescence intensity of (+) and IgG4 antigen fluorescence intensity of (+++) were detected in 63.6% and 86.4% of all patients, respectively. All had some degree of tubulointerstitial injury and just over half (53.0%) had severe injury. In addition, glomerulosclerosis involving up to 25% of glomeruli, occurred in 39 patients (59.1%). Overall, it showed no significant differences in the degree of PLA2R antigen deposition, IgG4 deposition and tubulointerstitial injury, the proportion of glomerulosclerosis and pathological stage between the two groups (p > 0.05). The pathological characteristics are available in .
Table 2. Comparison of pathological characteristics between the remission group and non-remission group.
Treatment outcomes
Of 67 elderly patients, 25 (37.3%) were treated with glucocorticoid combined with calcineurin inhibitors (including cyclosporine and tacrolimus) and 22 (32.8%) were treated with conservative therapy (including angiotensin II receptor blocker and angiotensin-converting enzyme inhibitor). At the same time, 13 patients (19.4%) were treated with the combination of glucocorticoid and cyclophosphamide (including intravenous cyclophosphamide and oral cyclophosphamide), and 1 patient (1.5%) was treated with the combination of glucocorticoid and mycophenolate mofetil. During at least one-year follow-up period, 6 patients (9.0%) had changed treatment scheme for only one time because of less effective treatment. Four patients had changed to receive combined glucocorticoid with cyclophosphamide therapy and one had changed to receive combined glucocorticoid and mycophenolate mofetil, who were early treated with combined glucocorticoid and calcineurin inhibitors. Meanwhile, one patient had changed to be treated with the combination of glucocorticoid and mycophenolate mofetil, who was early treated with combined glucocorticoid and cyclophosphamide.
Overall, 44 patients (65.7%) achieved remission by treatment. Three patients (4.5%) achieved complete remission and 41 patients (61.2%) achieved partial remission. Among the pairwise comparison of remission rates between different treatments, patients with combined treatment with glucocorticoid and cyclophosphamide or glucocorticoid and calcineurin inhibitors achieved higher remission rates compared with conservative treatment (glucocorticoid and cyclophosphamide vs. conservative treatment, 84.6% vs. 27.3%, p = 0.001; glucocorticoid and calcineurin inhibitor vs. conservative treatment, 88.0% vs. 27.3%, p < 0.001). In addition, 2 patients (4.4%) achieved complete remission and 36 patients (80.0%) achieved partial remission in the immunosuppressive group. Compared with conservative treatment, patients with immunosuppressive therapy achieved a higher remission rate (immunosuppressive group vs. conservative group, 84.4% vs. 27.3%, p < 0.001). A comparison of remission rates among different treatments is presented in and . Furthermore, there was no significant difference in the eGFR changing between the immunosuppressive treatment group and the conservative treatment group (3.3 vs. 0.2 mL/min/1.73m2, p = 0.852).
Figure 2. Comparison of remission rate in different treatment groups. Data information: In all relevant panels, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, no statistically significant difference. Data were expressed as n (%) and were compared by chi square test or Fisher exact probability methods. GC, glucocorticoid; CTX, cyclophosphamide; CNIs, calcineurin inhibitors; IST, immunosuppressive treatment.
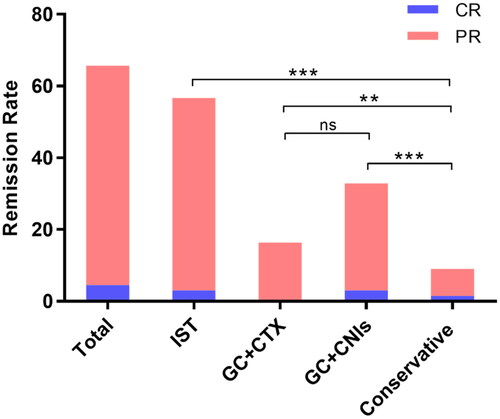
Table 3. Comparison of remission rates among different treatment groups.
