Abstract
Objective
By analyzing the clinical history, laboratory test indexes, and intraoperative ultrasound imaging data of patients receiving ultrasound-guided percutaneous transluminal angioplasty (UG-PTA) for the first time, the application value of UG-PTA in the treatment of peripheral stenosis of autogenous arteriovenous fistula (AVF) and the related factors affecting postoperative patency were investigated.
Methods
A total of 381 patients with dysfunction of radio-cephalic AVF were treated with UG-PTA from June 2017 to September 2019. According to the inclusion and exclusion criteria, 199 patients were included in this study. Baseline characteristics of patients, including demographic, clinical, and laboratory data, were collected. Kaplan–Meier’s survival curve was used to demonstrate the cumulative primary patency rate of UG-PTA. Univariate and multivariate Cox regression analysis was performed on clinical, anatomic, biochemical, and medication variables to identify the predictors of postintervention primary patency.
Results
The early technical success rate of UG-PTA was 98.4% (375/381). One hundred and ninety-nine patients, with an average age of 52.9 years, were analyzed, 97 of whom were males (48.7%). The median follow-up duration was 21 months. No major complication was observed. Postintervention primary patency rates were 87.7%, 75.8%, and 60.0% at 6, 12, and 24 months, respectively. A previously failed AVF (HR, 1.935, 95% CI 1.071–3.494; p = .029) and an increased level of parathyroid hormone (HR per 100 pg/mL increase, 1.105; 95% CI 1.014–1.203; p = .004) were identified as independent negative predictors of primary patency of UG-PTA.
Conclusions
UG-PTA is a safe and effective method for the treatment of peripheral stenosis of AVF. Previously failed AVF and elevated parathyroid hormone levels are associated with lower primary patency rate.
Introduction
With improved long-term outcomes and decreased rates of thrombosis, infection, hospitalization, and death, arteriovenous fistula (AVF) is regarded as the optimum vascular access (VA) for the majority of maintenance hemodialysis (MHD) patients [Citation1]. The most common cause of access failure in native AVFs, however, is stenosis [Citation2,Citation3]. Recently, surgery and percutaneous transluminal angioplasty (PTA) are mature options for the treatment of access stenosis. Compared with surgery, PTA offers less intrusive treatment, faster access to dialysis, and improved preservation of venous capital [Citation4]. PTA has been widely used in the treatment of VA stenosis. It is traditionally guided and monitored radiographically, although there are hazards of ionizing radiation exposure and unfavorable reactions to contrast media [Citation5]. With the wide use of color Doppler ultrasonography, ultrasound-guided PTA (UG-PTA) has become the mainstay treatment for VA dysfunction [Citation6]. UG-PTA can visualize the vascular structures without contrast medium and X-ray irradiation. Also, ultrasound is mobile and compact and can be operated in an office setting [Citation7].
In recent years, most of the studies on PTA in the treatment of VA stenosis are about the feasibility of traditional PTA and predictors on AVF dysfunction. Technical success rates of traditional PTAs are 81–87.5% [Citation8] and one-year primary patency rate is 42–50% [Citation9–11]. Previous studies showed that, albumin, older age, diabetes, high-sensitive C-sensitive protein, and longer lesion length were found associated with lower primary patency rates after traditional PTA [Citation12–14]. A few studies investigated the usefulness of UG-PTA. It is reported that the success rate of UG-PTA is 93–97.1% [Citation15–17], which is higher than that of the traditional ones. However, these studies on usefulness of UG-PTA have short follow-up time and relatively small sample size [Citation16,Citation18,Citation19]. Besides, the studies of affecting primary patency of AVF following UG-PTA were contradictory [Citation20–22].
From a therapeutic standpoint, it is crucial to be able to anticipate AVF dysfunction following the initial salvage with UG-PTA. It would enable medical professionals to watch these high-risk patients more carefully and organize planned actions [Citation20]. This study aimed to evaluate the safety and effectiveness of UG-PTA and identify the factors predicting AVF dysfunction after an initial UG-PTA.
