Abstract
Background
Methods for early prediction of the occurrence of acute kidney injury (AKI) were limited. The relationship between triglyceride glucose index (TyG) and the incidence of acute kidney injury in ICU patients is unclear. This study aims to explore the relationship between the two.
Methods
Based on their TyG index, participants from the Intensive Care Medical Information Market IV (MIMIC-IV) were divided into quartiles. A logistic regression model was constructed based on the risk of acute kidney injury as the main outcome, in order to detect a potential relationship that may exist between the TyG index and acute kidney injury in ICU patients. Finally, in order to confirm the relationship existing between the TyG index and the results, a restricted cubic spline model was used.
Results
In total, 54,263 patients were involved in our present study, of whom 48.2% were male. The occurrence of acute kidney injury was 25.1%. An independent relationship was observed between the TyG index and an increased risk of acute kidney injury through multivariate logistic regression analysis (OR, 1.28 [95% CI 1.22–1.35] p < 0.001). Q4 (5.344–9.911) of the TyG index quartiles was independently associated with an increase in the risk of acute kidney injury (OR, 1.43 [95% CI (1.32–1.54)] p < 0.001). Through the restricted cubic spline regression model, the risk of acute kidney injury was also demonstrated to increase linearly with an increase in the TyG index.
Conclusion
The triglyceride glucose index is related to the risk of acute kidney injury in ICU patients. In the future, in order to further validate this finding, larger prospective studies are needed.
1. Background
Acute kidney injury (AKI) is a commonly encountered issue among intensive care unit (ICU) patients, with an occurrence rate of 16–35% [Citation1–3]. According to conservative estimates, about 17 million people in the United-States are admitted to hospitals due to AKI every year, bringing more than $10 billion costs to the healthcare system [Citation4].
AKI has great impact on the mortality of ICU patients. Previous study has shown that AKI was a common in patients who are critically ill, with a hospital mortality rate of 62% that can be observed [Citation5]. A sudden decline in glomerular filtration rate (GFR) is usually observed in the early stages of AKI, but this kind of decrease is usually reversible [Citation6]. Therefore, we need an indicator for early diagnosis of AKI, so as to conduct clinical intervention as early as possible.
In the past few decades, the understanding of AKI has greatly improved, but early diagnosis remains a great challenge. The performance of traditional kidney function index and serum creatinine is very limited for the early diagnosis of acute kidney injury [Citation7]. We need to find more sensitive and rapid markers for the diagnosis of this disorder as early as possible.
Some studies have revealed that the triglyceride glucose index is associated with AKI [Citation8]. Nevertheless, the existing association between the TyG index and AKI among the ICU population is still unclear, so this study selected TyG index as an indicator to determine the extent of acute kidney injury. This study therefore conducted research on the association between the TyG index and AKI among ICU patients.
2. Methods
2.1. Subjects
This present study is a cross-sectional study. The data we analyzed were derived from the publicly available Intensive Care IV (MIMIC-IV) database [Citation9]. MIMIC-IV is an extensive and free database, which contains information about patients accepted to the ICU of a large tertiary care hospital in Boston, from 2008 to 2019.
The author (ZJ) joined and completed online the course on protecting human research participants proposed by the US National Institutes of Health’s, and therefore obtained permission to access the dataset. The certificate number is 53477982. The use of the database for research was then authorized by the review committee of MIT and Beth Israel Deaconess Medical Center, and a waiver of informed consent was also granted to us. We followed the STROBE guidelines for observational research.
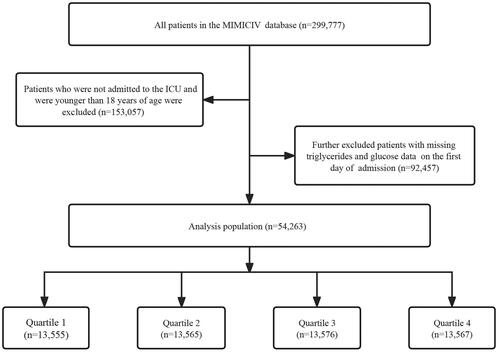
We included 299,777 patients from MIMIC-IV, and subsequently excluded patients who were not admitted to ICU and/or were less than 18 years of age. Afterwards, participants who did not have triglycerides (TG) and blood glucose data on their first day of admission were further excluded. 54,263 patients were finally involved in the ultimate study, and based on their TyG index quartiles on the first day of admission to the ICU, they were separated into four groups ().