The preceding analysis found that the levels of uPCR and uACR in the remission group were significantly higher than non-remission group (both p < 0.05) (See ). Therefore, further investigation was conducted and all patients were divided into three groups: uPCR ≥8000 mg/g (n = 22), 3500 mg/g ≤ uPCR <8000 mg/g (n = 23) and uPCR <3500 mg/g (n = 22), according to the level of uPCR. Likewise, depending on the level of serum albumin, all patients were divided into two groups: ALB > 30 g/L (n = 11) and ALB ≤ 30 g/L (n = 56). shows that the remission rate of patients whether in uPCR ≥8000 mg/g group or in 3500 mg/g ≤ uPCR <8000 mg/g group were significantly higher than those in uPCR <3500 mg/g group (77.3% vs. 36.4%, 82.6% vs. 36.4%, respectively, p = 0.002). Also, the remission rate in ALB ≤ 30 g/L group was significantly higher than those in ALB > 30 g/L group (71.4% vs. 36.4%, p = 0.038). Subsequent analysis revealed that the proportion of immunosuppressive therapy was significantly higher in uPCR ≥8000 mg/g group and 3500 mg/g ≤ uPCR < 8000 mg/g group compared with uPCR <3500 mg/g group (respectively, 90.9% and 87.0% vs. 22.7%, p < 0.001), as well higher in ALB > 30 g/L group than in ALB ≤ 30 g/L group (78.6% vs. 9.1%, p < 0.001). See for details.
Table 4. Comparison of remission rate in different proteinuria and albumin levels.
In this study, clinical and pathological data among three groups (including GC + CTX group, GC + CNIs group and conservative treatment group) were collected for further analysis. The data of other treatment schemes were not analyzed because of the small sample size. Compared with patients who underwent conservative treatment, the proportion of males, the levels of uPCR, uACR, BUN, Scr, CysC and PLA2R antigen-positive staining rate in kidney biopsy were higher in those who underwent combined treatment with glucocorticoid and cyclophosphamide, while the levels of eGFR, TP and ALB were lower (p < 0.05). There were no significant differences in age, SBP, DBP, TG, TC, LDL, HDL, HGB and UA between the two groups (p > 0.05). In addition, patients who received combined treatment with glucocorticoid and calcineurin inhibitors had higher levels of uPCR, uACR, TC and lower levels of TP, ALB than those who received conservative treatment (p < 0.05). There were no significant differences in gender, age, SBP, DBP, eGFR, BUN, Scr, CysC, TG, LDL, HDL, HGB, UA and PLA2R antigen-positive staining rate (p > 0.05). There were no significant differences in the incidence of comorbidities, the degree of IgG4 deposition, tubulointerstitial injury, glomerulosclerosis and pathological stage among the three groups (p > 0.05). A comparison of clinicopathological features among the three groups is available in . Furthermore, there were significant differences in PCR, ACR, ALB before and after treatment among the immunosuppressive group, GC + CTX group and GC + CNIs group, nevertheless, no significant difference in the conservative group (p < 0.01). There were no significant differences in eGFR before and after treatment among all groups (p > 0.05). See for details.
Figure 3. Comparison of PCR, ACR, ALB and eGFR before and after treatment. Data information: (A): comparison of PCR before and after treatment; (B): comparison of ACR before and after treatment; (C): comparison of ALB before and after treatment; (D): comparison of eGFR before and after treatment. In all relevant panels, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, no statistically significant difference. Data are presented as median (lower quartile, upper quartile) and compared by non-parametric test (Mann-Whitney U test). GC, glucocorticoid; CTX, cyclophosphamide; CNIs, calcineurin inhibitors; IST, immunosuppressive treatment.
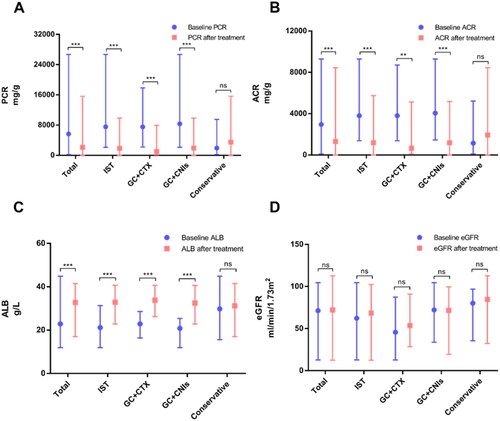
Table 5. Comparison of clinicopathological features among different treatment groups.