Materials and methods
Study design
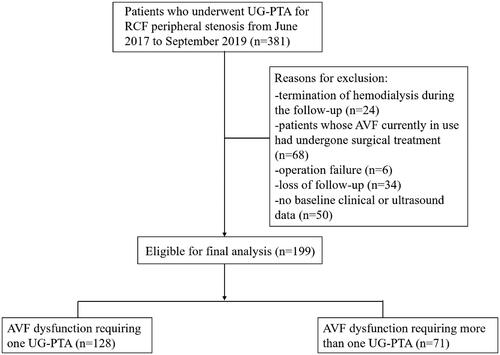
This study population was derived from a retrospective cohort with MHD patients treated with PTA due to peripheral stenosis of native AVFs from 1 June 2017 to 30 September 2019 at the Department of Nephrology from a tertiary teaching hospital. Inclusion criteria: (1) patients with native radio-cephalic fistulas, (2) mature AVFs, and (3) patients receiving UG-PTA. The main exclusion criteria were as follows: (1) operation failure; (2) termination of hemodialysis during the follow-up due to death, transplant, or the beginning of peritoneal dialysis; (3) no baseline clinical or ultrasound data; (4) patients whose AVF currently in use had undergone surgical treatment; (5) loss of follow-up. Ultimately, this study enrolled and analyzed 199 follow-up cases. This retrospective study was approved by the institutional review board, and a waiver of informed consent was obtained (TJ-IRB20210114). The investigations conform to the principles outlined in the Declaration of Helsinki. All patients were informed on the nature of the study and consented to its specifics.
Data collection
The hospitalization data of patients when they first operated UG-PTA were collected as the baseline data. The following clinical variables were recorded: patient age, gender, smoking status, coronary artery disease, diabetes, body mass index, and previous AVF failure. Patient records were searched for medication information. The medications listed below were documented: insulin, antiplatelet therapy, and statins. Laboratory parameters (e.g., parathyroid hormone, blood lipids, serum calcium, serum phosphorus, albumin, coagulation factors, and hemoglobin) were collected. The location, length, the presence of more than one stenosis, the grade of stenosis, and the flow volume of the brachial artery in the mid-upper arm were anatomical variables. Ultrasound examination was performed before operation. All patients were followed every three months in-person by telephone or at the outpatient clinic. Blood flow and venous pressure during dialysis, bleeding time after puncture and puncture difficulty were asked by telephone so as to judge whether the patients need outpatient follow-up or reintervention. The terminal points of follow-up were AVF abandonment, death, or kidney transplant.
Endovascular procedure
All patients were subjected to a physical examination, which included palpation and auscultation of the AVF. The pre-, intra-, and postoperative ultrasonic evaluation was performed by the same nephrologist. Using spectral ultrasonography, the brachial artery flow volume in the middle to upper arm of patients was assessed. A single entrance needle was used to access all arteriovenous (AV) fistulas while using duplex guiding. The stenotic segment was reached by inserting a guidewire via the vascular sheath using a 6–8 Fr introducer sheath (Terumo, Somerset, NY). Afterwards, 40 U/kg BW of intravenous heparin was administered. To accomplish total effacement, the balloon (New York, USA, CONQUEST, BD) was pushed into the stenosis and inflated for 60 s. The operator chose the matching balloons to be roughly 1 mm larger than the nearby typical fistula segment.
