Abstract
The safety of sodium-glucose co-transporter 2 (SGLT2) inhibitors in elderly patients with diabetic kidney disease (DKD) is still controversial. This study aimed to analyze the safety of SGLT2 inhibitors in elderly patients with type 2 diabetes mellitus (T2DM) and DKD. We systematically searched PubMed, Embase, Web of Science, and the Cochrane Library from inception to March 2023. Randomized controlled trials (RCTs) were included. Data including patient characteristics and interesting outcomes were extracted, and the dichotomous data and continuous variables were evaluated using risk ratio (RR) with 95% confidence intervals (CIs) and mean difference (MD) with 95% CIs, respectively. A total of 14 RCTs with 59874 participants were finally included. There were 38,252 males (63.9%) and 21,622 females (36.1%). The patients’ mean age was > 64.6 years. SGLT2 inhibitors could delay the further decline of estimated glomerular filtration rate (eGFR) when eGFR ≥ 60 ml/min/1.73m2 (MD: 2.36; 95%CI [1.15–3.57]). SGLT2 inhibitors in elderly patients with eGFR < 60 ml/min/1.73m2 (RR: 0.86; 95%CI [0.67–1.11]) may have a relatively increased risk of acute kidney injury compared to eGFR ≥ 60 ml/min/1.73m2. SGLT2 inhibitors increased the incidence of genital mycotic infections (RR: 3.47; 95%CI [2.97–4.04]) and diabetic ketoacidosis (RR: 2.25; 95%CI [1.57–3.24]). Except for genital mycotic infections and diabetic ketoacidosis, other adverse reactions were few, indicating that SGLT2 inhibitors are relatively safe for elderly patients with T2DM and DKD. Safety and renoprotection may be diminished when SGLT2 inhibitors are used in elderly patients with eGFR < 60 ml/min/1.73m2.
1. Introduction
Diabetic kidney disease (DKD) is one of the most common complications of diabetes. About 40% of type 2 diabetes mellitus (T2DM) patients suffer from DKD [Citation1]. The annual incidence of proteinuria in type 1 diabetes is approximately 2% to 3%, and approximately 8% in type 2 diabetes. The annual incidence of eGFR reduction below 60 mL/min/1.73m2 is approximately 2% to 4% [Citation2]. DKD is also a major cause of end-stage kidney disease (ESKD). In the United States, ESKD caused by DKD accounts for about 47%, and in countries such as Malaysia and Singapore, it is even as high as 60% [Citation3]. People with diabetes are ten times more likely to develop ESKD than adults without diabetes [Citation4]. In the U.S. adult population, age-standardized 10-year cumulative all-cause mortality was 7.7% in those without diabetes or chronic kidney disease (CKD) and 31.1% in those with diabetes and CKD [Citation5]. In 2017, approximately 219451 people died from DKD worldwide. Since 1990, DKD deaths account for 29% of all CKD male deaths and 32% of all CKD female deaths [Citation6]. The pathophysiology of DKD is complex and includes metabolic pathways (such as polyol pathway, hexosamine pathway, protein kinase C pathway, and advanced glycation end-product-related pathway) and hemodynamic factors (systemic and glomerular hypertension). These induce activation of intracellular signaling pathways, oxidative stress, hypoxia, dysregulation of autophagy, and epigenetic changes, which lead to renal inflammation and fibrosis [Citation7]. Current clinical trials have confirmed that SGLT2 inhibitors have a significant cardio-renal protective effect [Citation8,Citation9]. In patients with type 2 diabetes, the prevalence of DKD increases with age, accompanied by decreased eGFR and increased urinary protein [Citation10]. This study aimed to analyze the safety of SGLT2 inhibitors in elderly patients with T2DM and DKD.
2. Methods
This study is a systematic review and meta-analysis to evaluate the safety of SGLT2 inhibitors compared with placebo in elderly patients with T2DM and DKD. It was conducted and reported according to PRISMA (Preferred Reporting Item for Systematic Reviews and Meta-Analyses) guidelines [Citation11]. This meta-analysis has been registered at International Prospective Register of Systematic Reviews. The registration number is CRD42022331381. A protocol was not prepared.
