Abstract
Introduction
To establish a prediction model to predict immunosuppressive medication (IM) nonadherence in kidney transplant recipients (KTRs) based on a combined theory framework.
Methods
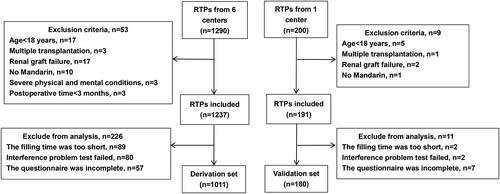
This polycentric, cross-sectional study included 1191 KTRs from October 2020 to February 2021 in China, with 1011 KTRs enrolled in the derivation set and 180 in the external validation set. Variables selected based on the combined theory of planned behavior (TPB)/health belief model (HBM) theory were analyzed by the least absolute shrinkage and selection operator (LASSO). Internal 10 cross-validation was conducted to determine the optimal lambda value. The receiver operating characteristic (ROC) curve, specificity, and sensitivity were used to evaluate the prediction model, and further assessment was run by external validation.
Results
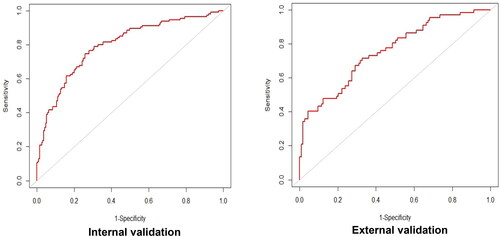
IM nonadherence rate was 38.48% in the derivation set and 37.22% in the validation set. The LASSO model was developed with eight predictors for IM nonadherence: age, preoperative drinking history, education, marital status, perceived barriers, social support, perceived behavioral control, and perceived susceptibility. The model demonstrated acceptable discrimination with the area under the ROC curve of 0.797 (95% CI: 0.745–0.850) in the internal validation set and 0.757 (95% CI: 0.684–0.829) in the external validation set. The specificity and sensitivity in the internal validation and external validation set were 0.741, 0.748, 0.673, and 0.716, respectively.
Conclusions
The LASSO model was developed to guide identifying high-risk nonadherent patients and timely and effective interventions to improve their prognosis and survival.
Introduction
Kidney transplant is the most effective therapy for end-stage kidney disease [Citation1]. Strict adherence to immunosuppressive medication (IM) postoperatively is crucial to prevent transplant rejection and remain the normal function of the transplanted kidney, thus avoiding allograft loss. Although the short-term 1-year survival rate was reported to be exceeding 95% [Citation2], the long-term survival of kidney transplant recipients (KTRs) has not improved synchronously mainly due to medication nonadherence [Citation3]. Medication nonadherence may be defined as ‘deviation from the prescribed medication regimen sufficient to influence adversely the regimen’s intended effect’ [Citation4]. IM nonadherence is common among KTRs, and its prevalence varies across studies due to different definitions and measurements. A recent review reported an average prevalence of IM nonadherence between 36% and 55% [Citation5]. IM nonadherence contributes to approximately 60% of acute rejection and is associated with a sevenfold increased risk for graft loss compared to adherent KTRs [Citation6–8]. In addition, IM nonadherence also incurs a significant economic burden and is reported to account for 3–10% of total healthcare costs in the US annually [Citation9].
Early identifying and predicting IM nonadherence is essential in designing effective interventions and improving postoperative outcomes among KTRs. The WHO summarized all possible factors of IM nonadherence, including (1) socioeconomic, (2) patient-related, (3) disease-related, (4) treatment-related, and (5) health provider and system [Citation10]. Based on this classification, a wide range of factors have been identified that may contribute to IM nonadherence, including socioeconomic status, health beliefs, social support, medicine doses, side effects, etc. [Citation5]. Due to the large variety of influencing factors for medication nonadherence, it is crucial to establish scientific and accurate prediction models based on a well-developed theoretic framework to guide future interventions to improve medication adherence. Although several models have been developed to predict medication nonadherence among KTRs, their clinical application is restricted by certain limitations. For instance, Paterson et al. [Citation11] developed a latent variable model of medication adherence in KTRs but only included psychosocial and neurocognitive factors; thus, it was not able to capture a full picture of all potential influencing factors. Zhu et al. [Citation12] developed a prediction model based on machine learning technology and presented the predicted results by formulation, which was complex to apply to clinical practice. So far, few models were practical and well-suited to predict IM nonadherence in KTRs, and an innovative model that is theory-based, comprehensive, and easy to apply is needed.