Definitions of main descriptive variables
The supplying artery, the juxta-anastomosis defined as the section of the fistula vein within 2 cm of the arteriovenous anastomosis (juxta-AVF), and the outflow vein (OFV) were identified as the three segments of the AVF in terms of lesion site [Citation13]. The period between the first PTA and the second PTA, AVF failure, or study end, whichever occurs first, is referred to as postintervention primary patency. Similarly, regardless of the number of additional interventions, postintervention secondary patency is defined as the interval from the first PTA until AVF failure or study conclusion [Citation23]. Individuals were considered to have AVF dysfunction if their venous lumen diameter decreased by more than 50%, their access blood flow decreased by more than 25% from the previous assessment, and their peak systolic velocity (PSV) ratio between stenotic and pre-stenotic area was larger than 2 [Citation16]. Complications were classified as major or minor according to the criteria of the Society of Cardiovascular and Interventional Radiology [Citation24]. The patients were followed monthly at the outpatient clinic or via phone according to their preference or convenience. Data were collected on access outcomes, alternative VAs, and interventions.
Statistical analyses
For normally distributed data, continuous variables were reported as mean ± standard deviation, and for skewed data, median with interquartile range (IQR). Percentage and frequency were used to express categorical variables. The parametric t-test or the nonparametric Mann–Whitney test were used to compare the two groups. A one-way ANOVA or the Kruskal–Wallis test was used to analyze multiple comparisons among three groups. When comparing categorical variables, χ2 or Fisher’s exact test was used as appropriate. Postintervention primary and secondary patency rates were calculated using Kaplan–Meier’s curves. Predictors of primary patency were evaluated by uni- and multivariate analyses using Cox proportional hazards regression. A two-tailed p < .05 was considered statistically significant. All data were recorded and analyzed by SPSS software (version 23; IBM, Armonk, NY).
Results
According to the inclusion criteria, 381 consecutive MHD cases who underwent a UG-PTA were identified. After applying the exclusion criteria above, a total of 199 patients were included. Details are summarized in . The subjects were divided into two groups according to the number of UG-PTAs. shows the baseline clinical characteristics. Ninety-seven cases (48.7%) were males, and the mean age was 52.9 ± 13.5 years. The median follow-up time was 21 months. Of the 199 patients with initial UG-PTAs, 71 (35.7%) required further PTAs during the follow-up period. Compared with patients who underwent UG-PTA once, patients underwent UG-PTA more than once were more likely to have alcohol consumption, and more often to have previously failed AVF (p < .05). Regarding laboratory findings, there were significant differences between the two groups in calcium–phosphate product (3.7 vs. 4.0, p = .035), high-sensitive C-reactive protein level (2.7 mg/mL vs. 5.0 mg/mL, p = .01), PTH level (203.0 pg/mL vs. 338.1 pg/mL, p = .012), platelet-larger cell ratio (28.8 vs. 25.9, p = .024), and mean platelet volume level (10.6/fL vs. 10.2/fL, p = .022). Medication treatment did not differ between the two groups ().
Table 1. Baseline characteristics (at first UG-PTA) by number of PTAs during follow-up.
Ultrasound examination data before and during UG-PTA were recorded. After comparison, we found that brachial artery flow volume of pre- and postoperative was lower in patients who underwent PTA more than once, but there was no statistically significant change (p > .05). No distinction was observed between the two groups in balloon size, location of lesions, and length of lesions. The stenosis at the OFV was the main lesion of patients who underwent one PTA. In the other group, the major lesions were at the OFV and juxta-AVF zone, accounting for 88.7% ().
Table 2. Ultrasonographic examinations of the 199 procedures.
In our study, the early technical success of UG-PTA was found to be 98.4% (375/381). Four patients had failed guidewire recanalization and two of them had residual stenosis larger than 30%. Among the 199 patients included, complications occurred in 22 (11.1%) cases (): in particular, 20 minor hematoma cases and two case of thrombosis needing thrombolysis and subsequent CVC installation for the next dialysis sessions were found. No major complications such as pseudo-aneurysm, micro-embolism, vascular rupture, and balloon rupture that required transition to surgery or fluoroscopic guided intervention were observed. Cutting balloons were preferred to conventional ones when obvious calcification or intima-media thickness (>1.5 mm) was observed under ultrasound. Postintervention primary patency rates were 87.7%, 75.8%, and 60% at 6, 12, and 24 months, respectively. Secondary patency rates were 94.5%, 84.4%, and 76% at 6, 12, and 24 months, respectively ().