2.1. Search strategy
We searched PubMed, Embase, Web of Science, and the Cochrane Library. Eligible studies on the treatment of DKD with SGLT2 inhibitors published in English from the establishment of the database to March 2023 were collected. The search terms included ‘Sodium-Glucose Transporter 2 Inhibitors,’ ‘Diabetic Kidney Disease,’ and ‘randomized controlled trial.’ The end date of the literature search is March 31, 2023. The detailed search strategy is available in the online Supplementary Table.
2.2. Study selection
Two reviewers (YL and CA) independently screened the search results and retrieved relevant studies for further evaluation. Full-text articles retrieved were reviewed in parallel by two reviewers (YL and CA) according to pre-determined criteria for inclusion in the study. Kappa statistic was calculated to measure agreement between two authors during the systematic search. The kappa of the agreement was 0.864. We included RCTs in elderly patients (defined as age ≥ 60 years) with T2DM and DKD (defined as the presence of albuminuria and/or reduced eGFR in the absence of signs or symptoms of other primary causes of kidney damageCitation12] comparing SGLT2 inhibitors with placebo and reporting changes in eGFR and/or urinary albumin/creatinine ratio (UACR) and/or blood pressure. Only the first paper reporting renal outcomes was included for multiple papers from the same study. Disagreements were resolved by discussion and/or consultation with the third reviewer (PLL).
2.3. Inclusion and exclusion criteria
Studies meeting these criteria were considered eligible: (1) Included participants were elderly patients with T2DM and DKD; (2) The treatment group was treated with SGLT2 inhibitors (doses were not restricted), and the control group was treated with placebo; (3) At least one of the following outcome measures was reported: eGFR, UACR, adverse effects, and cardiorenal outcomes; (4) Randomized controlled trials or clinical trials published in English. The exclusion criteria were as follows: (1) Meta-analyses, reviews, case reports, conferences, animal studies, etc. (2) Papers reporting results incompletely; (3) Failed to provide effective outcome indicators and data for statistical analysis; (4) Studies in which patient information is not extractable; (5) Repeated publications of the same research.
2.4. Data extraction and validity assessment
Two reviewers (YL and CA) independently recorded the following characteristics from the included studies using standard data extraction tools and making tables: study characteristics (authors, year, study design, and follow-up duration), participant characteristics (sample size, age), diabetes duration, baseline hemoglobin A1c (HbA1c) level, baseline blood pressure, eGFR and/or UACR), therapeutic intervention (type, dose, and duration of SGLT2 inhibitor), control group (placebo or active control), relevant outcomes (mean and standard deviation (SD) of changes in eGFR or UACR or blood pressure in treatment and control groups and relative risk (RR) values for adverse events in treatment and control groups). During data extraction, we calculated SD based on standard error (SE) or 95% confidence intervals (CIs) or Inter Quartile Range (IQR) if studies did not directly report SD. If the data were displayed graphically without numerical values, we used the image extraction software WebPlotDigitizer version 4.5 to extract the desired data points. Any disagreements were resolved in consultation with a third reviewer (PLL) to reach a consensus.
2.5. Risk of bias assessment
Study quality was evaluated by two authors (YL and CA) independently using the ‘Risk of bias’ assessment tool from the Cochrane Handbook for Systematic Reviews of Interventions, version 6.3 (2022) [Citation13]. We evaluated the risk of bias in a random sequence generation, allocation concealment, the blinding of participants and researchers, the blinding of outcome assessments, selective reporting, incomplete outcome data, and other bias.
