Abstract
Anti-PD-1/PD-L1 antibodies are widely used in anti-cancer therapy. While they have improved cancer prognoses, immune-related adverse events, which can cause acute kidney injury (AKI), cannot be ignored. The purpose of this retrospective cohort study was to assess the incidence, risk factors, and prognosis of AKI associated with anti-PD-1/PD-L1 antibodies. Patients who received anti-PD-1/PD-L1 antibody treatment at our hospital between January 2018 and December 2022 were enrolled. Clinical information, combined medications, concomitant diseases, tumor types, and laboratory indicators were collected from patient records, and the incidence of AKI was determined. The risk factors for AKI were assessed using univariate and multivariate logistic regression analyses. Overall, 1418 patients were enrolled. The median follow-up time was 112 days and 92 (6.5%) developed AKI. The median time from the initial anti-PD-1/PD-L1 antibody treatment to AKI was 99.85 days. Head and neck cancer and combined use of diuretics, non-steroidal anti-inflammatory drugs (NSAIDs), lower hemoglobin level, and other types of chemotherapeutic drugs were independent risk factors for AKI. The complete recovery, partial recovery, non-recovery, and unknown AKI rates were 7.6%, 28.3%, 52.2%, and 11.9%, respectively. Kidney biopsies were performed on two patients with AKI and pathology confirmed diagnosis of acute tubulointerstitial nephritis. In this cohort, AKI was not uncommon in patients treated with anti-PD-1/PD-L1 antibodies; therefore, it is necessary to monitor renal function and identify AKI early, especially in patients with head and neck tumors. Improving anemia and minimizing the use of diuretics, NSAIDs, and chemotherapeutics may reduce AKI.
Introduction
Immune checkpoint inhibitors (ICIs) are more commonly used in the treatment of cancer and have become indispensable in the field of cancer immunotherapy [Citation1], substantially improving the prognosis and overall survival of patients with various cancers [Citation2,Citation3]. Immune checkpoint blockade enhances antitumor immunity by blocking innate immune checkpoints, including cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) or downregulated expression of its ligand, programmed cell death ligand 1 (PD-L1). ICIs not only boost the immune system of patients to fight disease but also enhance the immune response of the body, resulting in immune-related adverse reactions (irAEs), which can potentially affect any tissue, including the skin, gut, endocrine glands, liver, and lungs [Citation3–5]. When this adverse reaction occurs in the kidney, it can cause acute kidney injury (AKI) [Citation6].
AKI is a clinical syndrome characterized by a sudden decline in the glomerular filtration rate (GFR), manifested by increased serum creatinine levels or oliguria [Citation7], which increases the risk of death, cardiovascular events, and progression to chronic kidney disease [Citation8]. Several studies have reported variable incidence of AKI associated with treatment with anti-PD-1/PD-L1 antibodies. A previous meta-analysis based on clinical trials showed an incidence of 2.2% [Citation9]. With the increasing application of anti-PD-1/PD-L1 antibodies, the incidence of ICI-related AKI, in reality, may be higher, ranging from 3–18%, as reported in the recent literature [Citation10–15]. Some studies have shown that using proton pump inhibitors (PPIs), non-steroidal anti-inflammatory drugs (NSAIDs), and antibiotics at baseline is associated with the development of AKI [Citation11,Citation16], but others have not suggested a role for such risk factors [Citation12,Citation14]. As previous studies have reported inconsistent incidence and risk factors for AKI associated with anti-PD-1/PD-L1 antibody treatment, we believe that further research on this topic is warranted.
Anti-PD-1/PD-L1 antibodies have been introduced in clinical practice relatively recently. In 2014, the first anti-PD-1 antibody was approved by the United States Food and Drug Administration (FDA) for the treatment of patients with cancer [Citation17,Citation18]. Only in 2018 did anti-PD-1/PD-L1 antibodies become available in China [Citation19]. Therefore; few studies have investigated the relationship between anti-PD-1/PD-L1 antibodies and AKI, particularly in China. Furthermore, AKI incidence and associated risk factors remain controversial. Consequently, we aimed to explore the incidence, risk factors, and prognosis of AKI in patients with cancer treated with anti-PD-1/PD-L1 antibodies to facilitate the early clinical identification of AKI to ensure prompt intervention measures to reduce the occurrence of AKI.
Materials and methods
Patient population
We conducted a retrospective cohort study involving all patients treated with anti-PD-1/PD-L1 antibodies at Zhejiang Provincial People’s Hospital between January 2018 and December 2022. The exclusion criteria were as follows: (1) absence of baseline or follow-up creatinine values; (2) patients with end-stage renal disease (ESRD) or on dialysis before receiving anti-PD-1/PD-L1 antibodies; (3) patients who have undergone kidney transplantation; and (4) patients considered to have obstructive AKI or hemodynamic disorder AKI after adequate evaluation. The definition of Hemodynamic AKI is AKI occurring in the setting of dehydration (circulatory collapse, persistent diarrhea, frequent vomiting, etc.), ischemic or septic ATN conditions. Patients were followed up for as long as 12 months from the day of the first anti-PD-1/PD-L1 antibody treatment until an AKI event or death. The study was approved by the Ethical Research Committee of Zhejiang Provincial People’s Hospital (Approval NO: 2022 − 049). Due to the retrospective nature of this cohort study, informed consent was waived. No patients had received interventions, and the data were analyzed anonymously.