Figure 2. AVF survival curves after 6-, 12-, and 24-months follow-up. (a) Primary patency. (b) Secondary patency.
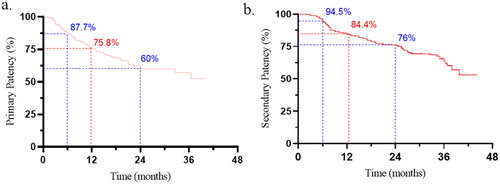
Table 3. Success and complication rates.
No significant difference was observed in primary patency in gender or age, but it was found decreased in patients with previously failed AVF. The receiver operating characteristics (ROC) analyses were performed to determine a threshold value for PTH, and the cutoff value was 394 pg/mL. We found that, patients with lower level of PTH (<394 pg/mL) exerted higher post-intervention access cumulative patency rates (). Patients with previously failed AVF were associated with a lower cumulative incidence for primary patency (). By performing multivariable regression analysis of univariate predictors, reduced postintervention primary patency was linked to two different factors. When compared to non-previously failed AVF, those who had previously failed AVF at the time of the first UG-PTA had shorter postintervention primary patency (HR, 1.935; 95% CI, 1.071–3.494; p = .029). A similar association was observed for PTH (HR per 100 pg/mL increase, 1.105; 95% CI 1.014–1.203; p = .004) ().
Figure 3. Kaplan–Meier’s cure of estimated primary patency. (a) Comparisons between PTH < 394 pg/mL and ≥394 pg/mL. (b) Comparison between non-previously failed AVF and previously failed AVF.
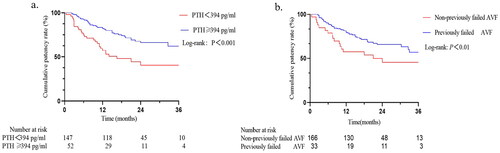
Table 4. Univariate and multivariate analyses of the effect of baseline patient characteristics on primary patency.
Discussion
This study retrospectively analyzed the predictors of long-term patency in patients with AVF after their first UG-PTAs. Also, the early therapeutic success rate was 98.4%, and postintervention primary patency rates at 6, 12, and 24 months were 87.7%, 75.8%, and 60.0%, respectively. First of all, UG-PTA is a safe and effective technology to deal with peripheral stenosis of AVFs. One hundred and ninety-nine patients with native radio-cephalic AVFs had no severe complications that required transition to surgery or fluoroscopic guided interventions. Through detailed records and analysis of a series of indicators such as clinical, ultrasonic indicators, laboratory tests, and medication history of all patients, it was found that the previously failed AVF and PTH were independent risk factors affecting the primary patency of UG-PTA. As a novel finding, this study demonstrated that PTH was associated with a lower primary patency rate. Few researchers have explored both the safety and postintervention factors of UG-PTA in one study. Its comparability is further constrained by some differences in the study design, the type of treated AVF (prosthetic vs. native), and the PTA equipment.
Vascular access complications are one of the main causes of the increase in morbidity and mortality in MHD patients [Citation25]. Thrombosis and stenosis are two common factors contributing to AVF dysfunction. The presence of stenosis may also lead to thrombosis [Citation26]. As a relatively mature method to deal with AVF stenosis, PTA has become the optimal choice of interventional nephrologists due to its advantages of simple operation, small trauma, and no consumption of vascular resources [Citation6]. Conventional fluoroscopic PTA will expose both the medical staff and the patients to X-rays and there is a risk of contrast agent allergy. Since the ultrasound equipment is portable and somewhat small, it can be used during day-surgery, day-hospital or hospitalized patient procedures. In this study, the success rate of UG-PTA was 98.4%, and the complication rate was 11.1%, and there was no serious complication. Primary patency rates at 6, 12, and 24 months were 87.7%, 75.8%, and 60.0%, respectively. These results are comparable to those reported in other literature [Citation15,Citation16], and also demonstrate the safety and effectiveness of UG-PTA.