2.6. Statistical analysis
Statistical analysis was performed using Review Manager 5.4 (The Nordic Cochrane Center, The Cochrane Collaboration) and STATA statistical software (version 16.0). Statistical significance was set at p < 0.05 for all analyses. For continuous variables, we used mean difference (MD) with 95% CIs. For dichotomous variables, RR with 95% CIs was used. The Cochrane Q test and the I2 statistic (defined as the test used to quantify heterogeneity and calculate the proportion of variation due to heterogeneity rather than chance) were used to quantify statistical heterogeneity [Citation14]. A fixed effects model was used if I2 < 50%, whereas a random effects model was used if I2 ≥ 50% [Citation15]. We explored the sources of heterogeneity through subgroup analysis. Sensitivity analyses were performed by excluding single studies one by one for meta-analyses with high heterogeneity [Citation16]. For meta-analyses with more than 10 studies, Egger’s tests were used to assess the possibility of publication bias.
2.7. Statement of ethics
All analyses were based on previously published research, so an ethics statement is not applicable.
3. Results
3.1. Study selection and characteristics
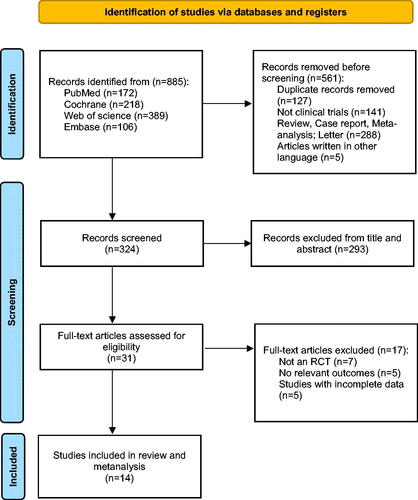
The detailed flow diagram of the study selection process is presented in . A total of 885 articles were retrieved from PubMed, Embase, Web of Science, and the Cochrane Library. After excluding duplicates and other studies that did not meet inclusion criteria, 324 records were screened. 293 records were excluded after reviewing the title and abstract. After a full-text evaluation of 31 eligible studies, 7 studies were excluded that did not qualify for RCTs, 5 studies lacked outcomes of relevant, and 5 studies did not report complete data. Ultimately, we included 14 studies that met the inclusion criteria [Citation17–30].
A total of 59874 patients were included in 14 RCTs, and the baseline characteristics of each trial are shown in . All studies have been published since 2016, and the number of participants in different studies ranged from 34 to 17160. The patients were 38252 males (63.9%) and 21622 females (36.1%). The mean age of all patients was over 64.6 years. All patients were previously diagnosed with type 2 diabetes, and most received background antidiabetic medications in addition to intervention therapy. Of these, 4 studies compared canagliflozin to placebo, 4 studies compared dapagliflozin to placebo, 2 studies compared empagliflozin to placebo, 2 studies compared ertugliflozin to placebo, and the remaining 2 studies compared sotagliflozin, bexagliflozin with placebo. 14 studies (59874 participants) reported changes in eGFR, 8 studies (23945 participants) reported changes in UACR, and 10 studies (27354 participants) reported changes in SBP. Trial durations ranged from 18 to 240 weeks. In the included studies, the mean baseline eGFR was 44.5 to 85.2 mL/min/1.73m2, and the mean baseline UACR was 13.36 to 1054 mg/g. The mean baseline SBP ranged from 126.4 to 142 mmHg.
Table 1. The basic characteristics of included studies.
3.2. The risk of bias
The included studies were assessed for risk of bias using ROB 2.0 according to the criteria recommended in the Cochrane Handbook version 6.3 [Citation13]. For all items assessed, 2 trials were rated as low risk, and 12 trials were rated as a possible risk. All trials were RCTs, and most described the process of bias arising from the randomization process, bias due to deviations from the intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. The possible risk is because there are multiple time-point measurements in the trials, some trials have multiple analyses for a certain outcome, and some trials do not (Figure S1).