Discussion
Membranous Nephropathy (MN) is one of the main causes of nephrotic syndrome in adults, which is characterized by the deposition of subepithelial immune complex and diffuse thickening of the glomerular basement membrane [Citation22]. The clinical presentation in elderly patients is similar to younger adults, edema being the presenting symptom in most patients because of the reduced elasticity of the skin and interstitial tissues [Citation23]. Most elderly patients with IMN presented clinically typical nephrotic syndrome characterized by massive proteinuria, hypoalbuminemia and hyperlipidemia. According to previous data, between 65% and 87% of elderly patients with membranous nephropathy presented with nephrotic syndrome [Citation19,Citation24,Citation25]. Meanwhile, they had a variety of comorbidities including hypertension, diabetes, coronary heart disease, cerebrovascular disease, etc. Compared to younger patients, elderly patients exhibited a higher frequency of diabetes and hypertension [Citation13,Citation16].
The majority of patients had unknown aetiology and were diagnosed with idiopathic membranous nephropathy (IMN). With the progressively increasing older population, ageing has gradually become a prominent public health problem around the world. The 2017 Global Burden of Disease Study showed that the growing burden of chronic kidney disease(CKD) might be driven mainly by population ageing [Citation12]. Therefore, this study paid attention to Chinese elderly patients with IMN, especially their pathological characteristics and initial treatment. In this study, a total of 67 patients with IMN were enrolled, which had a median age of 69.0 years with a 1.39:1 male-to-female ratio. The mean eGFR was 66.49 mL/min/1.73m2 and the median of uPCR and uACR was 5676.7 and 2951.6 mg/g, respectively. Totally, 44 patients, accounting for 65.7% achieved remission including 4.5% complete remission and 61.2% partial remission within 1-year follow-up after renal biopsy.
As is well known, the mechanism of IMN is still not clear. Beck et al. [Citation26] first reported that phospholipase A2 Receptor (PLA2R) is the major antigen of idiopathic membranous nephropathy in 2009, which is detected in 70% to 80% of patients with IMN. It is rarely found in patients with secondary membranous nephropathy or other glomerular disease [Citation27]. In this study, of 67 elderly patients with IMN, the PLA2R antigen-positive staining rate in renal tissue accounted for 80.3%, which is similar to previous studies. It also showed that IgG4 deposition was positive in 97% of patients and the proportion of patients with fluorescence intensity (+++) was high at 86.4%. IgG4 deposition was found to be negative or weakly positive in only two patients. PLA2R can locate on normal podocytes and at the same time, it can co-locate with IgG4 and then form finely granular immune deposits on the glomerular basement membrane and surrounding vascular wall in patients with IMN. Deposition of IgG4 under immunofluorescence examination is commonly found in patients with IMN [Citation28], which is also seen in a few patients with IgG4-related renal diseases. Deposition of IgG1, IgG2, or IgG3 is more common in secondary MN [Citation29]. The deposition of IgG4 in IMN is mainly related to the humoral immune response mediated by Th2 cells, leading to the increase of IgG4 in B cells [Citation30]. It suggests that the deposition of PLA2R and IgG4 in renal tissue can be used to identify idiopathic membranous nephropathy and secondary membranous nephropathy.
Due to older age and a range of comorbidities, elderly patients tend to develop glomerulosclerosis, tubular degeneration, tubular atrophy, interstitial fibrosis and other lesions in renal tissue. In 2019, Maria J Stangou et al. [Citation31] proposed that glomerulosclerosis and tubulointerstitial injury were positively correlated with the severity of proteinuria in patients with primary membranous nephropathy (PMN), which were independent factors of renal impairment. In our work, the pathological stages of all patients were mainly stage II and all patients had tubulointerstitial injury demonstrated as tubular degeneration, tubular atrophy, interstitial fibrosis and interstitial inflammation. Severe tubulointerstitial injury and a percentage of glomerular sclerosis ranging from 0% to 25% were found in more than 50% of patients. Meanwhile, they also found that patients diagnosed as PMN with glomerulosclerosis and tubulointerstitial injury benefited from immunosuppressive treatment compared with those who did not receive immunosuppressive therapy, and this beneficial effect was stable during long-term follow-up. Similarly, in our study, elderly patients with glomerulosclerosis and tubulointerstitial injury could achieve a higher urinary protein remission rate and benefit from early initial immunosuppressive treatment. Thus, they presented a novel, simplified and comprehensive FSTIV score based on optical microscopic observation, which was defined as focal segmental sclerosis (FSGS), tubular atrophy (TA), interstitial fibrosis (IF) and vascular hyalinosis (VH). In addition, previous studies suggested that age over 60 years, low serum albumin concentration and severe tubulointerstitial injury are independent risk factors for progressing to end-stage renal disease (ESRD) in patients with IMN [Citation32]. To conclude, this suggests that FSTIV score could be used to preliminarily judge the severity of the disease, and formulate appropriate treatment plans for elderly patients with IMN on the basis of optical microscopic examination of renal biopsy, so as to delay the progression to ESRD.