The least absolute shrinkage and selection operator (LASSO) regression removes unimportant variables via the regression coefficients and penalizing the size of the parameters [Citation13]. As such it is commonly used in studies in fields with large numbers of explanatory variables to reduce the variable space. The LASSO-based model has been used to diagnose and predict diseases widely [Citation14]. The potential utilization of LASSO regression outside its usual application in the area of variable selection has been proved, especially for real-time forecasting [Citation15]. In terms of a variety of factors, the application of a prediction model based on LASSO in medication adherence monitoring among KTRs is promising.
Zhang et al. have developed a combined theory of planned behavior (TPB)/health belief model (HBM) which showed improved predictive abilities than any single model in predicting IM nonadherence [Citation16]. The HBM model is a useful framework for understanding the influencing factors of medication nonadherence and includes four major constructs: perceived susceptibility, perceived severity, perceived benefits, and perceived barriers [Citation17]. A previous application of the HBM model among KTRs has shown that perceived barriers and perceived severity were associated with IM nonadherence [Citation18]. The TPB model is another important framework that has been widely used in the prediction and change of health-related behaviors and consists of four constructs: attitudes, perceived behavioral control, subjective norms, and intentions [Citation19,Citation20]. However, as a psychosocial-cognitive model, the TPB model did n’ot include any demographic and clinical factors that have been demonstrated to affect IM nonadherence. A combined TPB/HBM theory (shown in ) [Citation16] that added the HBM variable to the TPB model has been shown to enhance the predicted power of IM nonadherence in KTRs.
Figure 1. The combined TPB/HBM theory [Citation16].
![Figure 1. The combined TPB/HBM theory [Citation16].](/cms/asset/9e313a59-a9fe-443e-b491-e998e8d6a80c/irnf_a_2238832_f0001_b.jpg)
This study aimed to establish a prediction model for IM nonadherence by the LASSO approach based on the combined TPB/HBM theory, which will help identify high-risk IM nonadherence patients and guide targeted interventions to improve adherence.
Materials and methods
This study was performed with explicit adherence to Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD).
Study population
This polycentric, cross-sectional study was conducted on 1191 KTRs admitted to seven different hospitals in China from October 2020 to February 2021. The inclusion criteria were as follows: (1) age ≥18 years; (2) no graft failure after kidney transplant; (3) no exercise taboo (such as paralysis, osteoarthropathy, and cardiopulmonary disease); KTRs who were not Mandarin-speaking, had multiple transplantations, with cognition/mental disorders, and with postoperative time <3 months were excluded. A total of 1011 KTRs were enrolled in the derivation set and 180 in the validation set [Citation12]. The flowchart of enrollment is illustrated in .
This study was approved by the Ethics Committee of the Third Xiangya Hospital (Changsha, China) (no.: 2019-S61). Written informed consent was obtained from all the participants.
Defined criteria for IM nonadherence
In our study, IM nonadherence was determined with the Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) [Citation21], measuring the implementation adherence in the last four weeks via four items: taking adherence, drug holidays, timing adherence, and self-initiated dose reductions. Patients with any answer option as ‘yes’ are determined as nonadherent. The Chinese version of BAASIS has been validated in transplant recipients (Cronbach’s α, 0.755) [Citation22,Citation23].
Data collection and instruments
Based on the literature review, which showed self-efficacy and social support were also factors for IM nonadherence [Citation11,Citation24], and the combined theory model, the following questionnaires were used to collect the relevant data:
General Data Questionnaire
The self-designed General Data Questionnaire collected the demographic characteristics and clinical characteristics.
Beliefs about Medication Questionnaire (BMQ)
The BMQ developed by Horne and Weinman [Citation25] includes four subscales of medication beliefs under the general and specific domains: general harm (five items), general overuse (three items), specific necessity (five items), and specific concerns (six items). The items are structured on a five-point Likert scale, in which the higher scores indicate stronger belief. The difference between the necessity and concern indicates beliefs about medication. Cronbach’s α of the specific necessity and specific concerns subscales were 0.813 and 0.706, respectively [Citation26].