3.3. Clinical outcomes
3.3.1. eGFR
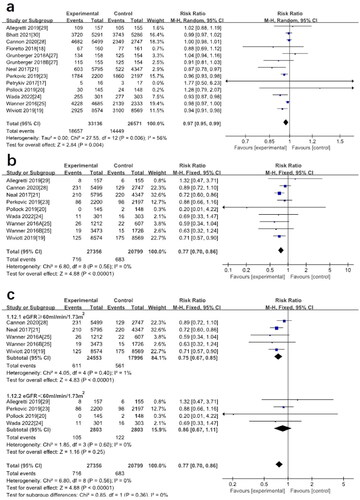
Changes in eGFR from baseline to endpoint and their MD ± SD of changes were extracted from 14 studies. There was no significant difference in eGFR levels in DKD patients treated with SGLT2 inhibitors compared to placebo (MD: 0.70; 95%CI [-0.51–1.92], p = 0.26) (). Because of the large heterogeneity of studies (I2=96%), we used a random-effects model.
Figure 2. (a) Forest plot for the mean difference of the change in eGFR comparing SGLT2 inhibitors with placebo. (b) Subgroup analysis of the effect of SGLT2 inhibition on eGFR based on baseline eGFR levels. (c) Subgroup analysis of the effect of SGLT2 inhibition on eGFR based on different SGLT2 inhibitors. (Wanner2016A: the group of Empagliflozin 10 mg, Wanner2016B: the group of Empagliflozin 25 mg, Grunberger2018A: the group of Ertugliflozin 5 mg, Grunberger2018B: the group of Ertugliflozin 15 mg).

To further explore the sources of heterogeneity, we performed a subgroup analysis of baseline eGFR levels. It was found that SGLT2 inhibitors may have a protective effect on the renal function when eGFR was between 60 and 90 mL/min/1.73m2 (MD: 2.36; 95%CI [1.15–3.57], p = 0.0001, I2=86%). In general, there was no significant difference from placebo (). We also performed a subgroup analysis of different SGLT2 inhibitors and found that taking canagliflozin (MD: 3.68; 95%CI [1.47–5.88], p = 0.001, I2=93%) and empagliflozin (MD: 2.84; 95%CI [2.05–3.63], p < 0.00001, I2=0%) may improve eGFR, while dapagliflozin (MD: −1.61; 95%CI [-4.77–1.55], p = 0.32, I2=86%), and ertugliflozin (MD: −0.56; 95%CI [-3.81–2.69], p = 0.74, I2=91%) may reduce eGFR levels as shown in . By subgroup analysis, we found that different SGLT2 inhibitors may be the source of heterogeneity.
3.3.2. Adverse events
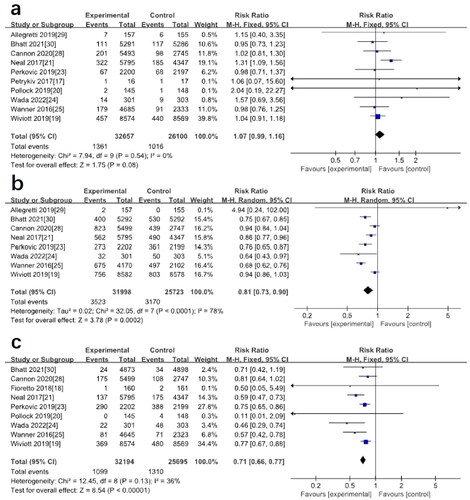
A total of 12 studies reported data on adverse events involving 59707 patients, with no significant difference in the incidence of adverse events between the SGLT2 inhibitor group and placebo (RR: 0.97; 95%CI [0.95–0.99], p = 0.004, I2=56%) (), there may be heterogeneity between studies. In a meta-analysis of data from 8 studies, the incidence of acute kidney injury (AKI) was lower in elderly patients with T2DM and DKD after treatment with SGLT2 inhibitors than in controls (RR: 0.77; 95%CI [0.70–0.86], p < 0.00001, I2=0%) (). Further subgroup analysis found no decrease in the risk of AKI when eGFR < 60 mL/min/1.73m2 (RR: 0.86; 95%CI [0.67–1.11], p = 0.25, I2=0%) (). However, when eGFR ≥ 60 mL/min/1.73m2, the incidence of AKI decreases (RR: 0.75; 95%CI [0.67–0.85], p < 0.00001, I2=1%) ().