Previous studies identified aging, diabetes, hemoglobin, location and degree of initial stenosis as potential indicators of postintervention primary patency after fluoroscopy-guided PTA [Citation13,Citation20,Citation27,Citation28]. Our study suggested demographic factors and diabetes were not associated with postintervention patency, which may be related to different treatments, selected populations, AVF type, and stenosis sites. Meanwhile, several of these studies were single-center reports with tiny subject populations, which lacked multivariate statistical analysis and intervention data. According to the literature, the negative role of diabetes is still not a consistent conclusion in postintervention primary patency after PTA. There was no difference in primary AVF maturity rate between nondiabetics and diabetics but lower primary patency in diabetics, which was published in the early 1990s [Citation29]. A previous publication including 395 AVFs indicated that diabetic patients had lower maturation rate and patency rate after VA construction than non-diabetic patients [Citation30]. One study demonstrated that glycemic management as measured by HbA1c may play a significant role in the development of primary AVF failure among diabetic subjects [Citation31]. Heye et al. found patients with diabetes mellitus had a greater risk of developing early dysfunction after PTA [Citation32]. Other studies have not shown that diabetes reduces fistula patency after traditional PTA [Citation12,Citation13,Citation27].
Arterial media calcification causes vessel stiffness, vascular compliance loss, and calcification plaques [Citation33], all of which contribute to vascular stenosis and AVF dysfunction [Citation34]. Vascular calcification could be caused by abnormalities of bone and mineral metabolism, the imbalance between calcification promoter and inhibitor, various arterial diseases and traditional cardiovascular risk factors [Citation35]. Lyu et al. [Citation36] reported that AVF failure was linked to calcification markers like OPN, fetuin-A, and BMP7 [Citation36]. Our study found that higher PTH level was a risk factor for AVF dysfunction after UG-PTA. Secondary hyperparathyroidism (sHPT) is caused by impaired renal function, which coexists with disturbed phosphate, calcium, and vitamin D metabolism. Patients with chronic kidney disease (CKD) are at high risk of dying because of these changes in mineral metabolism, which are linked to the calcification of soft tissues like arteries, heart valves, and myocardium [Citation34]. As an important hormone regulating mineral metabolism, PTH plays an important role in vascular calcification. A previous study found PTH was an independent predictor of coronary artery calcifications progression in MHD patients [Citation37]. Other publications suggested lower PTH was linked to more extensive calcification in calcium-treated patients, whereas greater PTH was linked to calcification in subjects treated with sevelamer [Citation38,Citation39]. The mechanisms linking PTH and vascular calcification are not fully understood. According to Chertow et al. [Citation39], calcium may negatively affect the balance of skeletal and extraskeletal calcification in MHD patients, either directly or indirectly (through PTH). Nakao et al. [Citation40] reported PTH receptor 1 is primarily responsible for the anabolic effects of PTH on osteogenic cells via the protein kinase A (PKA) signaling pathway. Another study indicated that PTH serves a crucial role in promoting the osteoblastic differentiation of human umbilical vein endothelial cells [Citation41]. Based on prior studies, individuals with higher coronary artery calcium (CAC) are at high risk for cardiovascular disease (CVD) events, non-CVD outcomes, and mortality than those with lower CAC [Citation42]. Vascular calcification is associated with an increased risk of mortality in patients with CKD [Citation34], so it is of great significance to pay early attention to mineral metabolism in MHD patients and control blood phosphorus, calcium, and parathyroid hormone levels.
In the present study, a previously failed AVF was another risk factor of primary patency after UG-PTA. A previous study demonstrated that only reliable predictor of postintervention secondary patency loss was previously unsuccessful and abandoned AVF [Citation12]. No similar finding was seen in any of the other studies that evaluated the predictors of postintervention primary patency [Citation14,Citation16,Citation43]. It is probable that several factors relating to patient selection and standard surgical procedures played a part in the current study.