Theory of Planned Behavior
The TPB questionnaire developed by Chisholm et al. was widely used to assess the following five TPB variables related to intentions to adhere to medication: attitudes, intentions, perceived behavioral control, subjective norms, and past behavior [Citation20]. Cronbach’s α of each variable was 0.744, 0.703, 0.668, 0.762, and 0.861 [Citation27].
Health Belief Model
The HBM questionnaire developed by Rosenstock [Citation28] is a reliable scale to assess perceived susceptibility, perceived severity, perceived benefits, and perceived barriers. It has been widely applied to predict and intervene in IM nonadherence in KTRs [Citation18,Citation29]. The items are developed on a five-point Likert scale, in which higher scores indicate stronger health beliefs in KTRs. Cronbach’s α coefficient was 0.823.
Perceived Social Support Scale (PSSS)
The PSSS scale has mainly been used to measure the perceived adequacy of support from the following three sources: family, friends, and significant others [Citation30]. Higher scores indicate better social support. Cronbach’s α was 0.840 [Citation31].
General Self-Efficacy Scale (GSES)
The GSES developed by Schwarzer and Born [Citation32] includes 10 items to assess individuals’ perception of one’s competence to cope with stressful or challenging demands. The items are developed on a four-point Likert scale, in which higher scores indicate higher self-efficacy. Cronbach’s α was 0.871 [Citation33].
Eligible participants completed the questionnaire independently under the guidance of the researchers. The data collector was unclear about the research content and the identity information of patients was hidden, which reduces the selection bias of data. Before the data collection, one medical student and two postoperative patients were invited to complete the questionnaire, with an average completion time of 6 min and 48 s. Those with less than 25% of the average completion time were excluded. Due to special circumstances, some KTRs could not come to the follow-up outpatient clinic and were investigated in the form of an online questionnaire.
Derivation and validation dataset
The final dataset consisted of 35 variables collected from 1011 samples, including 21 characteristic variables () and 14 dimensions involved in the scales (general harm, general overuse, beliefs about medication, attitudes, intentions, perceived behavioral control, subjective norms, past behavior, perceived susceptibility, perceived severity, perceived benefits, perceived barriers, social support, and GSES scores). The derivation dataset was divided into two parts using computer-generated random sampling at a fixed ratio: a 70% training dataset (with 707 samples) and a 30% test dataset (with 304 samples). To further validate the accuracy and robustness of the LASSO model, we used an external validation dataset (with 180 samples from other areas) to perform external validation.
Table 1. Baseline characteristics of the study population in the derivation and external validation set (%).
Model development and validation
We used the R package glmnet version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) to develop a penalized logistic regression LASSO model. The penalty regularization parameter lambda (λ) was determined by 10-fold cross validation with the cv.glmnet module [Citation34]. Based on the optimal parameters, the variables with nonzero coefficients were used to create the final LASSO model.
Data of the derivation set were split randomly with 30% of the data for internal validation. The areas under the receiver operating characteristic curves (AUC), specificity, and sensitivity were used to evaluate the performance of the model, and further external verification was performed by data of 180 KTRs in the validation set.
Statistical analysis
The sociodemographic characteristics were analyzed using descriptive statistics. Categorical variables were expressed as frequencies (percentages). The comparison of differences between the two groups was conducted by the Chi-square test. Statistical analysis was performed using IBM SPSS software, version 25.0 (SPSS Inc., Chicago, IL), and R software, version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) (http://www.r-project.org). The difference was statistically significant at p value <.05.
Results
Baseline characteristics of the study population
shows the demographic and clinical characteristics of two sets in the study population. In the derivation set, 19.6% of KTRs were older than 50 years, 56.3% were men, 71.1% of KTRS were married, 41.0% had a history of drinking, and 59.1% suffered from postoperative complications.
IM nonadherence among KTRs
In this study, 389 of the 1011 KTRs (38.48%) were determined to be nonadherent in the derivation set, while 67 of the 180 KTRs (37.22%) were nonadherent in the external validation set. Adherence to IM measured by BAASIS in the derivation set is shown in . Specifically, missing the prescribed medication time was the most common cause of IM nonadherence, which had the highest rate of 27.8%. The second most common cause was missing one dose of IM, with a rate of 21.6%. 3.3% of the participants completely stopped the intake of IM without doctors’ advice.