2.2. Data collection
Baseline characteristics were extracted using The Structured Query Language (SELECT) of Navicat Premium (Version 16), including gender, age, ethnicity, laboratory indicators, and comorbidities. The triglyceride glucose index was calculated using the following method: ln [fasting TG (mg/dl)]×Fasting blood glucose (mg/dl)]/2 [Citation10]. Acute myocardial infarction, atrial fibrillation, cerebral infarction, chronic kidney disease, diabetes, heart failure, hyperlipidemia, hypertension and respiratory failure were defined by the ICD-9 or ICD-10 codes. In MIMIC-IV, variables with missing data are common, and missing values for all the screening variables were <5%, so interpolation was not used.
2.3. Main results and clinical definitions
The risk of acute kidney failure was the main outcome of this study. Conforming to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, AKI is established as a serum creatinine (SCr) level that is ≥0.3 mg/dL above baseline within 48 h, or urinary output is <0.5 mL/kg/h for 6 h [Citation11]. Lacking of the data on urinary output in MIMIC-IV, therefore, AKI was diagnosed by SCr level in this study.
2.4. Statistical analysis
The distribution of baseline data was shown as different result groups for patients involved in this study. Classified data were expressed in numbers (percentages), while continuous data were expressed in mean ± standard deviation or median (interquartile range), as appropriate. The analysis of variance test or the rank sum test was used for the examination of the differences in continuous variables. To compare the characteristics of the subjects in the result group, the Chi-square test or the Fisher’s exact test for categorical variables was used.
Due to the small percentage of missing data for hemoglobin, lymphocytes, neutrophils, platelet count, potassium, red blood cells, serum creatinine, sodium, urea nitrogen, and white blood cells (the missing rate varied from 0.03% to 1.79%), no interpolation method was utilized. Assessment of the independent association between the TyG index and the risk of acute kidney injury was conducted using a multivariate logistic regression analysis. The triglyceride glucose index was input as a categorical variable (quartile) and a continuous variable (a risk ratio (OR) was calculated for each additional unit). Four models were used in the regression analysis. Adjustments were made to the multivariable models, as follows: No adjustments were made to Model 1; adjustments were made for Model 2 according to age, gender, and ethnicity; Model 3 was adjusted according to Model 2 by adding glucose, triglycerides, hemoglobin, lymphocytes, neutrophils, platelet count, potassium, red blood cells, serum creatinine, sodium, urea nitrogen, and white blood cells; Model 4 was adjusted according to Model 3 by adding acute myocardial infarction, atrial fibrillation, cerebral infarction, chronic kidney disease, diabetes, heart failure, hyperlipidemia, hypertension, and respiratory failure.
The possible linear relationship between the triglyceride glucose index and the risk of acute kidney injury was explored using a restricted quadratic spline model. The lowest TyG index value quartile was used as a reference group for analysis, and the covariates described above (Model 4) were adjusted. By using the quartile level as a sequential variable, the P value of the trend was obtained.
We further conducted a hierarchical analysis based on age, gender, atrial fibrillation, and respiratory failure, so as to determine the robustness of the TyG index in predicting the risk of acute kidney failure. To examine the interaction between the triglyceride glucose index and the variables that were used for stratification, the likelihood-ratio test was used. A two-tailed test was conducted in our study, and a value of p < 0.05 was considered as statistically significant. The R statistical software package (http://www.R-project.org, R Foundation) and the Fengrui statistical software version 1.7.1 [Citation12] were used for all analyses.
3. Results
A total of 54,263 patients were eventually involved in this study. The average age of the involved patients was 59.3 ± 17.7 years old, and 26,140 (48.2%) were male. Among all the enrolled participants, the average triglyceride glucose index value was 10.4 ± 0.7. The incidence of acute kidney injury was 25.1%.
3.1. Baseline characteristics
Based on the TyG index quartiles, the baseline characteristics of the patients are shown in . Participants were separated into quartiles based on the level of their triglyceride glucose index at admission (quartiles - Q1: 5.344–9.911; Q2: 9.911–10.328; Q3: 10.328–10.81; Q4: 10.81–16.337). The mean values of the TyG index were 9.6 ± 0.3, 10.1 ± 0.1, 10.6 ± 0.1, and 11.4 ± 0.6 for the four groups, respectively. Compared with lower groups, patients with higher TyG index had a more elevated prevalence of acute myocardial infarction, chronic kidney disease, diabetes, hyperlipidemia, hypertension, higher levels of blood glucose, triglycerides, neutrophils, serum creatinine, urea nitrogen, white blood cells, and lower levels of lymphocytes. The incidence of acute kidney injury (18.5% vs. 22.6% vs. 26.1% vs. 33.2%, p < 0.001) gradually increased with the increase of TyG index.