This study was designed retrospectively, which has inherent disadvantages. First, retrospective information on medication use incorrectly assumes patient compliance. The complete data on PTH levels 3 and 6 months before and after UG-PTA could not be obtained, which makes it difficult to perform a statistical analysis of PTH trajectory and patency of the UG-PTA. Second, other unmeasured ultrasonographic examinations, such as vascular diameter and vascular calcification, may also play an important role in AVF dysfunction. Last but not least, the duration of the follow-up was relatively short (median follow-up of 18 months). Future research will elucidate the problems with UG-PTA for peripheral AVF stenosis.
Conclusions
UG-PTA is safe and effective for the treatment of AVF patients with peripheral stenosis. A previously failed AVF and increased PTH levels are the risk factors for reduced postintervention primary patency. Being aware of these risk factors is helpful to maintaining the function of AVFs.
Consent form
Written informed consent has been obtained from the patients to publish this paper.
Author contributions
FH and GX designed and supervised the study. XX, QL, YY, and YW collected data from patients. FH performed the operations. XX, XZ, and CZ analyzed the data and wrote the manuscript.
Acknowledgements
The authors thank all of our colleagues working in the Department of Nephrology, Tongji Hospital.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data underlying this article will be shared on reasonable request with the corresponding authors.
Additional information
Funding
References
- Woodside KJ, Bell S, Mukhopadhyay P, et al. Arteriovenous fistula maturation in prevalent hemodialysis patients in the United States: a national study. Am J Kidney Dis. 2018;71(6):1–9. doi: 10.1053/j.ajkd.2017.11.020.
- Riella MC, Roy-Chaudhury P. Vascular access in haemodialysis: strengthening the Achilles’ heel. Nat Rev Nephrol. 2013;9(6):348–357. doi: 10.1038/nrneph.2013.76.
- Hakim R, Himmelfarb J. Hemodialysis access failure: a call to action. Kidney Int. 1998;54(4):1029–1040. doi: 10.1046/j.1523-1755.1998.00122.x.
- Long B, Brichart N, Lermusiaux P, et al. Management of perianastomotic stenosis of direct wrist autogenous radial-cephalic arteriovenous accesses for dialysis. J Vasc Surg. 2011;53(1):108–114. doi: 10.1016/j.jvs.2010.08.007.
- Schmidli J, Widmer MK, Basile C, et al., Editor’s choice – vascular access: 2018 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(6):757–818. doi: 10.1016/j.ejvs.2018.02.001.
- Nalesso F, Garzotto F, Petrucci I, et al. Standardized protocol for hemodialysis vascular access assessment: the role of ultrasound and color Doppler. Blood Purif. 2018;45(1–3):260–269. doi: 10.1159/000485590.
- Napoli M, Bacchini G, Scarpati L, et al. Ultrasound guided interventional procedures on arteriovenous fistulae. J Vasc Access. 2021;22(1 Suppl.):91–96. doi: 10.1177/1129729820977380.
- Sofocleous CT, Schur I, Koh E, et al. Percutaneous treatment of complications occurring during hemodialysis graft recanalization. Eur J Radiol. 2003;47(3):237–246. doi: 10.1016/s0720-048x(02)00087-6.
- Turmel-Rodrigues L, Pengloan J, Bourquelot P. Interventional radiology in hemodialysis fistulae and grafts: a multidisciplinary approach. Cardiovasc Intervent Radiol. 2002;25(1):3–16. doi: 10.1007/s00270-001-0082-y.
- Flu H, Breslau PJ, Krol-van Straaten JM, et al. The effect of implementation of an optimized care protocol on the outcome of arteriovenous hemodialysis access surgery. J Vasc Surg. 2008;48(3):659–668. doi: 10.1016/j.jvs.2008.04.002.