Table 2. Adherence to IM measured by BAASIS.
Results of the derivation LASSO model
A total of 35 variables were determined to fit the LASSO regression model and plotted the coefficients at different log lambda values (). Internal 10 cross-validation was conducted to determine the optimal lambda value (λ = 0.03329, ). Variables with coefficients shrunk to zero were eliminated from the model. Eight variables with nonzero coefficients were identified, including age (18–20 years; 21–30 years), marital status (married), education, preoperative drinking history, perceived behavioral control, perceived susceptibility, perceived barriers, and social support with coefficients 1.135590464, 1.839251090, −0.440658433, 0.033095056, 0.223995291, −0.034962893, −0.025246225, −0.055747898, and −0.008602059 (). Finally, the LASSO model was developed by using the data of eight variables in the derivation dataset for predicting IM nonadherence. It was worth noting that the coefficients of LASSO did not represent the true coefficients in the true model as they were penalized.
Figure 3. Determination of optional lambda for LASSO model with 10-fold cross-validation. (A) The distribution of the lasso coefficient of variables. (B) The log lambda (λ) value is selected by cross-validation. The vertical dotted line at the left shows the value of lambda. min and the one at the right shows the value of lambda. 1se.
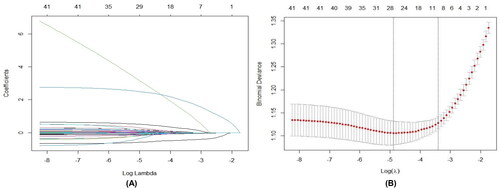
Table 3. The coefficients of selected variables in the LASSO model.
Model validation
The ROC curves in the derivation set and external validation set are shown in . The model demonstrated acceptable discrimination with an AUC of 0.797 (95% CI: 0.745–0.850) in the internal validation set and 0.757 (95% CI: 0.684–0.829) in the external validation set. The prediction model for IM nonadherence has favorable performance and enormous potential. The specificity and sensitivity in the internal validation set were 0.741 and 0.748, while they were 0.673 and 0.716 in the external validation set, respectively ().
Table 4. Assessment of the LASSO model.
Comparative analysis of logistic regression models
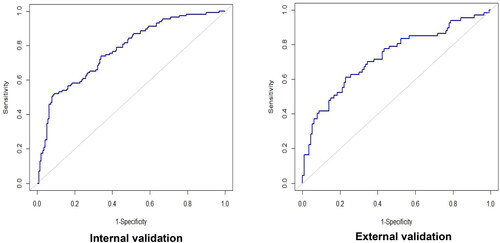
Furthermore, we also developed another prediction model for IM nonadherence by the traditional logistic regression. The logistic regression models were developed on the training dataset, and the performance was assessed on the test dataset and external validation dataset. The AUC of 0.774 (95% CI: 0.720–0.827) in the test set (external validation) and 0.728 (95% CI: 0.649–0.807) in the external validation set (). Based on the ROC curve, the LASSO model with eight variables achieved better performance compared with the traditional logistic regression model.
Discussion
IM nonadherence among KTRs has become a global challenge [Citation35]. Improving adherence achieves greater health benefits than discovering any new therapy according to WHO [Citation9]. The development of an accurate prediction tool is essential to identify high-risk nonadherent patients and provide timely and effective interventions to prevent future graft loss and improve their prognosis and survival. The highlight of the study is the first to use a LASSO model to predict IM nonadherence in KTRs, with advantages in methodology by avoiding the potential issue of model overfitting caused by too many influencing factors for IM nonadherence. Another strength is that the selection of potential factors of IM nonadherence was based on the combined TPB/HBM theory, which conceptualizes psychosocial factors related to health behaviors and health behavior change and has been well demonstrated to have favorable predicting power. In addition, the large sample size can reduce systematic errors and provide more robust results with more statistical power.
In the model, 1/2 predictors enrolled are objective indicators that are easy to obtain and less prone to measurement bias, including age, marital status, education, and preoperative drinking history. The other predictors (perceived barriers, perceived behavioral control, perceived susceptibility, and social support) can also be reliably assessed using reliable questionnaires. Thus, the practical and quantitative model is convenient for clinical application.