Table 1. Baseline characteristics of critical patients grouped according to TyG index quartiles.Table Footnotea
3.2. Univariate logistic regression analysis of the incidence rate of acute kidney failure
TyG index was a risk factor associated with AKI (). However, univariate analysis also showed that gender, age, glucose, triglycerides, hemoglobin, lymphocytes, neutrophils, platelet count, potassium, red blood cells, serum creatinine, sodium, urea nitrogen, white blood cells, acute myocardial infection, atrial fibrillation, cerebral infarction, chronic kidney disease, diabetes, heart failure, hyperlipidemia, hypertension, and respiratory failure were factors associated with AKI (p < 0.05).
Table 2. Univariate logistic regression analysis of incidence rate of acute renal failure.
3.3. Logistic regression analysis of multifactorial TyG index and acute kidney injury
After that adjustments have been made in the multivariate analysis, the TyG index was found to be significantly associated with AKI (). For each unit growth in the triglyceride glucose index, the incidence of AKI grew by 1.28-fold (p < 0.001). After TyG index quartiles stratification, the incidence of AKI in the highest TyG index subgroup (Q4) increased by 1.43-fold compared with the subgroup with the lowest TyG index (Q1) (p < 0.001).
Table 3. Multivariate logistic regression analyses of TyG index and incidence of acute kidney failure.
Table 4. Effect size of TyG index on the incidence of acute kidney failure in each subgroup.
3.4. Restricted cubic spline regression model
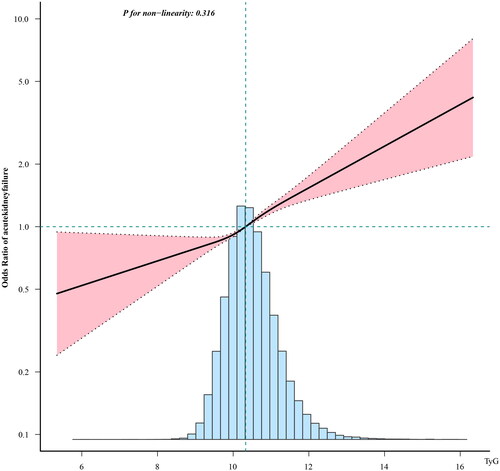
The risk of AKI increased linearly with the growth of the TyG index ().
Figure 2. Linear dose-response relationship between triglyceride-glucose index and incidence of acute kidney failure. Adjustment factors included sex, age, race, glucose, triglycerides, hemoglobin, lymphocytes, neutrophils, platelet count, potassium, red blood cells, serum creatinine, sodium, urea nitrogen, white blood cells, acute myocardial infarction, atrial fibrillation, cerebral infarction, chronic kidney disease, diabetes, heart failure, hyperlipidemia, hypertension, and respiratory failure. The black line and pink line represent the estimated values and their corresponding 95% confidence intervals, respectively.
3.5. Hierarchical analysis of the incidence of acute kidney injury according to TyG index
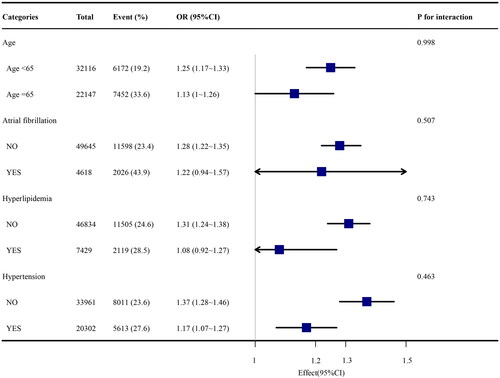
We further evaluated the risk predictors of TyG index of the main outcomes for different subgroups of the patients, including age, atrial fibrillation, and respiratory failure. In the subgroup of patients without respiratory failure, it was found that the TyG index was remarkably associated with a more elevated risk of AKI (OR, 1.3 [95% CI (1.23–1.38)] p < 0.001).
4. Discussion
From what we know, this present research is the first one to have explored the relationship that may exist between the TyG index and the risk of AKI in ICU patients. The key finding emerging from our results shows that, even after adjusting the potential confounding variables, ICU patients with increased TyG index have a higher risk of AKI. Most importantly, a novel, simple, and effective biomarker is provided through this study, for the early warning of AKI in ICU patients.