It is generally known that the natural course of idiopathic membranous nephropathy varies from person to person. About 1/3 of patients achieve spontaneous remission [Citation33], and 1/3 of patients have persistent proteinuria but stable renal function. However, approximately 1/3 of patients progressed to ESRD within 5 to 10 years [Citation34]. Patients with proteinuria <3.5 g/d and normal eGFR are evaluated at low risk of progressive renal impairment and do not need to receive immunosuppressants because of a spontaneous response rate of 30% to 40% and side effects of immunosuppressive therapy. But immunosuppressive treatment should be considered in patients with a moderate and high risk of progressive renal impairment. Treatment plans for idiopathic membranous nephropathy include conservative therapy, various immunosuppressants and rituximab, a novel anti-CD20 antigen monoclonal antibody [Citation35]. In general, elderly patients tend to prefer conservative treatment as they have poor immunity and multiple comorbidities while immunosuppressive therapy has its side effects and expensive medical expenditures, long-term monitoring and management. The present study found that patients with higher proteinuria and lower serum albumin had a higher remission rate because of a higher immunosuppressive treatment rate (see and ). Further analysis showed that patients with combined treatment with glucocorticoid and cyclophosphamide or glucocorticoid and calcineurin inhibitor achieved higher remission rates and had higher levels of uPCR, uACR as well as lower levels of TP, ALB compared with conservative treatment (see and ]. Meanwhile, there was no statistically significant difference in the 1-year progression rate in eGFR between the immunosuppressive treatment group and the conservative treatment group. Similarly, a nationwide cohort study in Japan reported that early immunosuppressive therapy achieved higher remission rates in elderly patients with IMN [Citation17]. Remission of proteinuria not only improves the quality of life but also is a good predictor of long-term prognosis [Citation36–38]. It reveals that we could induce the remission of proteinuria in the early stage and improve long-term prognosis in elderly patients with IMN. However, on the one hand, it has been documented that immunosuppressive therapy increases the risk of infection in elderly patients and conservative treatment is recommended for those with a high risk of infection and with limited life expectancy [Citation19]. On the other hand, older patients with IMN have distinct clinical courses and responses to numerous immunosuppressive drugs so the treatment modality is particularly important.
KDIGO guideline did not recommend that patients with MN received glucocorticoid alone as it has limited therapeutic effect in older patients and is associated with higher complication rates including sodium retention with edema, hypertension and worse infection [Citation13,Citation18]. Conversely, the Japanese evidence-based clinical practice guideline for nephrotic syndrome 2020 [Citation39] suggested that corticosteroid monotherapy could be one of the first-line therapies of MN. Other initial treatments involved combination treatment with corticosteroids and immunosuppressive agents, mizoribine, or adrenocorticotropic hormone (ACTH). 2021 KDIGO guideline [Citation35] stated that rituximab and calcineurin inhibitors can increase the complete and partial remission rate and are preferred as initial treatment in patients with MN and maintained kidney function because of their beneficial side-effect profile. It is proven that only alkylating agents can prevent kidney failure in patients with reduced eGFR even though they had more-frequent and more-severe effects than rituximab or CNIs. As for alkylating agents, cyclophosphamide is preferred over chlorambucil as RCT and several cohort studies suggested fewer side effects with cyclophosphamide. There is moderate-quality evidence that compared with CNIs, rituximab could achieve more persistent remissions [Citation40] while the use of CNIs is at risk of a high relapse rate. Previous cohort study with a longer follow-up period in Korea showed that a combination of corticosteroids and cyclophosphamide in elderly patients with MN had a similar remission rate and fewer side-effects (including mortality, renal outcomes and infections) compared with other immunosuppressive treatments [Citation13]. Currently, there are not a lot of studies on the comparison of different immunosuppressive agents in the elderly. Overall, immunosuppressive therapy acts as a double-edged sword that can induce remission of proteinuria to delay renal function progression in elderly patients with IMN, nevertheless, it can lead to short-term and long-term side effects.