Clinical collection and definitions
The clinical data of each patient, including age, sex, body mass index (BMI), blood pressure, smoking history, drinking history, concomitant diseases, combined medication, tumor type, and laboratory data, were collected using the electronic medical record system. Concomitant diseases included chronic kidney disease (CKD), diabetes, hypertension, cardiovascular diseases, chronic obstructive pulmonary disease, and liver cirrhosis. Combined drugs included PPIs, renin-angiotensin-aldosterone system inhibitors (RAASis), NSAIDs, H2 receptor antagonists, iohexol, nephrotoxic antibiotics, diuretics, and chemotherapy drugs used in combination regimens, such as bevacizumab, cyclophosphamide, oxaliplatin, carboplatin, and cisplatin. Nephrotoxic antibiotics include vancomycin, aminoglycoside antibiotics, amphotericin B, and sulfonamides antibiotics. Diuretics include furosemide, torasemide, and spironolactone. The tumor types were divided into lung cancer, hepatobiliary and pancreatic system tumors, urologic neoplasms, urological neoplasms, gastrointestinal tract tumors, melanoma, reproductive system tumors, head-neck tumors, and breast cancer. The laboratory data included baseline creatinine values, eGFR values, serum albumin levels, anemia indicators, liver function indicators, lipid levels, and inflammatory indicators. Combination medication was referred to medication administered during anti-PD-1/PD-L1 antibody therapy. Extrarenal immune-related adverse events (IRAEs) included thyroid dysfunction, skin rash, drug-induced liver injury, fever, pneumonia and others.
The baseline creatinine level was defined as the creatinine level measured closest to the time of the first anti-PD-1/PD-L1 antibody treatment. Within 12 months after the first dose of anti-PD-1/PD-L1 antibody, AKI was defined as at least a 1.5-fold increase in the serum creatinine level from baseline within 7 days according to the Kidney Disease Improvement Global Outcomes (KDIGO) criteria for AKI and was graded according to the magnitude of the increase [Citation10,Citation20]. The estimated glomerular filtration rate (eGFR) was calculated using the eGFR-EPIformula. We analyzed the risk factors of AKI in patients treated with an anti-PD-1/PD-L1 antibody. Moreover, we conducted a statistical analysis of the outcomes of patients who experienced AKI. The outcome was determined by the degree of decrease in serum creatinine levels within 90 days after AKI as follows: (1) full recovery: serum creatinine dropped to baseline levels or below; (2) partial recovery: blood creatinine level decreased by ≥25% from the peak but was higher than the baseline blood creatinine level; (3) not recovered: serum creatinine levels decreased by <25% from the peak value, or patients were still dependent on dialysis treatment [Citation21]. Two nephrologists independently reviewed the etiology in all AKI cases, and a third physician resolved diagnostic discrepancies.
Statistical analysis
All continuous variables are represented by median or interquartile range. The t-test was used to compare groups for data conforming to the normal distribution, and the rank sum test was used to assess data that did not conform to a normal distribution. Categorical variables are expressed as counts and percentages, and the chi-square test was used to compare groups. Univariate and multivariate logistic regression analyses were used to determine risk factors for AKI, and variables with p-values < 0.05 in univariate logistic analysis were analyzed in multivariate logistic analysis. R software (version 3.6.2, R Foundation for Statistical Computing., Auckland, NZ) and IBM SPSS Statistics (version 26.0, IBM Corp., Armonk, NY, USA) were used for statistical analysis. Statistical significance was set at p < 0.05.
Results
We identified a total of 2065 patients who had been treated with anti-PD-1/PD-L1 antibodies, including 482 patients without baseline creatinine values, 131 patients without follow-up creatinine values, 4 patients with baseline estimated GFR (eGFR) <15 mL/min/1.73 m2, and 30 patients who were considered to have obstructive or hemodynamic AKI (). Overall, 1418 patients were included in our cohort, including 1025 males (72.3%), and the median age was 66 years (interquartile range [IQR]: 58–72) with a baseline eGFR value of 99.43 (IQR: 83.3–117.68) mL/min/1.73 m2. The proportion of patients who smoked and drank alcohol was not low: 424 patients (29.9%) had a history of smoking and 295 patients (20.8%) had a history of alcohol intake. The most common type of tumor was lung cancer 462 (32.6%), followed by gastrointestinal tumor in 379 (26.7%) and 323 (22.8%) cases of hepatobiliary pancreas tumor. Approximately 32.5% of patients had a history of hypertension. A total of 214 patients (15.1%) had diabetes, and only 13 patients (0.9%) had preexisting chronic kidney disease before treatment was initiated. Regarding laboratory measures, the baseline serum uric acid value was 303 (IQR: 244.3–371.0) µmol/L, and the hemoglobin and albumin levels were 11.9 (IQR: 10.6–13.2) g/dL and 36.1 (IQR: 32.9–39.3) g/L, respectively. A large proportion of patients (87.2%) also received PPI treatment, while 1013 (71.4%) patients had received anti-PD-1/PD-L1 antibody treatment combined with other types of chemotherapy agents. More than half of patients (55.5%) received iohexol during anti-PD-1/PD-L1 antibody therapy. Moreover, 62 (4.4%) patients died during follow-up.