The LASSO model selected eight predictors for IM adherence, including age, marital status, education, preoperative drinking history, perceived barriers, social support, perceived behavioral control, and perceived susceptibility. However, the coefficients of LASSO were not completely equivalent to the true coefficients in the true model; because LASSO minimized prediction error subject to the constraint that the model was not too complex and tended to omit covariates with small coefficients [Citation36,Citation37].
Younger age was related to medication nonadherence [Citation38,Citation39]. Interestingly, Chisholm et al. [Citation40] found that older recipients were targeted as nonadherent. Older recipients face some issues (co-morbidities, social isolation, and physical limitations) leading to two contradictory outcomes: nonadherence; or better awareness of their limits and closer attention to drug therapy [Citation41]. This discrepancy may be explained by the different cultures in different studies and warrants further research. Patients with preoperative drinking histories are more likely to forget to take medicine due to impaired episodic memory and cognitive ability caused by alcoholism [Citation42]. Greater social support is a protective factor for IM adherence [Citation43]. Compared to married patients, those who are single, divorced, or widowed are more likely to live alone and lack support from family members for medication supervision and reminder [Citation44]. Besides, the KTRs with lower education were more adherent, which was inconsistent with some studies [Citation45,Citation46]. The diversity of the sample or class condition of groups might cause different findings. But Lin et al. [Citation47] observed that such patients were better monitored and managed, which was possible for the reasons for better adherence in this population.
In this study, perceived barriers, perceived behavioral control, and perceived susceptibility were determined as important predictors for IM nonadherence, which partially supports the combined TPB/HBM model contributing to the overall influence for IM nonadherence. Patients who perceive more barriers are more concerned about the frequency, side effects, and price of IM; they also hold more negative health beliefs about IM, which all contribute to IM nonadherence [Citation48]. This finding suggests that future interventions to improve IM adherence may benefit from changing their perceived barriers. Perceived behavioral control tends to be an important predictor of nonadherence [Citation20]. Greater perceived behavioral control played an active part in the intentions to self-efficacy and medicine adherence [Citation49]. In addition, the more susceptibility KTRs perceived to transplant rejection, the more adherent they were. The people who perceived more susceptibility were afraid of experiencing rejection, which would lead to renal failure, dependence on dialysis, and low quality of life [Citation50]. Perceived susceptibility has been identified as a major predictor of IM adherent behaviors [Citation29].
It is worth mentioning that postoperative time was not an independent predictor in our study. Similar to our study, Chisholm-Burns et al. [Citation51] revealed the same result. Nonetheless, previous studies have reported postoperative time was indeed related to IM nonadherence [Citation52,Citation53]. It’ is still controversial whether IM nonadherence is associated with postoperative time. In the future, the role of time on nonadherence and its correlation with IM nonadherence need further evaluation with larger population cohorts.
In addition, combined with the preoperative psychosocial assessment [Citation54], the prediction model contributed to predicting the risk of IM nonadherence in advance. In our study, the factors for IM nonadherence were divided into modifiable and non-modifiable factors. For non-modifiable factors (age, marriage status, preoperative drinking history, etc.), we can target patients with these characteristics and monitor nonadherence earlier. Modifiable perceived barriers or behavioral control can be ameliorated by providing education about IM necessity and medication-taking self-efficacy, which guides timely and effective interventions to improve IM adherence. For KTRs with a higher predicted risk, early interventions are conducive to improving their prognosis and survival.
Limitations
First, sample source areas and places were based on the available resources rather than random access; so, the system error was unable to avoid. Second, all data were collected by self-reported questionnaires, which could cause certain subjective biases. There is no real gold standard for the evaluation of IM adherence. Even the BASSIS may well have biases: the participants might conceal nonadherence or lack of data on measurable objective clinical endpoints. Besides, KTRs under the age of 18 were not included in this study, but this group was also reported to be at high risk for adherence difficulties from some previous research.
Conclusions
We developed a LASSO model based on the combined TPB/HBM theory to predict IM nonadherence among KTRs, which guides identifying high-risk nonadherent recipients and timely and effective interventions to improve their prognosis and survival.