Currently, plasma creatinine levels are the basis for the diagnosis of AKI. However, an increase in creatinine levels can only be observed within a few days after kidney injury. According to a previous study, serum creatinine is not a reliable indicator of acute kidney injury, which may result in an underestimation of the number of patients who will develop acute kidney injury during the acute phase [Citation13]. Study showed that compared with SCr, urine output may be a more sensitive indicator for the diagnosis of AKI [Citation14]. However, the differential diagnosis of oliguria in critically ill patients is difficult due to the inability to monitor GFR and/or RBF in real time. Therefore, the diagnostic performance of serum creatinine in acute kidney injury is not good enough. Urine Biomarker [TIMP-2] × [IGFBP7] was proposed to predict the occurrence of AKI [Citation15], but previous studies have found that urinary TIMP-2 × IGFBP7 levels could only predict the occurrence of stage 2–3 AKI. In addition, urinary TIMP-2 × IGFBP7 levels was varied for time of urine collection [Citation16]. Previous markers such as NGAL [Citation17] and KIM-1 [Citation18] have defects such as low sensitivity and poor specificity, and cannot be widely used in clinical practice. For this reason, a new indicator should be sought to predict the risk of this disorder in ICU patients at an early stage. The TyG index was an accessible and reliable test for estimating insulin resistance (IR), which closely mirrors the glucose clamp technique, the gold standard test in the assessment of insulin sensitivity [Citation19].
As a new clinical surrogate index for IR, The TyG index was often used to predict cardiovascular risk. Studies from our country and abroad have revealed that the TyG index is related to the risk and development of kidney diseases. According to the report of Song et al. the rise of the TyG index is associated with atherosclerosis and kidney tubular epithelial cell damage [Citation20]. Shi’s research found that there is a strong connection between the TyG index and the decrease in Egfr [Citation21]. According to the study by Liu et al. adults with higher TyG index have a higher urinary albumin to creatinine ratio (UACR) [Citation22]. According to a Korean study, proteinuria is particularly associated with high TyG index in patients with RHF [Citation23]. Professor Li’s research on TyG index and kidney injury in middle-aged and elderly people has shown that there is a positive and independent relationship between the TyG index and worsening renal function [Citation24]. Qin’s research showed that, in patients with diabetes, high TyG levels are positively associated with the occurrence of contrast induced acute kidney injury (CI-AKI) [Citation8]. In spite of all these findings, there are currently limited data on the association between the triglyceride glucose index and acute kidney injury.
Hyperglycemia is frequent in critically ill patients, and can occur even in those who don’t have diabetes [Citation25,Citation26]. Currently, research on the mechanism of hyperglycemia in ICU patients continues. Studies from the past have shown that stress hyperglycemia combined with high liver glucose output and insulin resistance are common in ICU due to inflammation and neuroendocrine disorders [Citation27]. Therefore, in ICU patients, the proportion of hyperglycemia is very high. Moreover, existing study have shown that hyperglycemia is an independent predictor of AKI [Citation28]. Hyperglycemia can cause glomerular blood flow and vascular permeability abnormalities, including capillary obstruction, tissue hypoxia and glomerulosclerosis, which would lead to proteinuria and then AKI [Citation29,Citation30].
Abnormal blood lipids have already been proven to be essential in the development and progression of kidney disease [Citation31]. Although the mechanism behind this phenomenon has not yet been fully clarified, it has been suggested that lipids may damage the blood vessels, mesangial cells, and tubular cells of the kidneys. Moorhead et al. were the first to propose the “lipid nephrotoxicity hypothesis” [Citation32]. Currently, studies have demonstrated that hypertriglyceridemia is an independent risk factor of early AKI [Citation33].
TyG index can simultaneously monitor changes in triglycerides and glucose, and it is also a reliable indicator for IR. We hypothesis that IR can affect the pathogenesis of AKI through the following aspects. First, hyperglycemia and hyperinsulinemia itself can increase urinary protein excretion by inducing glomerular hyperfiltration, impairing endothelial function, and increasing vascular permeability [Citation29,Citation30]. Second, hyperinsulinemia can increase the production of reactive oxygen species in tissues, induce oxidative stress, and promote inflammation [Citation34,Citation35]. Thirdly, Metabolic acidosis caused by hyperglycemia can stimulate the excessive secretion of TNF-α, interleukin-6 (IL-6), and plasminogen activator inhibitior-1 which cause increased vascular permeability, and leukocyte and platelet activation resulting in an inflammatory and pro-thrombotic state[Citation36].