Incidence and characteristics of AKI
During the study period, 92 (6.5%) patients experienced AKI events, and the proportion of patients with AKI stages I, II, and III were 75%, 18.5%, and 6.5%, respectively. The median time from the first treatment with anti-PD-1/PD-L1 antibodies to AKI was 99.85 (IQR: 32.8 8–155.72) days. The baseline eGFR of the AKI group was 103.82 (IQR: 84.06–123.84) mL/min/1.73 m2, and no patient had a history of chronic kidney disease before treatment with the anti-PD-1/PD-L1 antibodies in the AKI group. The patients with AKI and non-AKI were of similar ages, BMI, blood pressure, smoking history, mortality rate, concomitant diseases, baseline eGFR levels, and lipid levels in blood tests. The proportion of patients with head and neck cancer was significantly higher in the AKI group (15.2%) than in the non-AKI group (3.8%; p < 0.001). Patients in the AKI group were more likely to have urinary leukocytosis than those in the non-AKI group (8.25 [3.2, 18.5] vs. 4.7 [2.0, 13.0]; p = 0.004); however, the urine specific gravity was similar (1.02 [1.01, 1.02] vs. 1.02 [1.01, 1.02]; p = 0.073). Patients with high blood glucose levels, low serum albumin levels, and anemia were more likely to develop AKI. In the AKI group, 70 (76.1%) patients received NSAIDs, and 45 (48.9%) patients were treated with diuretics, which was significantly different from concomitant treatments in the non-AKI group (p < 0.01) (). Extrarenal IRAEs were less common in the AKI group than in the non-AKI group (1.3% vs. 9.6%). Furthermore, Hypothyroidism was less common in AKI (0.8% vs. 4.8%). Drug-induced liver injury, Fever, Pneumonia and others were not significantly different between the two groups (Supplementary Table 1).
Table 1. Baseline characteristics between AKI and non-AKI cohorts.
Risk factors for AKI in patients receiving anti-PD-1/PD-L1 antibodies
Univariate logistic analysis revealed that alanine aminotransferase, aspartate aminotransferase, uric acid, serum albumin, baseline eGFR level, blood glucose, serum lymphocyte count, hemoglobin level, sex, head and neck cancer, and combined use of NSAIDs, diuretics, and patients receiving chemotherapy were associated with the AKI (p < 0.05). A multivariate logistic regression analysis was conducted using the variables statistically significant in the univariate analysis. The results showed that lower hemoglobin level (odd ratio [OR]: 0.871; 95% confidence interval [CI]: 0.766–0.991; p = 0.037), head and neck tumors (OR: 5.036; 95%CI: 2.531–10.021; p = 0.001), combined use of diuretics (OR: 2.123; 95%CI: 1.331–3.389; p = 0.002), NSAIDs (OR: 2.078; 95% CI: 1.236–3.494; p = 0.006), and chemotherapy (OR: 2.076; 95% CI: 1.155–3.729; p = 0.015) were independent risk factors for AKI ().
Figure 1. Flowchart of the study design. PD-1: programmed death-1; PD-L1: programmed death ligand-1; AKI: acute kidney injury; ZJPH: Zhejiang Provincial people’s Hospital; scr: serum creatinine; ESRD: end-stage renal disease.
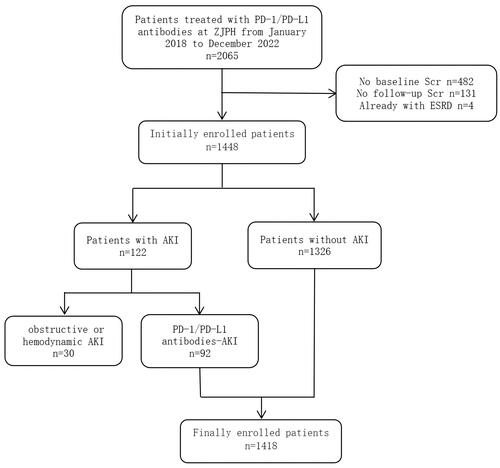
Figure 2. Multivariate logistic regression analysis of risk factors for AKI in patients receiving anti-PD-1/PD-L1 antibodies. NSAIDs: non-steroidal anti-inflammatory drugs; eGFR: estimated glomerular filtration rate; ALT: alanine transaminase; AST: Aspartate aminotransferase; UA: uric acid; ALB: albumin; glu: Fasting blood-glucose; LymphJ: Lymphocyte count; Hb: Hemoglobin.