Author contributions
Study design: Lei Dong and Jia Liu. Data collection: Xiao Zhu. Built the machine learning models: Lei Dong, Hongyu Zhao, and Qin Zhao. Manuscript drafting: Lei Dong. Revisions to the paper: Jia Liu, Shan Liu, and Lina Gong. Supervision: Jia Liu.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data supporting the conclusions of this article will be made available by the authors upon reasonable request. Requests to access these datasets should be directed to [email protected].
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction http://dx.doi.org/10.1080/0886022X.2024.2356372
Additional information
Funding
References
- Axelrod DA, Schnitzler MA, Xiao H, et al. An economic assessment of contemporary kidney transplant practice. Am J Transplant. 2018;18(5):1–10. doi: 10.1111/ajt.14702.
- Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–1226. doi: 10.1056/NEJMoa1302506.
- Álvarez-Rangel LE, Martínez-Guillén P, Granados-Ventura L, et al. Long-term patient and graft survival in kidney transplant recipients. Rev Med Inst Mex Seguro Soc. 2019;57(6):348–356.
- Fine RN, Becker Y, De Geest S, et al. Nonadherence consensus conference summary report. Am J Transplant. 2009;9(1):35–41. doi: 10.1111/j.1600-6143.2008.02495.x.
- Gokoel S, Gombert-Handoko KB, Zwart TC, et al. Medication non-adherence after kidney transplantation: a critical appraisal and systematic review. Transplant Rev. 2020;34(1):100511. doi: 10.1016/j.trre.2019.100511.
- Low JK, Crawford K, Manias E, et al. A compilation of consumers’ stories: the development of a video to enhance medication adherence in newly transplanted kidney recipients. J Adv Nurs. 2016;72(4):813–824. doi: 10.1111/jan.12886.
- Zhu Y, Zhou Y, Zhang L, et al. Efficacy of interventions for adherence to the immunosuppressive therapy in kidney transplant recipients: a meta-analysis and systematic review. J Investig Med. 2017;65(7):1049–1056. doi: 10.1136/jim-2016-000265.
- Mathis AS. Managed care implications of improving long-term outcomes in organ transplantation. Am J Manag Care. 2015;21(1 Suppl.):S24–S30.
- Brown MT, Bussell J, Dutta S, et al. Medication adherence: truth and consequences. Am J Med Sci. 2016;351(4):387–399. doi: 10.1016/j.amjms.2016.01.010.
- World Health Organisation. Adherence to long-term therapies. Geneva, Switzerland; 2003.
- Paterson T, O’Rourke N, Shapiro RJ, et al. Medication adherence in renal transplant recipients: a latent variable model of psychosocial and neurocognitive predictors. PLOS One. 2018;13(9):e0204219. doi: 10.1371/journal.pone.0204219.
- Zhu X, Peng B, Yi Q, et al. Prediction model of immunosuppressive medication non-adherence for renal transplant patients based on machine learning technology. Front Med. 2022;9:796424. doi: 10.3389/fmed.2022.796424.
- Kang J, Choi YJ, Kim IK, et al. LASSO-Based machine learning algorithm for prediction of lymph node metastasis in T1 colorectal cancer. Cancer Res Treat. 2021;53(3):773–783. doi: 10.4143/crt.2020.974.
- Ahmed F, Khan AA, Ansari HR, et al. Systems biology and LASSO-based approach to decipher the transcriptome-interactome signature for predicting non-small cell lung cancer. Biology. 2022;11(12):1752. doi: 10.3390/biology11121752.
- Chen Y, Chu CW, Chen MIC, et al. The utility of LASSO-based models for real time forecasts of endemic infectious diseases: a cross country comparison. J Biomed Inform. 2018;81:16–30. doi: 10.1016/j.jbi.2018.02.014.
- Zhang P, Zhu X, Yan J, et al. Identification of immunosuppressive medication nonadherence factors through a combined theory model in renal transplant recipients: 6–12. Front Pharmacol. 2021;12:655836. doi: 10.3389/fphar.2021.655836.
- Jones CJ, Smith H, Llewellyn C. Evaluating the effectiveness of health belief model interventions in improving adherence: a systematic review. Health Psychol Rev. 2014;8(3):253–269. doi: 10.1080/17437199.2013.802623.