- Greenberg JI, Suliman A, Angle N. Endovascular dialysis interventions in the era of DOQI. Ann Vasc Surg. 2008;22(5):657–662. doi: 10.1016/j.avsg.2008.03.006.
- Neuen BL, Gunnarsson R, Baer RA, et al. Factors associated with patency following angioplasty of hemodialysis fistulae. J Vasc Interv Radiol. 2014;25(9):1419–1426. doi: 10.1016/j.jvir.2014.05.020.
- Higashiura W, Takara H, Kitamura R, et al. Factors associated with secondary functional patency after percutaneous transluminal angioplasty of the early failing or immature hemodialysis arteriovenous fistula. Cardiovasc Intervent Radiol. 2019;42(1):34–40. doi: 10.1007/s00270-018-2083-0.
- Bautista AB, Suhocki PV, Pabon-Ramos WM, et al. Postintervention patency rates and predictors of patency after percutaneous interventions on intragraft stenoses within failing prosthetic arteriovenous grafts. J Vasc Interv Radiol. 2015;26(11):1673–1679. doi: 10.1016/j.jvir.2015.08.008.
- Leskovar B, Furlan T, Poznič S, et al. Ultrasound-guided percutaneous endovascular treatment of arteriovenous fistula/graft. Clin Nephrol. 2017;88(13):61–64. doi: 10.5414/CNP88FX15.
- Granata A, Maccarrone R, Di Lullo L, et al. Feasibility of routine ultrasound-guided percutaneous transluminal angioplasty in the treatment of native arteriovenous fistula dysfunction. J Vasc Access. 2021;22(5):739–743. doi: 10.1177/1129729820943076.
- Wakabayashi M, Hanada S, Nakano H, et al. Ultrasound-guided endovascular treatment for vascular access malfunction: results in 4896 cases. J Vasc Access. 2013;14(3):225–230. doi: 10.5301/jva.5000126.
- Cho S, Lee YJ, Kim SR. Clinical experience with ultrasound guided angioplasty for vascular access. Kidney Res Clin Pract. 2017;36(1):79–85. doi: 10.23876/j.krcp.2017.36.1.79.
- Bacchini G, Cappello A, La Milia V, et al. Color Doppler ultrasonography imaging to guide transluminal angioplasty of venous stenosis. Kidney Int. 2000;58(4):1810–1813. doi: 10.1046/j.1523-1755.2000.00344.x.
- Yap Y-S, Chi W-C, Lin C-H, et al. Factors affecting patency of arteriovenous fistula following first percutaneous transluminal angioplasty. Clin Exp Nephrol. 2021;25(1):80–86. doi: 10.1007/s10157-020-01958-w.
- Neuen BL, Gunnarsson R, Webster AC, et al. Predictors of patency after balloon angioplasty in hemodialysis fistulas: a systematic review. J Vasc Interv Radiol. 2014;25(6):917–924. doi: 10.1016/j.jvir.2014.02.010.
- Chen L, Zhang W, Tan J, et al. Morphological lesion types are associated with primary and secondary patency rates after high-pressure balloon angioplasty for dysfunctional arteriovenous fistulas. Blood Purif. 2022;51(5):425–434. doi: 10.1159/000516883.
- Sidawy AN, Gray R, Besarab A, et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg. 2002;35(3):603–610. doi: 10.1067/mva.2002.122025.
- Aruny JE, Lewis CA, Cardella JF, et al. Quality improvement guidelines for percutaneous management of the thrombosed or dysfunctional dialysis access. J Vasc Interv Radiol. 1999;10(4):491–498. doi: 10.1016/s1051-0443(99)70071-0.
- Campos RP, Do Nascimento MM, Chula DC, et al. Stenosis in hemodialysis arteriovenous fistula: evaluation and treatment. Hemodial Int. 2006;10(2):152–161. doi: 10.1111/j.1542-4758.2006.00087.x.
- Stolic R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Med Princ Pract. 2013;22(3):220–228. doi: 10.1159/000343669.