Recovery of patients with AKI
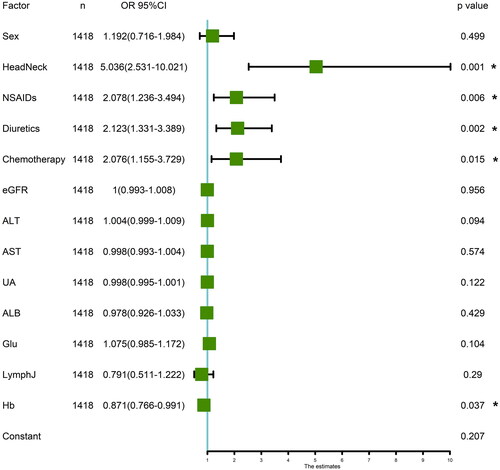
Ninety-two patients with AKI were followed up for at least 90 days: 7 patients (7.6%) achieved a complete recovery, 26 patients (28.3%) achieved partial recovery, 48 patients (52.2%) showed no recovery, and serum creatinine data was missing for 11 patients (11.9%) (). There were 69 patients (75%) in AKI stage 1, 17 patients (18.5%) in AKI stage 2, and 6 patients (6.5%) in AKI stage 3. Of the 69 patients with AKI stage 1, 6 patients (8.7%) achieved complete recovery and 18 patients (26.1%) achieved partial recovery, while others did not recover (56.5%) or were missing on follow-up (8.7%). Among the 17 patients with AKI stage 2, one patient (5.9%) recovered completely, 6 patients (35.3%) recovered partially, 6 patients (35.3%) did not recover. Of the 6 patients with AKI stage 3, none completely recovered, 2 patients (33.3%) achieved a partial recovery, and 3 patients (50%) did not recover ().
Figure 3. Recovery of renal function in patients with AKI. (A) Among a total of 92 patients with AKI, 7 patients (7.6%) recovered completely, 26 patients (28.3%) recovered partially, 48 patients (52.2%) did not recover; (B) There were 69 patients (75%) in AKI stage 1, 17 patients (18.5%) in AKI stage 2, and 6 patients (6.5%) in AKI stage 3. Of the 69 patients with AKI stage 1, 6 patients (8.7%) achieved complete recovery and 18 patients (26.1%) achieved partial recovery, while others did not recover (56.5%) or missed follow-up (8.7%). Among the 17 patients with AKI stage 2, one patient (5.9%) recovered completely, 6 patients (35.3%) recovered partially, 6 patients (35.3%) did not recovery. Among the 6 patients with AKI stage 3, no patient completely recovered, 2 patients (33.3%) partially recovered, and 3 patients (50%) did not recover. (C) Of the 92 patients with AKI, 58 (63%) continued the PD1 therapy. (D) In 92 patients with AKI and each stage, the number of patients with glucocorticoid use, continued using PD1/PDL1, and recovery of AKI within 90 days were shown, respectively.
Less then one third patients received corticosteroids and all 6 patients with AKI stage 3 received glucocorticoid therapy. The median duration of glucocorticoid use was 12.4 days, and the median total dose was 263.6 mg (), but there was no significant difference between different AKI stages. Close to two-thirds of patients were restarted with PD1 after AKI (). However, the differences between different AKI stages were also not significant.
Table 2. Subsequent major drug use after AKI.
Pathology
A renal biopsy was obtained from two patients with AKI, and the pathology findings showed that the main lesions were acute interstitial nephritis (AIN) and acute tubular injury ().
Figure 4. Pathological results of renal biopsy from two cases with AKI. (A–C) Renal tissue specimens from case 1, show the main lesion was acute tubulointerstitial injury; (D–F) from case 2, show acute interstitial nephritis as the main lesion. A: Hematoxylin and eosin (HE) stain shows the brush border of the renal tubules epithelial cells was desquamated, a few red cell casts were seen in the lumen (arrows, 10 × 40); B: Periodic acid-Schiff (PAS) stain shows a small amount of inflammatory cell infiltration in the renal interstitium (arrows, 10 × 40); C: PAS stain shows mild glomerular mesangial cell proliferation with increased mesangial matrix, and segmental endothelial cell swelling and proliferation (arrows, 10 × 40); D: HE stain shows renal interstitial inflammatory cell infiltration, including monocytes, lymphocytes, plasma cells, eosinophils, and neutrophils (arrows, 10 × 40). E: PAS stain shows that the renal tubules are extruded, the basement membrane is thickened, and the epithelial cells are swollen (arrows, 10 × 40); F: PAS stain shows mild glomerular mesangial cell proliferation and mesangial matrix increasing (arrows, 10 × 40).