Figure 3. (a) Forest plot for the comparison of adverse events between SGLT-2 inhibitors and placebo. (b) Forest plot for the comparison of AKI between SGLT-2 inhibitors and placebo. (c) Subgroup analysis of the effect of SGLT2 inhibition on AKI based on baseline eGFR levels.
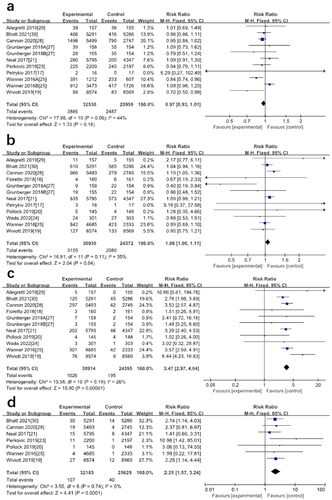
A total of 58489 patients (32530 in the SGLT2 inhibitors group and 25959 in the placebo group) participated in 9 studies on the hypoglycemic outcome. Meta-analysis results showed no significant difference in the incidence of hypoglycemia between patients on SGLT2 inhibitors and those on placebo (RR: 0.97; 95%CI [0.93–1.01], p = 0.18, I2=44%) (). 11 studies reporting data related to adverse events of urinary tract infections (UTIs), the meta-analysis showed no significant difference in the probability of UTIs using SGLT2 inhibitors compared with placebo (RR: 1.06; 95%CI [1.00–1.11], p = 0.04, I2=35%) (). We extracted data on events consistent with genital mycotic infections from baseline to end of follow-up from 10 studies. The results showed that the SGLT2 inhibitor group was significantly different from placebo, and genital mycotic infections are more likely to occur with SGLT2 inhibitors (RR: 3.47; 95%CI [2.97–4.04], p < 0.00001, I2=26%) (). 7 studies reported diabetic ketoacidosis. Participants who were treated with SGLT2 inhibitors had a significantly higher risk of diabetic ketoacidosis than those treated with placebo (RR: 2.25; 95%CI [1.57–3.24], p < 0.0001, I2=0%) (). Results of meta-analysis showed that the use of SGLT2 inhibitors did not increase the incidence of fractures (RR: 1.07; 95%CI [0.99–1.16], p = 0.08, I2=0%) ().
Figure 4. (a) Forest plot for the comparison of hypoglycemia between SGLT-2 inhibitors and placebo. (b) Forest plot for the comparison of UTIs between SGLT-2 inhibitors and placebo. (c) Forest plot for the comparison of genital mycotic infections between SGLT-2 inhibitors and placebo. (d) Forest plot for the comparison of diabetic ketoacidosis between SGLT-2 inhibitors and placebo.
3.3.3. Cardiac and renal outcomes
Eight studies reported cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure or unstable angina. SGLT2 inhibitors were effective in reducing the incidence of MACE compared with placebo (RR: 0.81; 95%CI [0.73–0.90], p = 0.0002, I2=78%) (). Renal composite outcomes (including doubling of serum creatinine, renal impairment/failure, renal death, ESKD, etc.) were reported in 9 studies. Meta-analysis showed that SGLT2 inhibitors significantly reduced the risk of renal composite outcomes (RR: 0.71; 95%CI [0.66–0.77], p < 0.00001, I2=36%) ().
3.4. Sensitivity analysis and publication bias
For meta-analyses of I2 > 50%, we have deleted one of the studies one by one to conduct a sensitivity analysis of eGFR, adverse events, and MACE. Finally, the results of these meta-analyses have not changed significantly (Figure S2), suggesting that the meta-analysis results obtained in this study are reliable. We conducted Egger’s tests on meta-analyses incorporated into at least 10 studies, including eGFR (p = 0.328), adverse events(p = 0.444), hypoglycemia(p = 0.965), UTIs(p = 0.601), genital mycotic infections(p = 0.750), and fractures(p = 0.734). The results of Egger’s tests indicate that the risk of bias was low (P for Egger’s tests all > 0.05) (Table S2). The funnel plots are shown in Figure S3.