- Xia M, Yan J, Liu S, et al. Beliefs of immunosuppressive medication among Chinese renal transplant recipients, as assessed in a cross-sectional study with the Basel Assessment of Adherence to Immunosuppressive Medications Scale. Transplant Proc. 2019;51(3):742–748. doi: 10.1016/j.transproceed.2018.10.029.
- Li C, Wang C, Zhang N, et al. Effects of health education based on theory of planned behavior on treatment compliance among maintenance hemodialysis patients. Chin J Mod Nurs. 2019;25(8):1029–1032.
- Chisholm MA, Williamson GM, Lance CE, et al. Predicting adherence to immunosuppressant therapy: a prospective analysis of the theory of planned behaviour. Nephrol Dial Transplant. 2007;22(8):2339–2348. doi: 10.1093/ndt/gfm149.
- Dobbels F, Berben L, De Geest S, et al. The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: a systematic review. Transplantation. 2010;90(2):205–219. doi: 10.1097/TP.0b013e3181e346cd.
- Shang Y, Teng S, Liu H, et al. Reliability and validity of Chinese version of Basel Assessment of Adherence to Immunosuppressive Medication Scale in accessing liver transplant recipients. J Nurs Admin. 2017;17(1):17–19.
- Liu N, Liu J, Zhong Z, et al. Cross-cultural adaptation and validation of the 2020 Basel Assessment of Adherence to Immunosuppressive Medications Scale. J Nurs Sci. 2021;36(16):6–9.
- Zhao SM, Dong FF, Qiu HZ, et al. Quality of life, adherence behavior, and social support among renal transplant recipients in China: a descriptive correlational study. Transplant Proc. 2018;50(10):3329–3337. doi: 10.1016/j.transproceed.2018.05.026.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–567. doi: 10.1016/s0022-3999(99)00057-4.
- Liu Y, Li Z, Han M, et al. The reliability and validity of the Chinese version of Beliefs about Medical Questionnaire among elderly patients with depressive disorder. Chin J Nurs. 2014;49(4):389–393.
- Teng S. Using the theory of planned theory (TPB) to investigate the factors of immunosuppressive medication adherence among liver transplant recipients. Beijing (China): Beijing University of Chinese Medicine; 2016. p. 71.
- Rosenstock IM. Why people use health services. Milbank Mem Fund Q. 1966;44(Suppl. 3):94–127. doi: 10.2307/3348967.
- Kung PC, Yeh MC, Lai MK, et al. Renal transplant recipients: the factors related to immunosuppressive medication adherence based on the health belief model. J Nurs Res. 2017;25(5):392–397. doi: 10.1097/JNR.0000000000000181.
- Blumenthal JA, Burg MM, Barefoot J, et al. Social support, type a behavior, and coronary artery disease. Psychosom Med. 1987;49(4):331–340. doi: 10.1097/00006842-198707000-00002.
- Zhang F, Zhu S, Deng P. Evaluation of Perceived Social Support Scale used in study of social support among hospitalized patients in China. Chin Nurs Res. 2018;32(13):2048–2052.
- Schwarzer R, Born A. Optimistic self-beliefs: Assessment of general perceived self-efficacy in thirteen cultures. World Psychol. 1997;3(1-2):177–190.
- Shen J, Tanf D. Use of General Self-Efficacy Scale (GSES) in Chinese aged people. Chin J Clin Psychol. 2004;12(4):342–344.
- Bartholomew TS, Tookes HE, Spencer EC, et al. Application of machine learning algorithms for localized syringe services program policy implementation–Florida, 2017. Ann Med. 2022;54(1):2137–2150. doi: 10.1080/07853890.2022.2105391.
- Lemstra M, Nwankwo C, Bird Y, et al. Primary nonadherence to chronic disease medications: a meta-analysis. Patient Prefer Adherence. 2018;12:721–731. doi: 10.2147/PPA.S161151.
- Xi LJ, Guo ZY, Yang XK, et al. Application of LASSO and its extended method in variable selection of regression analysis. Zhonghua Yu Fang Yi Xue Za Zhi. 2023;57(1):107–111.
- Wurm MJ, Rathouz PJ, Hanlon BM. Regularized ordinal regression and the ordinalNet R package. J Stat Softw. 2021;99(6). doi: 10.18637/jss.v099.i06.