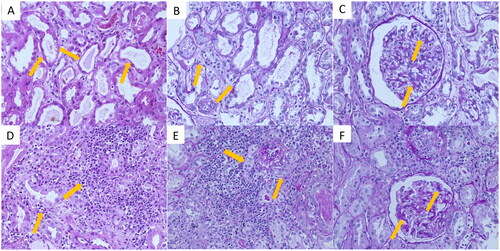
Case 1: A 69-year-old man diagnosed with liver cancer had a normal baseline creatinine (61.3 μmol/L) and eGFR (119.05 mL/min/1.73 m2) levels and received tislelizumab injection (anti-PD-1 antibody). His concomitant drugs includes pantoprazole, enalapril, celecoxib, iohexol, furosemide, cisplatin and cephalosporin antibiotics. The creatinine level increased to 288.3 μmol/L after 83 days of treatment initiation, and the renal biopsy showed the brush border of the renal tubular epithelial cells was shed, and a few red blood cell casts were seen in the lumen. The renal interstitium was diffusely edematous, and there was evidence of multifocal mononuclear, lymphocyte, and plasma cell infiltration in the interstitium. The glomerular mesangial area was mildly to moderately widened; mesangial cells proliferated; the mesangial matrix increased, and segmental endothelial cells were swollen and proliferated. The pathological impression was acute tubulointerstitial injury (). The serum creatinine level continued to increase despite the administration of glucocorticoids, and the patient was treated with hemodialysis without recovery of renal function.
Case 2: A 65-year-old man who underwent radical surgery for lung cancer had a normal baseline creatinine level (84 μmol/L) and an eGFR of 83.66 mL/min/1.73 m2. The patient was subsequently treated with camrelizumab and tislelizumab for lung cancer recurrence with multiple organ metastases. His concomitant drugs includes PPIs, NSAIDs, iohexol, furosemide, cyclophosphamide, oxaliplatin and cephalosporin antibiotics. The serum creatinine level increased to 291 μmol/L after 316 days of treatment. A renal biopsy was performed, and the pathology demonstrated that there was edema in the renal interstitium, with infiltration of diffuse monocytes, lymphocytes, plasma cells, and multifocal eosinophilic and neutrophilic cells. The renal tubules presented extrusions and multifocal tubular atrophy, basement membrane thickening, and localized brush border shedding. The glomeruli appeared nearly normal. The pathological impression was AIN (). After treatment with glucocorticoids, the creatinine level gradually decreased until 90 days after the onset of AKI; creatinine level was 128 μmol/L, indicating a partial recovery of renal function.
Discussion
This study is one of the largest retrospective cohort studies to assess AKI-related events in patients with cancer treated with anti-PD-1/PD-L1 antibodies. Overall, we enrolled 1418 oncology patients who received at least one dose of anti-PD-1/PD-L1 antibody treatment. During the follow-up period, 92 patients experienced AKI, with an incidence of AKI of 6.5%. The median time from the start of anti-PD-1/PD-L1 antibody treatment to AKI was 99.85 days. Head and neck cancer and combination therapy with chemotherapy drugs, NSAIDs, and diuretics, and lower hemoglobin levels were independent risk factors for AKI among patients with cancer treated with anti-PD-1/PD-L1 antibodies. The complete recovery, partial recovery, non-recovery, and unknown AKI rates were 7.6%, 28.3%, 52.2%, and 11.9%, respectively. Renal biopsy were performed on two patients with AKI, showing that the main pathological change was acute tubulointerstitial nephritis. In our study, the incidence of AKI associated with anti-PD-1/PD-L1 antibodies was 6.5%, which was similar to that reported by Ji et al. [Citation10] and Sorah et al. [Citation22]. However, previous studies have reported a higher incidence of AKI, ranging from 14.2 to 18% [Citation12–15]. A possible reason for this difference is that we screened for the etiology of AKI and excluded hemodynamic and obstructive causes. Furthermore, we did not include treatment with other ICIs such as CTLA-4 antagonists, which have been reported to cause higher rates of adverse events [Citation23,Citation24].