4. Discussion
In this systematic review and meta-analysis, we found that the effect of SGLT2 inhibitors on eGFR in elderly patients with T2DM and DKD was not significantly different from placebo. This is consistent with Yu’s conclusion [Citation31]. By subgroup analysis, it was shown that different baseline eGFR and different drugs might be sources of heterogeneity. The results of our subgroup analysis showed that when eGFR ≥ 60 mL/min/1.73m2, SGLT2 inhibitors could delay the decline rate of eGFR and protect renal function. When eGFR < 60 mL/min/1.73m2, SGLT2 inhibitors have no significant renoprotective effect and should be used cautiously. We found a small decrease in eGFR within 4 weeks of SGLT2 inhibitors use in the studies of Fioretto, Wanner, and Bhatt [Citation18,Citation25,Citation30], within 3 weeks in the Wada study [Citation24] and 6 weeks in the Allegretti study [Citation29]. The studies of Fioretto and Allegretti showed that after a short-term decline in eGFR, eGFR would recover but not reach the baseline level [Citation18,Citation29]. Wada and Wanner’s study found that after a short-term decline in eGFR, eGFR would recover slightly and continue to decline slowly [Citation24,Citation25]. Bhatt’s study found that after 4 weeks, the rate of decline in eGFR slowed compared to the control group [Citation30]. This showed that after using SGLT2 inhibitors, most patients would experience a transient decline in eGFR, but long-term use could slow the decline in eGFR and protect renal function. Due to the small number of studies included, we were unable to determine whether high-dose SGLT2 inhibitors would have a significant effect on eGFR. 8 studies found that patients with DKD significantly reduce body weight and body mass index (BMI) after using SGLT2 inhibitors [Citation18–22,Citation26,Citation27,Citation29].
We conducted a meta-analysis of adverse events in studies of the safety of SGLT2 inhibitors, which showed no significant difference in adverse events after using SGLT2 inhibitors compared with placebo. Five major adverse events were analyzed: hypoglycemia, UTIs, genital mycotic infections, diabetic ketoacidosis, and fracture. The incidence of hypoglycemia was significantly less than that of placebo, indicating that SGLT2 inhibitors have a lower risk of hypoglycemia. The common adverse effects of SGLT2 inhibitors are UTIs and genital mycotic infections, which may be related to the mechanism of increasing urine glucose. Of the included studies, 11 studies reported relevant data [Citation17–21,Citation24,Citation25,Citation27–30]. UTIs occurred in 3155 patients taking SGLT2 inhibitors, 10.20% of the trial group, compared with 2080 patients in the placebo group, 8.53% of the placebo group. The analysis results showed no significant difference in UTIs between SGLT2 inhibitors and placebo, suggesting that SGLT2 inhibitors are less likely to cause UTIs. Genital mycotic infections occurred in 1026 patients taking SGLT2 inhibitors, 3.32% of the trial group, and 195 patients in the placebo group, 0.8% of the placebo group, suggesting that SGLT2 inhibitors can significantly increase the risk of genital mycotic infections. Fewer elderly patients had other adverse reactions, such as dizziness, hypotension, etc., which may be related to the combination of other hypoglycemic drugs in diabetic patients.