TyG index has the advantages of being simple, easy to access, and low in cost. Triglyceride and glucose levels can be obtained through routine measurements, and the cost of these measurements is very low. Therefore, we chose TyG index to analyze the risk of AKI in ICU patients. To be noticed, in the subgroup of patients without respiratory failure, it was found that the TyG index was remarkably associated with a more elevated risk of AKI. It is believed that this is the result of survivor bias. In other subgroups, significant interactions were not observed (P value of interaction >0.05) ().
According to the above data, TyG index seems to have high reliability and effectiveness as an indicator for predicting the incidenceof AKI. Our results showed that high TyG levels are closely related to an increased incidence rate. AKI ultimately leads to chronic kidney insufficiency, uremia, or death. Our results might help identify the high-risk patients in clinic, and take action in advance to prevent patient developing AKI. So far, there have been no clinical studies on the association between acute kidney injury and TyG markers in ICU patients. Our investigation has filled this gap.
4.1. Study limitations
Firstly, this is a horizontal survey, so a causal relationship cannot be proven. However, we have conducted multifaceted and rigorous statistical methods with great vigilance, resulting in effective and reliable outcomes. This was also a retrospective study, in order to validate the relationship between TyG and AKI, further cohort studies are necessary. Secondly, all data came from the United States. In addition, urinary output was not mentioned in the database, so the definition of AKI in the present paper only used the SCr criterion of KDIGO 2012 definition. Therefore, the results may not be fully applicable to other countries. However, we have registered patients of different races, so this study is still representative.
5. Conclusion
In ICU patients, it was noticed that the occurrence of AKI increased with the rise of the TyG index. Therefore, the measurement of the TyG index may be helpful for risk stratification and early prediction of AKI in ICU patients. For this reason, more research is necessary to ascertain whether the TyG index can be used as a key intervention element to improve the clinical incidence rate of this particular population.
Ethical approval and consent
This study was conducted in accordance to the Declaration of Helsinki, and the MIMIC-IV database has also been approved by the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center. Copies of these data can be found in the MIMIC database. Therefore, this trial does not require ethical approval or informed consent.
Authors contribution
ZHJ was in charge of data extraction, data analysis, and redaction of the papers. KZ reviewed the main knowledge points of the manuscript, and modifications were made when necessary. The submission of the paper was approved by all authors.
Acknowledgements
We appreciate Dr. Jie Liu of the Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital for statistics, study deign consultations and editing the manuscript.
Data availability statement
The dataset generated and analyzed in this study can be obtained from the authors upon reasonable request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Thakar CV, Christianson A, Freyberg R, et al. Incidence and outcomes of acute kidney injury in intensive care units: a veterans administration study. Crit Care Med. 2009;37(9):1–9. doi: 10.1097/CCM.0b013e3181a5906f.
- Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35(8):1837–1843; quiz 1852. doi: 10.1097/01.CCM.0000277041.13090.0A.
- Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35(10):1692–1702. doi: 10.1007/s00134-009-1530-4.
- Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740.
- Chang C-H, Fan P-C, Chang M-Y, et al. Acute kidney injury enhances outcome prediction ability of sequential organ failure assessment score in critically ill patients. PLoS One. 2014;9(10):e109649. doi: 10.1371/journal.pone.0109649.
- Micarelli D, Cristi E, Taddei AR, et al. A case of acute renal failure with multiple origins of the renal injury. CEN Case Rep. 2020;9(4):437–441. doi: 10.1007/s13730-020-00505-6.
- Lei L, Li LP, Zeng Z, et al. Value of urinary KIM-1 and NGAL combined with serum cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep. 2018;8(1):7962. doi: 10.1038/s41598-018-26226-6.
- Qin Y, Tang H, Yan G, et al. A high triglyceride-glucose index is associated with contrast-induced acute kidney injury in chinese patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2020;11:522883. doi: 10.3389/fendo.2020.522883.
- Johnson A, Bulgarelli L, Pollard T, et al. Mimic-IV. Version 1.0). PhysioNet. 2021;101(23):e215–e220. doi: 10.13026/s6n6-xd98.
- Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034.
- Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454.
- Yang Q, Zheng J, Chen W, et al. Association between preadmission metformin use and outcomes in intensive care unit patients with sepsis and type 2 diabetes: a cohort study. Front Med (Lausanne). 2021;8:640785. doi: 10.3389/fmed.2021.640785.
- Forni LG, Darmon M, Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855–866. doi: 10.1007/s00134-017-4809-x.