There are several shortages in this study. Firstly, this was single-center retrospective study with small amounts of patients and a short follow-up period. Secondly, due to limited follow-up data, treatment including therapeutic dosing and usage may be not sufficiently detailed. In the future, we will increase the sample size and promote multi-center cooperation as well as necessary larger prospective studies.
Conclusion
Most elderly patients diagnosed with IMN had multiple comorbidities, and the membranous Churg’s stage II was most common. The glomerular PLA2R and IgG4 antigen deposition were frequently detected accompanied by glomerulosclerosis and severe tubulointerstitial injury. For high-risk elderly patients with severe proteinuria, early initial immunosuppressive therapy could achieve higher urinary protein remission rate. Therefore, it is crucial for clinicians to balance the risks and benefits of immunosuppressive therapy based on clinical and pathological characteristics and develop individualized treatment regimen for elderly patients with IMN.
Ethical approval
All participants gave written informed consent. This study was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital, registration number KY-Q-2022-193-01.
Author contributions
W. Liu, W. Hao, and W. Hu designed and conducted the study. J. Liang and F. Xia collected analyzed the data. Z. Zhao and Y Wu verified the data. F. Yu and X. Fang assisted in interpreting the data. All authors critically edited the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download PDF (111.8 KB)Acknowledgment
The authors thank all patients for their participation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sinico RA, Mezzina N, Trezzi B, et al. Immunology of membranous nephropathy: from animal models to humans. Clin Exp Immunol. 2016;183(2):1–11.
- Zhou Q, Yang X, Wang M, et al. Changes in the diagnosis of glomerular diseases in east China: a 15-year renal biopsy study. Ren Fail. 2018;40(1):657–664.
- Tang L, Yao J, Kong X, et al. Increasing prevalence of membranous nephropathy in patients with primary glomerular diseases: a cross-sectional study in China. Nephrology (Carlton). 2017;22(2):168–173.
- Xu X, Ning Y, Shang W, et al. Analysis of 4931 renal biopsy data in Central China from 1994 to 2014. Ren Fail. 2016;38(7):1021–1030.
- Jin B, Zeng C, Ge Y, et al. The spectrum of biopsy-proven kidney diseases in elderly chinese patients. Nephrol Dial Transplant. 2014;29(12):2251–2259.
- Nie P, Lou Y, Wang Y, et al. Clinical and pathological analysis of renal biopsies of elderly patients in northeast China: a single-center study. Ren Fail. 2021;43(1):851–859.
- Pan X, Xu J, Ren H, et al. Changing spectrum of biopsy-proven primary glomerular diseases over the past 15 years: a single-center study in China. Contributions to Nephrology. 2013;181:22–30.
- Hou JH, Zhu HX, Zhou ML, et al. Changes in the spectrum of kidney diseases: an analysis of 40,759 Biopsy-Proven cases from 2003 to 2014 in China. Kidney Dis (Basel). 2018;4(1):10–19.
- Hu R, Quan S, Wang Y, et al. Spectrum of biopsy proven renal diseases in Central China: a 10-year retrospective study based on 34,630 cases. Sci Rep. 2020;10(1):10994.
- Cybulski M, Cybulski L, Krajewska-Kulak E, et al. Illness acceptance, pain perception and expectations for physicians of the elderly in Poland. BMC Geriatr. 2017;17(1):46.
- Tonelli M, Riella MC. World kidney day 2014: CKD and the aging population. Am J Kidney Dis. 2014;63(3):349–353.
- Fraser SDS, Roderick PJ. Kidney disease in the global burden of disease study 2017. Nat Rev Nephrol. 2019;15(4):193–194.
- Bae E, Lee SW, Park S, et al. Treatment and clinical outcomes of elderly idiopathic membranous nephropathy: a multicenter cohort study in korea. Arch Gerontol Geriatr. 2018;76:175–181.
- Zhang XD, Cui Z, Zhang MF, et al. Clinical implications of pathological features of primary membranous nephropathy. BMC Nephrol. 2018;19(1):215.