The risk of cardiovascular diseases is higher in elderly patients with type 2 diabetes over 60 years old [Citation32]. Through a meta-analysis, we found that MACE was decreased after using SGLT2 inhibitors, which indicated that SGLT2 inhibitors could delay the progression of cardiovascular diseases. Our study found that SGLT2 inhibitors reduced the renal composite outcome and the number of deaths due to renal failure by reducing proteinuria and delaying the decline in eGFR. This conclusion is the same as the previous meta- analyses [Citation33,Citation34]. We found that SGLT2 inhibitors reduced the risk of AKI among all elderly patients. However, subgroup analysis showed that renal function may affect the effect of SGLT2 inhibitors. When eGFR < 60 mL/min/1.73m2, the safety and renoprotective effects of SGLT2 inhibitors may be diminished. Therefore, the pros and cons should be considered in elderly patients with renal insufficiency.
DKD has become one of the most serious complications in elderly patients with T2DM. We included six large and eight small to medium-sized studies with randomized controlled trials with participants of multiple races. We used clinical epidemiological principles and methods to critically evaluate the studies, screened those that met the inclusion criteria, synthesized the data quantitatively using meta-analysis, and identified commonalities and differences between studies by pooling the results of multiple studies. It provided more comprehensive and reliable evidence to guide the use of SLGT2 inhibitors in elderly patients with T2DM and DKD in clinical practice. The safety and efficacy of SGLT2 inhibitors, which can be used in adult patients with T2DM and DKD, have been validated in trials and approved by experts [Citation35]. The use of SGLT2 inhibitors in elderly patients should be different from that of young and middle-aged patients because of more chronic diseases and deterioration of physical functions. Through this systematic review and meta-analysis, we found that basic eGFR levels in elderly patients with T2DM and DKD may affect the safety and renoprotective effects of SGLT2 inhibitors. It means that the safety and renoprotective effects of SGLT2 inhibitors may also decrease with the decline of basic renal function.
Despite our rigorous methodology and careful selection of relevant papers, our research has many deficiencies. Fixed-effects model should be used only if it is reasonable to assume that all studies share the same, one common effect. A fixed-effects model is inappropriate if statistical heterogeneity exists between effect sizes. Fixed-effects models consider only sampling error, with larger weights for larger samples and smaller weights for smaller samples. Therefore, the effect size of a large sample size study is almost determinative for the final combined effect size [Citation36]. We only included 14 studies with a total of 59874 patients. Some studies have only 18 weeks of follow-up, which may not be enough to detect the effect of SGLT2 inhibitors on eGFR. Some studies have small numbers of participants, and the results may be subject to error, leading to inaccurate analyses. There are many types of SGLT2 inhibitors, and we only included studies on dapagliflozin, canagliflozin, empagliflozin, ertugliflozin, sotagliflozin, and bexagliflozin. This does not accurately reflect the safety of all SGLT2 inhibitors.
5. Conclusions
SGLT2 inhibitors may have a renal protective effect on DKD elderly patients with eGFR ≥ 60 mL/min/1.73m2 and can delay the decline of eGFR. When eGFR < 60 mL/min/1.73m2, SGLT2 inhibitors may increase the risk of AKI. SGLT2 inhibitors may diminish safety and renoprotective effects in elderly patients with poor basic renal function and should be selected cautiously in clinical practice. In addition to genital mycotic infections and diabetic ketoacidosis, the incidence of other adverse events (hypoglycemia, UTIs, fracture, etc.) was low, which means that long-term use of SGLT2 inhibitors in DKD elderly patients may be safe. SGLT2 inhibitors reduced MACE and renal composite outcomes in the participants, indicating that SGLT2 inhibitors can delay the progression of cardiovascular diseases and DKD. Although there may be a short-term decreased eGFR and AKI risk in elderly patients, SGLT2 inhibitors have long-term cardio-renal protective effects. Due to insufficient trial data, we could not analyze the effects of other adverse effects associated with SGLT2 inhibitors in elderly patients. With the increasing use of SGLT2 inhibitors in clinical practice, the role of SGLT2 inhibitors in delaying DKD progression and reducing the incidence of end-stage renal disease in elderly patients is still worthy of further study.
Author contributions
All authors contributed to the meta-analysis design, data analysis, and the manuscript’s drafting, review, and final approval.
Supplemental Material
Download PDF (508.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:1.