- Cheung CY, Chan KM, Tang G, et al. Immunosuppressive medication adherence in kidney transplant recipients during the COVID-19 pandemic: a cross-sectional study in Hong Kong. Transplant Proc. 2021;53(8):2447–2450. doi: 10.1016/j.transproceed.2021.08.018.
- Gaynor JJ, Ciancio G, Guerra G, et al. Graft failure due to noncompliance among 628 kidney transplant recipients with long-term follow-up: a single-center observational study. Transplantation. 2014;97(9):925–933. doi: 10.1097/01.TP.0000438199.76531.4a.
- Chisholm MA, Lance CE, Mulloy LL. Patient factors associated with adherence to immunosuppressant therapy in renal transplant recipients. Am J Health Syst Pharm. 2005;62(17):1775–1781. doi: 10.2146/ajhp040541.
- Belaiche S, Décaudin B, Dharancy S, et al. Factors relevant to medication non-adherence in kidney transplant: a systematic review. Int J Clin Pharm. 2017;39(3):582–593. doi: 10.1007/s11096-017-0436-4.
- Le Berre AP, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res. 2017;41(8):1432–1443. doi: 10.1111/acer.13431.
- Sabbatini M, Garofalo G, Borrelli S, et al. Efficacy of a reduced pill burden on therapeutic adherence to calcineurin inhibitors in renal transplant recipients: an observational study. Patient Prefer Adherence. 2014;8:73–81. doi: 10.2147/PPA.S54922.
- Chisholm-Burns MA, Spivey CA, Wilks SE. Social support and immunosuppressant therapy adherence among adult renal transplant recipients. Clin Transplant. 2010;24(3):312–320. doi: 10.1111/j.1399-0012.2009.01060.x.
- Griva K, Davenport A, Harrison M, et al. Non-adherence to immunosuppressive medications in kidney transplantation: intent vs. forgetfulness and clinical markers of medication intake. Ann Behav Med. 2012;44(1):85–93. doi: 10.1007/s12160-012-9359-4.
- Jindal RM, Neff RT, Abbott KC, et al. Association between depression and nonadherence in recipients of kidney transplants: analysis of the United States Renal Data System. Transplant Proc. 2009;41(9):3662–3666. doi: 10.1016/j.transproceed.2009.06.187.
- Lin SY, Fetzer SJ, Lee PC, et al. Predicting adherence to health care recommendations using health promotion behaviours in kidney transplant recipients within 1–5 years post-transplant. J Clin Nurs. 2011;20(23–24):3313–3321. doi: 10.1111/j.1365-2702.2011.03757.x.
- Ranahan M, Von Visger J, Kayler LK. Describing barriers and facilitators for medication adherence and self-management among kidney transplant recipients using the information-motivation-behavioral skills model. Clin Transplant. 2020;34(6):e13862. doi: 10.1111/ctr.13862.
- Yang L, Liu HX, Hu Y, et al. Exploration of adherence to the immunosuppressive medication in kidney transplant recipients based on theory of planned behavior. Clin Nurs Res. 2022;31(6):1189–1198. doi: 10.1177/10547738221096550.
- Orr A, Orr D, Willis S, et al. Patient perceptions of factors influencing adherence to medication following kidney transplant. Psychol Health Med. 2007;12(4):509–517. doi: 10.1080/13548500701294556.
- Chisholm-Burns M, Pinsky B, Parker G, et al. Factors related to immunosuppressant medication adherence in renal transplant recipients. Clin Transplant. 2012;26(5):706–713. doi: 10.1111/j.1399-0012.2011.01589.x.
- Villeneuve C, Rousseau A, Rerolle JP, et al. Adherence profiles in kidney transplant patients: causes and consequence. Patient Educ Couns. 2020;103(1):189–198. doi: 10.1016/j.pec.2019.08.002.
- De Geest S, Burkhalter H, Bogert L, et al. Describing the evolution of medication nonadherence from pretransplant until 3 years post-transplant and determining pretransplant medication nonadherence as risk factor for post-transplant nonadherence to immunosuppressives: the Swiss Transplant Cohort Study. Transpl Int. 2014;27(7):657–666. doi: 10.1111/tri.12312.
- Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497–1504. doi: 10.1097/TP.0b013e3181a440ae.