- Wen M, Li Z, Li J, et al. Risk factors for primary arteriovenous fistula dysfunction in hemodialysis patients: a retrospective survival analysis in multiple medical centers. Blood Purif. 2019;48(3):276–282. doi: 10.1159/000500045.
- Wu C-C, Wen S-C, Yang C-W, et al. Baseline plasma glycemic profiles but not inflammatory biomarkers predict symptomatic restenosis after angioplasty of arteriovenous fistulas in patients with hemodialysis. Atherosclerosis. 2010;209(2):598–600. doi: 10.1016/j.atherosclerosis.2009.10.021.
- Lin SL, Huang CH, Chen HS, et al. Effects of age and diabetes on blood flow rate and primary outcome of newly created hemodialysis arteriovenous fistulas. Am J Nephrol. 1998;18(2):96–100. doi: 10.1159/000013315.
- Huijbregts HJT, Bots ML, Wittens CHA, et al. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol. 2008;3(3):714–719. doi: 10.2215/CJN.02950707.
- Afsar B, Elsurer R. The primary arteriovenous fistula failure-a comparison between diabetic and non-diabetic patients: glycemic control matters. Int Urol Nephrol. 2012;44(2):575–581. doi: 10.1007/s11255-011-9978-x.
- Heye S, Maleux G, Vaninbroukx J, et al. Factors influencing technical success and outcome of percutaneous balloon angioplasty in de novo native hemodialysis arteriovenous fistulas. Eur J Radiol. 2012;81(9):2298–2303. doi: 10.1016/j.ejrad.2011.09.004.
- London GM, Guérin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18(9):1731–1740. doi: 10.1093/ndt/gfg414.
- Torres PA, De Broe M. Calcium-sensing receptor, calcimimetics, and cardiovascular calcifications in chronic kidney disease. Kidney Int. 2012;82(1):19–25. doi: 10.1038/ki.2012.69.
- Cozzolino M, Gallieni M, Brancaccio D. The mechanisms of hyperphosphatemia-induced vascular calcification. Int J Artif Organs. 2008;31(12):1002–1003. doi: 10.1177/039139880803101203.
- Lyu B, Banerjee T, Scialla JJ, et al. Vascular calcification markers and hemodialysis vascular access complications. Am J Nephrol. 2018;48(5):330–338. doi: 10.1159/000493549.
- Malluche HH, Blomquist G, Monier-Faugere M-C, et al. High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients on dialysis. J Am Soc Nephrol. 2015;26(10):2534–2544. doi: 10.1681/ASN.2014070686.
- Chertow GM, Burke SK, Raggi P, et al. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–252. doi: 10.1046/j.1523-1755.2002.00434.x.
- Chertow GM, Raggi P, Chasan-Taber S, et al. Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant. 2004;19(6):1489–1496. doi: 10.1093/ndt/gfh125.
- Nakao Y, Koike T, Ohta Y, et al. Parathyroid hormone enhances bone morphogenetic protein activity by increasing intracellular 3′, 5′-cyclic adenosine monophosphate accumulation in osteoblastic MC3T3-E1 cells. Bone. 2009;44(5):872–877. doi: 10.1016/j.bone.2009.01.370.
- Cheng Z-Y, Ye T, Ling Q-Y, et al. Parathyroid hormone promotes osteoblastic differentiation of endothelial cells via the extracellular signal-regulated protein kinase 1/2 and nuclear factor-kappaB signaling pathways. Exp Ther Med. 2018;15(2):1754–1760. doi: 10.3892/etm.2017.5545.
- Peng AW, Dardari ZA, Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571–1583. doi: 10.1161/CIRCULATIONAHA.120.050545.
- Romann A, Beaulieu MC, Rhéaume P, et al. Risk factors associated with arteriovenous fistula failure after first radiologic intervention. J Vasc Access. 2016;17(2):167–174. doi: 10.5301/jva.5000459.