- De Vlieger G, Forni L, Schneider A. New diagnostics for AKI in critically ill patients: what to expect in the future. Intensive Care Med. 2022;48(11):1632–1634. doi: 10.1007/s00134-022-06843-6.
- Vijayan A, Faubel S, Askenazi DJ, et al. Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis. 2016;68(1):19–28. doi: 10.1053/j.ajkd.2015.12.033.
- Wang Y, Zou Z, Jin J, et al. Urinary TIMP-2 and IGFBP7 for the prediction of acute kidney injury following cardiac surgery. BMC Nephrol. 2017;18(1):177. doi: 10.1186/s12882-017-0592-8.
- Yuan S-M. Acute kidney injury after cardiac surgery: Risk factors and novel biomarkers. Braz J Cardiovasc Surg. 2019;34(3):352–360. doi: 10.21470/1678-9741-2018-0212.
- Tanase DM, Gosav EM, Radu S, et al. The predictive role of the biomarker kidney molecule-1 (KIM-1) in acute kidney injury (AKI) cisplatin-induced nephrotoxicity. Int J Mol Sci. 2019;20(20):5238. doi: 10.3390/ijms20205238.
- Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288.
- Zhao S, Yu S, Chi C, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern shanghai study. Cardiovasc Diabetol. 2019;18(1):95. doi: 10.1186/s12933-019-0898-x.
- Shi W, Liu S, Jing L, et al. Estimate of reduced glomerular filtration rate by triglyceride-glucose index: insights from a general chinese population. Postgrad Med. 2019;131(4):287–294. doi: 10.1080/00325481.2019.1595983.
- Liu N, Liu C, Qu Z, et al. Association between the triglyceride-glucose index and chronic kidney disease in adults. Int Urol Nephrol. 2023;55(5):1279–1289. doi: 10.1007/s11255-022-03433-9.
- Oh D, et al. High Triglyceride-Glucose index with renal hyperfiltration and albuminuria in young adults: the korea national health and nutrition examination survey (KNHANES V, VI, and VIII). J Clin Med. 2022;11(21):6419. doi: 10.3390/jcm11216419.
- Lei L, Liang H, Qu Y, et al. Association between triglyceride-glucose index and worsening renal function in the elderly. Front Nutr. 2022;9:951564. doi: 10.3389/fnut.2022.951564.
- Olariu E, Pooley N, Danel A, et al. A systematic scoping review on the consequences of stress-related hyperglycaemia. PLoS One. 2018;13(4):e0194952. doi: 10.1371/journal.pone.0194952.
- Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response!. Crit Care. 2013;17(2):305. doi: 10.1186/cc12514.
- Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–1807. doi: 10.1016/S0140-6736(09)60553-5.
- Shacham Y, Gal-Oz A, Leshem-Rubinow E, et al. Admission glucose levels and the risk of acute kidney injury in nondiabetic ST segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. Cardiorenal Med. 2015;5(3):191–198. doi: 10.1159/000430472.
- Thomas JL, Pham H, Li Y, et al. Hypoxia-inducible factor-1α activation improves renal oxygenation and mitochondrial function in early chronic kidney disease. Am J Physiol Renal Physiol. 2017;313(2):F282–f290. doi: 10.1152/ajprenal.00579.2016.
- Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. doi: 10.2337/dc13-2112.
- Li J, Guan M, Li C, et al. The dipeptidyl peptidase-4 inhibitor sitagliptin protects against dyslipidemia-related kidney injury in apolipoprotein E knockout mice. Int J Mol Sci. 2014;15(7):11416–11434. doi: 10.3390/ijms150711416.
- Moorhead JF, et al. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2(8311):1309–1311.
- Wu C, Ke L, Tong Z, et al. Hypertriglyceridemia is a risk factor for acute kidney injury in the early phase of acute pancreatitis. Pancreas. 2014;43(8):1312–1316. doi: 10.1097/MPA.0000000000000180.
- Dimova R, Chakarova N, Grozeva G, et al. The relationship between glucose variability and insulin sensitivity and oxidative stress in subjects with prediabetes. Diabetes Res Clin Pract. 2019;158:107911. doi: 10.1016/j.diabres.2019.107911.
- Bolton CH, Downs LG, Victory JG, et al. Endothelial dysfunction in chronic renal failure: roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2001;16(6):1189–1197. doi: 10.1093/ndt/16.6.1189.
- Garg R, Chaudhuri A, Munschauer F, et al. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37(1):267–273. doi: 10.1161/01.STR.0000195175.29487.30.