- Kim Y, Yoon HE, Chung BH, et al. Clinical outcomes and effects of treatment in older patients with idiopathic membranous nephropathy. Korean J Intern Med. 2019;34(5):1091–1099.
- Lin C, Zheng D, Wang Y, et al. Clinical and pathological features of idiopathic membranous nephropathy in young people. Nephrology (Carlton). 2019;24(6):599–604.
- Yokoyama H, Yamamoto R, Imai E, et al. Better remission rates in elderly japanese patients with primary membranous nephropathy in nationwide real-world practice: the Japan nephrotic syndrome cohort study (JNSCS). Clin Exp Nephrol. 2020;24(10):893–909.
- Abrass CK. Treatment of membranous nephropathy in the elderly. Semin Nephrol. 2003;23(4):373–378.
- Zent R, Nagai R, Cattran DC. Idiopathic membranous nephropathy in the elderly: a comparative study. Am J Kidney Dis. 1997;29(2):200–206.
- Ehrenreich T, Porush JG, Churg J, et al. Treatment of idiopathic membranous nephropathy. N Engl J Med. 1976;295(14):741–746.
- Group K. KDIGO clinical practice guideline for glomerulonephritis. Kidney Inter Suppl. 2012;2(2):139–274.
- Alsharhan L, Beck LH.Jr. Membranous nephropathy: Core curriculum 2021. Am J Kidney Dis. 2021;77(3):440–453.
- Deegens JK, Wetzels JF. Membranous nephropathy in the older adult: epidemiology, diagnosis and management. Drugs Aging. 2007;24(9):717–732.
- O’Callaghan CA, Hicks J, Doll H, et al. Characteristics and outcome of membranous nephropathy in older patients. Int Urol Nephrol. 2002;33(1):157–165.
- Sumnu A, Gursu M, Ozturk S. Primary glomerular diseases in the elderly. World J Nephrol. 2015;4(2):263–270.
- Beck LH, Jr., Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21.
- Radice A, Pieruzzi F, Trezzi B, et al. Diagnostic specificity of autoantibodies to M-type phospholipase A2 receptor (PLA2R) in differentiating idiopathic membranous nephropathy (IMN) from secondary forms and other glomerular diseases. J Nephrol. 2018;31(2):271–278.
- Doi T, Mayumi M, Kanatsu K, et al. Distribution of IgG subclasses in membranous nephropathy. Clin Exp Immunol. 1984;58(1):57–62.
- Qu Z, Liu G, Li J, et al. Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol Dial Transplant. 2012;27(5):1931–1937.
- Kuroki A, Iyoda M, Shibata T, et al. Th2 cytokines increase and stimulate B cells to produce IgG4 in idiopathic membranous nephropathy. Kidney Int. 2005;68(1):302–310.
- Stangou MJ, Marinaki S, Papachristou E, et al. Histological grading in primary membranous nephropathy is essential for clinical management and predicts outcome of patients. Histopathology. 2019;75(5):660–671.
- Zhang BO, Cheng M, Yang M, et al. Analysis of the prognostic risk factors of idiopathic membranous nephropathy using a new surrogate end-point. Biomed Rep. 2016;4(2):147–152.
- Polanco N, Gutiérrez E, Covarsí A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21(4):697–704.
- Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol. 2003;23(4):324–332.
- KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4s):S1–s276.
- Cattran DC, Kim ED, Reich H, et al. Membranous nephropathy: quantifying remission duration on outcome. J Am Soc Nephrol. 2017;28(3):995–1003.
- Yamaguchi M, Ando M, Katsuno T, et al. Urinary protein and renal prognosis in idiopathic membranous nephropathy: a multicenter retrospective cohort study in Japan. Ren Fail. 2018;40(1):435–441.
- Chen X, Chen Y, Ding X, et al. Baseline proteinuria level is associated with prognosis in idiopathic membranous nephropathy. Ren Fail. 2019;41(1):363–369.
- Wada T, Ishimoto T, Nakaya I, et al. A digest of the Evidence-Based clinical practice guideline for nephrotic syndrome 2020. Clin Exp Nephrol. 2021;25(12):1277–1285.
- Fervenza FC, Appel GB, Barbour SJ, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381(1):36–46.