Using multivariate logistic regression analysis, we found that treatment with chemotherapeutic agents, NSAIDs, diuretics, lower hemoglobin levels and head and neck tumors were independent risk factors for AKI among patients with cancer treated with anti-PD-1/PD-L1 antibodies. Previous studies have found similar results [Citation10,Citation13,Citation16,Citation25]. NSAIDs are the most common drug-induced cause of AIN [Citation26]. The pathogenesis of AIN is often related to a type IV hypersensitivity reaction in which T cells play a central role; T cells are the most important lymphocytes in drug-induced AIN [Citation26,Citation27]. Normally, self-reactive T cells that leave the thymus are kept dormant by peripheral mechanisms, including CTLA-4 and PD-1 checkpoint signaling pathways. The tubule epithelium (TEC) expresses PD-L1, which is capable of protecting it from autoimmunity mediated by T cells [Citation28]. Interfering with checkpoint signaling by anti-PD-1/PD-L1 antibodies may lead to activation of self-reactive T cells, and thereby facilitate AKI occurrence [Citation6]. The use of diuretics can lead to an increased occurrence of AKI, which is possible because diuretics can cause decreased renal blood perfusion; hence, there is a risk of prerenal AKI [Citation29,Citation30], but this mechanism may not be the primary one because we excluded hemodynamic AKI. We speculate that treatment with antibodies to PD1 or PDL1 leads to activation of self-reactive T cells, which then leads to renal tubular epithelial cell dysfunction. The use of diuretics at this time is likely to increase the risk of urine casts obstruction in tubular. The exact details need to be confirmed by more basic experiments. Dumoulin et al. [Citation31] showed that combining chemotherapy and ICI therapy may lead to increased nephrotoxicity, which is consistent with our findings. Our study also showed that a lower hemoglobin level was a risk factor of AKI. Meanwhile, previous studies have reported that anemia increases the risk of AKI, possibly because it leads to decreased blood oxygen transport and insufficient effective circulation, which leads to increased oxygen consumption by renal tubular cells and promotes renal cell apoptosis [Citation32,Citation33]. Moreover, our study showed that head and neck tumors were independent risk factors for AKI, which has not been reported in previous studies. Most head and neck cancers are diagnosed in the advanced stages, with the involvement of lymph nodes in the local region [Citation34]. In approximately 10% of patients with locally advanced disease, distant metastases have already been developed [Citation35]. Furthermore, more than half of locally advanced head and neck cancers patients relapse despite aggressive local treatment with radical intent [Citation36]. These disease characteristics may have led to more intensive chemotherapy or immunotherapy in these patients, thereby increasing the incidence of adverse effects, including AKI. The results of our further analysis supported this inference. After further analysis, we found that compared with the non-head and neck cancer group, the head and neck cancer group had a higher proportion of alcohol consumption (33.8% vs 20.2%), and a higher proportion of combined use of other chemotherapy drugs (87.7% vs 70.7%). Further analysis by drug category showed that Platinum drugs were more commonly used in the head and neck cancer group 40 (61.5% vs. 30.6%). The Total number of PD1 and/or PDL1 doses administered was higher and the total number of doses was also higher (Supplementary Table 2). This novel finding could be considered a warning for clinical practice. Consequently, when patients with head and neck tumors receive treatment with anti-PD-1/PD-L1 antibodies, renal function should be monitored more frequently to evaluate the presence of adverse renal reactions.
Contrary to previous studies, we did not find that baseline eGFR level or PPI use was related to the occurrence of AKI using multivariate logistic regression analysis [Citation14,Citation37,Citation38]. We speculate that the possible reasons are as follows: the baseline eGFR levels of the patients included in our study were almost all within the normal range, and there was no comparison of cases with decreased eGFR levels. In addition, oncologists have become increasingly more aware of the nephrotoxicity of anti-PD-1/PD-L1 antibodies. When they initiate ICI therapy, patients with abnormal renal function are likely to be excluded. With regard to PPI use, a cohort with a larger sample size may be required, considering the high proportion (87.2%) of patients receiving PPI in our sample and the lack of negative controls. Seethapathy et al. [Citation11] also concluded that the role of PPI in AKI requires further clarification.
In our analysis, when evaluating the prognosis of 92 patients with AKI we found that more than half of the patients did not recover renal function, which is higher than that reported in previous studies [Citation37,Citation39]. This low rate of renal recovery prompted us to consider some potential causes, such as whether the occurrence of AKI was given sufficient attention by the attending physician, and whether the treatment and follow-up process lacked the of involvement of nephrologists. Our result showed less then one third patients with AKI received corticosteroids, and median duration of glucocorticoid use was less than two weeks, which means that the treatment of these patients was likely to be flawed, leading to a lower rate of AKI recovery. Therefore, early identification of risk factors for AKI, as in our study, and further increasing the attention, providing appropriate treatment strategies and monitoring frequency for potential AKI may improve the prognosis of AKI in the future. Using the hospital’s electronic medical record system, a renal dysfunction alert of the target population can be set up, so that nephrologists can be more actively involved in the management and follow-up of these patients. These approaches are expected to help improve the prognosis of patients with AKI and to prevent patients from losing the best anti-tumor treatment due to renal insufficiency.
Renal biopsies were performed on two patients in our cohort, and the pathological results suggested that the main lesions were AIN and acute tubular injury, which is consistent with previous studies [Citation6,Citation40–42]. In case 1, the patient experienced stage III AKI and was treated with steroid and hemodialysis. However, the patient’s outcome was still poor, and the patient also lost the opportunity to receive further anti-tumor therapy. Notably, the renal biopsy pathology of this patient revealed a few red blood cell casts were seen in the lumen, suggesting severe acute interstitial nephritis, which also reflects a possible role of renal biopsy in predicting clinical outcomes. This patient also serves as an example to underline that the adverse effects of AKI should not be ignored, and that early identification of AKI is important in clinical practice. Unfortunately, in clinical practice, patients were less willing to accept kidney biopsy. It has been reported in the literature that only 18.4% of AKI patients visited the nephrology department, and only about one-third of them received kidney biopsy [Citation43]. In our study cohort, the proportion was even lower. The major reason was that these patients were often in poor physical condition and cannot tolerate renal biopsy, or patients just refused the suggestion of renal biopsy. Nephrologists need to take actions to help patients recognize and understand this disease.