- Koye DN, Shaw JE, Reid CM, et al. Incidence of chronic kidney disease among people with diabetes: a systematic review of observational studies. Diabet Med. 2017;34(7):887–12.
- Saran R, Robinson B, Abbott KC, et al. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3 Suppl 1):A7–A8.
- Koye DN, Magliano DJ, Nelson RG, et al. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018;25(2):121–132.
- Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–308.
- Thomas B. The global burden of diabetic kidney disease: time trends and gender gaps. Curr Diab Rep. 2019;19(4):18.
- Sugahara M, Pak WLW, Tanaka T, et al. Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology (Carlton). 2021;26(6):491–500.
- Barutta F, Bernardi S, Gargiulo G, et al. SGLT2 inhibition to address the unmet needs in diabetic nephropathy. Diabetes Metab Res Rev. 2019;35(7):e3171.
- Giorgino F, Vora J, Fenici P, et al. Cardiovascular protection with sodium-glucose co-transporter-2 inhibitors in type 2 diabetes: does it apply to all patients? Diabetes Obes Metab. 2020;22(9):1481–1495.
- Russo GT, De Cosmo S, Viazzi F, et al. Diabetic kidney disease in the elderly: prevalence and clinical correlates. BMC Geriatr. 2018;18(1):38.
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- American diabetes association professional practice committee. 11. Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S175–S184.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. www.training.cochrane.org/handbook
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: q statistic or I2 index? Psychol Methods. 2006;11(2):193–206.
- Borenstein M, Hedges LV, Higgins JPT, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111.
- Patsopoulos NA, Evangelou E, Ioannidis JPA. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–1157.
- Petrykiv SI, Laverman GD, de Zeeuw D, et al. The albuminuria-lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes Metab. 2017;19(10):1363–1370.
- Fioretto P, Del Prato S, Buse JB, et al. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): the DERIVE study. Diabetes Obes Metab. 2018;20(11):2532–2540.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357.
- Pollock C, Stefánsson B, Reyner D, et al. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(6):429–441.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657.
- Takashima H, Yoshida Y, Nagura C, et al. Renoprotective effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, in type 2 diabetes patients with chronic kidney disease: a randomized open-label prospective trial. Diab Vasc Dis Res. 2018;15(5):469–472.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306.
- Wada T, Mori-Anai K, Kawaguchi Y, et al. Renal, cardiovascular and safety outcomes of canagliflozin in patients with type 2 diabetes and nephropathy in east and South-East asian countries: results from the canagliflozin and renal events in diabetes with established nephropathy clinical evaluation trial. J Diabetes Investig. 2022;13(1):54–64.
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334.
- Shimizu W, Kubota Y, Hoshika Y, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19(1):148.
- Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther. 2018;9(1):49–66.
- Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435.
- Allegretti AS, Zhang W, Zhou W, et al. Safety and effectiveness of bexagliflozin in patients with type 2 diabetes mellitus and stage 3a/3b CKD. Am J Kidney Dis. 2019;74(3):328–337.
- Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–139.
- Yu B, Dong C, Hu Z, et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021;100(8):e24655.
- Boonman-de Winter LJM, Rutten FH, Cramer MJM, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012;55(8):2154–2162.
- Cao H, Liu T, Wang L, et al. Comparative efficacy of novel antidiabetic drugs on cardiovascular and renal outcomes in patients with diabetic kidney disease: a systematic review and network meta-analysis. Diabetes Obes Metab. 2022;24(8):1448–1457.
- Kaze AD, Zhuo M, Kim SC, et al. Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: a meta-analysis. Cardiovasc Diabetol. 2022;21(1):47.
- Zelniker TA, Braunwald E. Clinical benefit of cardiorenal effects of Sodium-Glucose cotransporter 2 inhibitors: JACC state-of-the-Art review. J Am Coll Cardiol. 2020;75(4):435–447.
- Kanters S. Fixed- and Random-Effects models. Methods Mol Biol Clifton NJ. 2022;2345:41–65.