Our study also had some limitations that should be considered. First, we excluded patients with obstructive and hemodynamically impaired AKI from our screening of patients with AKI to be enrolled. Further, we could not identify coexisting cases because a renal biopsy was not performed, and there was no pathological evidence. Second, patients treated with anti-PD-1/PD-L1 antibodies are frequently treated with other drugs that have the potential to cause acute tubulointerstitial nephritis (ATIN), including NSAIDs, PPIs, etc. We do not have laboratory objective parameters to rule in anti-PD-1/PD-L1 antibodies mediated ATIN. Therefore, due to the such small number of kidney biopsy cases, our lineup may have resulted in the inclusion of AKI cases of non-anti-PD-1/PD-L1 antibodies induced ATIN. Third, this was a single-center retrospective study, and the results may have been subject to bias.
In conclusion, this study demonstrated that the incidence of AKI among patients with cancer treated with anti-PD-1/PD-L1 antibodies was 6.5%. The median time from the initial anti-PD-1/PD-L1 antibody treatment to AKI development was 99.85 days. Head and neck cancer and the combined use of NSAIDs, diuretics, lower hemoglobin levels, and combination regimens with chemotherapeutic drugs were independent risk factors for AKI. Complete or partial recovery was achieved in one-third of the 92 patients with AKI within 90 days in our cohort. Kidney biopsies were performed on two patients with AKI and pathology confirmed diagnosis of acute tubulointerstitial nephritis. In clinical practice, the occurrence of AKI among patients with cancer treated with anti-PD-1/PD-L1 antibodies requires increased attention, and it is necessary to monitor renal function and identify AKI early during anti-PD-1/PD-L1 antibody treatment. Further studies are necessary to accurately determine the predictors and biomarkers of AKI to provide a reference for future clinical studies.
Supplemental Material
Download PDF (189.6 KB)Supplemental Material
Download PDF (147.3 KB)Acknowledgments
We would like to thank our colleagues at Zhejiang Provincial People’s Hospital for their valuable contributions to this study. We are grateful for the technical support and data platform from Yidu Cloud Technology Company Ltd. We would like to thank Dr. Zenglei He for support with the statistical analysis. Lastly, we would like to thank Editage (www.editage.cn) for English language editing.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The datasets of this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:1–12. doi: 10.1146/annurev-pathol-042020-042741.
- Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18(6):345–362. doi: 10.1038/s41571-021-00473-5.
- Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481.
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016.
- Quach HT, Johnson DB, LeBoeuf NR, et al. Cutaneous adverse events caused by immune checkpoint inhibitors. J Am Acad Dermatol. 2021;85(4):956–966. doi: 10.1016/j.jaad.2020.09.054.
- Sprangers B, Leaf DE, Porta C, et al. Diagnosis and management of immune checkpoint inhibitor-associated acute kidney injury. Nat Rev Nephrol. 2022;18(12):794–805. doi: 10.1038/s41581-022-00630-8.
- Levey AS, James MT. Acute kidney injury. Ann Intern Med. 2017;167(9):ITC66–ITC80. doi: 10.7326/AITC201711070.
- Mercado MG, Smith DK, Guard EL. Acute kidney injury: diagnosis and management. Am Fam Physician. 2019;100(11):687–694.
- Manohar S, Kompotiatis P, Thongprayoon C, et al. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant. 2019;34(1):108–117. doi: 10.1093/ndt/gfy105.
- Ji MS, Wu R, Feng Z, et al. Incidence, risk factors and prognosis of acute kidney injury in patients treated with immune checkpoint inhibitors: a retrospective study. Sci Rep. 2022;12(1):18752. doi: 10.1038/s41598-022-21912-y.
- Seethapathy H, Zhao S, Chute DF, et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. 2019;14(12):1692–1700. doi: 10.2215/CJN.00990119.
- Meraz-Muñoz A, Amir E, Ng P, et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000467.
- Koks MS, Ocak G, Suelmann B, et al. Immune checkpoint inhibitor-associated acute kidney injury and mortality: an observational study. PLoS One. 2021;16(6):e0252978. doi: 10.1371/journal.pone.0252978.
- García-Carro C, Bolufer M, Bury R, et al. Acute kidney injury as a risk factor for mortality in oncological patients receiving checkpoint inhibitors. Nephrol Dial Transplant. 2022;37(5):887–894.
- Shimamura Y, Watanabe S, Maeda T, et al. Incidence and risk factors of acute kidney injury, and its effect on mortality among japanese patients receiving immune check point inhibitors: a single-center observational study. Clin Exp Nephrol. 2021;25(5):479–487. doi: 10.1007/s10157-020-02008-1.
- Seethapathy H, Zhao S, Strohbehn IA, et al. Incidence and clinical features of Immune-Related acute kidney injury in patients receiving programmed cell death ligand-1 inhibitors. Kidney Int Rep. 2020;5(10):1700–1705. doi: 10.1016/j.ekir.2020.07.011.
- Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. doi: 10.1172/JCI80011.
- Xia L, Liu Y, Wang Y. PD-1/PD-L1 blockade therapy in advanced Non-Small-Cell lung cancer: current status and future directions. Oncologist. 2019;24(Suppl. 1):S31–S41. doi: 10.1634/theoncologist.2019-IO-S1-s05.
- Zhe Lu, Guojun Zhang, Qunfeng Yao. The progress in cancer immunotherapy targeting PD-1/PD-L1 immune checkpoint. Journal of Modern Oncology. 2022;30(5):926–930.
- Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454.
- Ye Du, Luyu Fu, Yidan Guo, et al. Clinical characteristics of acute kidney injury in cancer patients receiving immune checkpoint inhibitors. Chin J Nephrol. 2022;38(9):802–810.
- Sorah JD, Rose TL, Radhakrishna R, et al. Incidence and prediction of immune checkpoint inhibitor-related nephrotoxicity. J Immunother. 2021;44(3):127–131. doi: 10.1097/CJI.0000000000000338.
- Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–2385. doi: 10.1093/annonc/mdx286.
- Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. doi: 10.1038/nrclinonc.2016.58.
- Gérard AO, Barbosa S, Parassol N, et al. Risk factors associated with immune checkpoint inhibitor-induced acute kidney injury compared with other immune-related adverse events: a case-control study. Clin Kidney J. 2022;15(10):1881–1887. doi: 10.1093/ckj/sfac109.
- Praga M, González E. Acute interstitial nephritis. Kidney Int. 2010;77(11):956–961. doi: 10.1038/ki.2010.89.
- Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol. 2010;6(8):461–470. doi: 10.1038/nrneph.2010.71.
- Ding H, Wu X, Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol. 2005;115(2):184–191. doi: 10.1016/j.clim.2005.01.005.
- Zhou L, Li Y, Gao Q, et al. Loop diuretics are associated with increased risk of Hospital-Acquired acute kidney injury in adult patients: a retrospective study. J Clin Med. 2022;11(13):3665.
- Scott J, Jones T, Redaniel MT, et al. Estimating the risk of acute kidney injury associated with use of diuretics and renin angiotensin aldosterone system inhibitors: a population based cohort study using the clinical practice research datalink. BMC Nephrol. 2019;20(1):481. doi: 10.1186/s12882-019-1633-2.
- Dumoulin DW, Visser S, Cornelissen R, et al. Renal toxicity from pemetrexed and pembrolizumab in the era of combination therapy in patients with metastatic nonsquamous cell NSCLC. J Thorac Oncol. 2020;15(9):1472–1483. doi: 10.1016/j.jtho.2020.04.021.
- Liang W, Yu CJ, Wang QY, et al. Anemia is associated with increased risk of contrast‑induced acute kidney injury: a systematic review and meta-analysis. Bioengineered. 2021;12(1):648–661. doi: 10.1080/21655979.2021.1883887.
- Han SS, Baek SH, Ahn SY, et al. Anemia is a risk factor for acute kidney injury and Long-Term mortality in critically ill patients. Tohoku J Exp Med. 2015;237(4):287–295. doi: 10.1620/tjem.237.287.
- Botticelli A, Cirillo A, Strigari L, et al. Anti-PD-1 and anti-PD-L1 in head and neck cancer: a network Meta-Analysis. Front Immunol. 2021;12:705096. doi: 10.3389/fimmu.2021.705096.
- Rothschild U, Muller L, Lechner A, et al. Immunotherapy in head and neck cancer - scientific rationale, current treatment options and future directions. Swiss Med Wkly. 2018;148:w14625. doi: 10.4414/smw.2018.14625.
- Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3305–3313. doi: 10.1200/JCO.2015.62.0963.
- Cortazar FB, Kibbelaar ZA, Glezerman IG, et al. Clinical features and outcomes of immune checkpoint Inhibitor-Associated AKI: a multicenter study. J Am Soc Nephrol. 2020;31(2):435–446. doi: 10.1681/ASN.2019070676.
- Gupta S, Short S, Sise ME, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9(10):e003467. doi: 10.1136/jitc-2021-003467.
- Qin Z, Liu K, Xu X, et al. Incidence, predictors and 6-month overall outcome of acute kidney injury in chinese patients receiving PD-1 inhibitors. Future Oncol. 2022;18(16):1951–1962. doi: 10.2217/fon-2021-1004.
- Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90(3):638–647. doi: 10.1016/j.kint.2016.04.008.
- Seethapathy H, Herrmann SM, Sise ME. Immune checkpoint inhibitors and kidney toxicity: advances in diagnosis and management. Kidney Med. 2021;3(6):1074–1081. doi: 10.1016/j.xkme.2021.08.008.
- Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45(2):160–169. doi: 10.1159/000455014.
- Oleas D, Bolufer M, Agraz I, et al. Acute interstitial nephritis associated with immune checkpoint inhibitors: a single-Centre experience. Clin Kidney J. 2021;14(5):1364–1370. doi: 10.1093/ckj/sfaa008.
