Sir,
We read with great interest the recently published meta-analysis by Limei Zhao and coworkers entitled ‘Iron metabolism-related indicators as predictors of the incidence of acute kidney injury after cardiac surgery: a meta-analysis’ [Citation1]. The study aimed to investigate the relationship of iron metabolism-related indicator markers and the incidence of acute kidney injury (AKI) after cardiac surgery (CS). The analysis included data on serum ferritin, hepcidin-25 (hepatic bactericidal protein), transferrin, and urine catalytic iron. The study found that patients with lower 24 h but not 6 h postoperative urinary hepcidin-25 levels (μg/L) were more likely to develop AKI. The authors acknowledge that only a few studies examined the relationship between markers of iron metabolism and CS-associated AKI, and few studies were consequently included in the analysis.
In a recent study, we compared the predictive value of plasma Neutrophil Gelatinase–Associated Lipocalin (NGAL), hepcidin-25, and plasma NGAL:hepcidin-25-ratio with that of serum creatinine and urinary output for primary-endpoint MAKE (acute kidney injury [AKI] stages 2 and 3, persistent AKI >48 h, acute dialysis, and in-hospital mortality) and secondary-endpoint AKI in 100 on-pump CS patients at intensive care unit (ICU) admission (corresponding to 6 h). We performed receiver operator characteristic (ROC) curve, logistic regression, and reclassification analyses [Citation2]. Out of 100 patients, 9 developed MAKE and 9 developed AKI, respectively.
Previously, our group and others provided evidence of elevated levels of hepcidin-25 in the urine of patients who did not develop AKI after cardiac surgery [Citation3–5]. This may possibly indicate a nephroprotective property through the interaction with reactive iron compounds since hepcidin-25 synthesis is physiologically increased by iron overload [Citation6]. In our study cohort, this pattern of higher hepcidin-25 levels in patients without AKI described for urine samples (P = 0.001 for 6 h and 24 h) [Citation5] could not be demonstrated in patients’ plasma samples at 6 h and even less so at 24 h after CS with P = 0.189 and P = 0.652, respectively () [Citation2]. This is of interest as it may suggest that urinary biomarkers might be more sensitive for histological damage (i.e., tubular injury) than plasma biomarkers. However, in direct comparison, the area under the 6 h ROC-curve (AUC) of 1-urine hepcidin-25 versus 1-plasma hepcidin-25 was superior for MAKE (0.60 (0.41–0.78) versus 0.83 (0.72–0.94), P = 0.010) but not for AKI (0.63 (0.42–0.84) versus 0.80 (0.68–0.91), P = 0.103) [Citation2].
Figure 1. Plasma- and corresponding urine biomarker concentrations prior to cardiac surgery (0 h), at ICU admission (6 h) and at 24 h after commencement of cardiopulmonary bypass for patients with AKI (dark blue box/whiskers) and without AKI (light blue box/whiskers). Boxes represent median (25–75 IQR), whiskers represent ± 1.5 ⋅ IQR, dots represent outliers, extreme outliers are selectively hidden to improve scale interpretation and comparability of AKI vs. AKI–free patients. Asterisks (*) denote P < 0.05. AKI: acute kidney injury; ICU: intensive care unit; IQR: inter quartile range; NGAL: Neutrophil gelatinase-associated Lipocalin; u/p: urine/plasma.
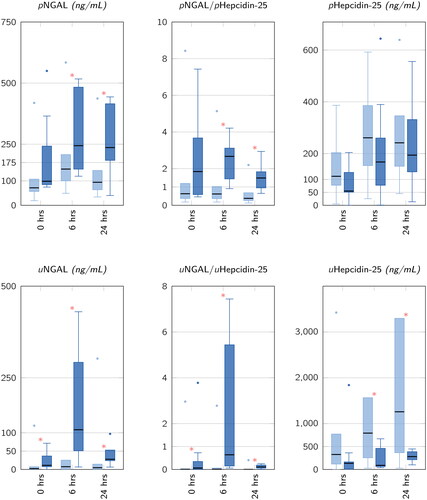
As the findings by Zhao et al. illustrated in their ‘Forest plot Figures 2 and 3’ are based on two or three studies only, the distributions of the hepcidin-25 levels in the timecourse after CS presented in this letter’s may have likely altered their calculations and conclusions drawn [Citation1].
Noteworthy, both hepcidin-25 and NGAL are involved as regulators in tubular iron metabolism [Citation7]:
As a tubuloprotective NGAL:siderophore:iron-complex, NGAL regulates the intrarenal iron metabolism [Citation6], is significantly upregulated after tubular damage, and may thus contribute to limiting kidney damage [Citation8].
In our cohort, the combination of plasma NGAL and plasma hepcidin-25 or urine NGAL and urine hepcidin-25 at ICU admission expressed as the NGAL:hepcidin-25-ratio improved the predictive value for the diagnosis of AKI over NGAL and hepcidin-25 alone with AUC values of 0.89 (0.82–0.97) and 0.89 (0.81–0.98) for urine and plasma, respectively [Citation2,Citation5]. Additionally, the urinary NGAL:hepcidin-25-ratio may identify patients at risk for postoperative need of renal replacement therapy [Citation9]. We acknowledge that the sample size used in this study is a limitation precluding the generalization of the findings.
Finally, we agree with Zhao et al. [Citation1] that more data on the involvement of hepcidin-25 and the interaction of iron metabolism in CS-associated AKI is needed to draw decisive clinical conclusions.
Ethical approval
The Institutional Review Board of Charité University of Berlin, Germany (approval no: ZS EK 11 654/07) approved the study registered as NCT00672334. The ethics committee granted permission to collect data and conduct biomarker measurements. Written informed consent was obtained from each patient.
Disclosure statement
Albert A has received honoraria speaking for Abbott Diagnostics on unrelated work. Haase M has received honoraria speaking for Abbott Diagnostics, Alere, Biosite Inc., and Siemens Healthineers. Albert C has received honoraria speaking for Siemens Healthineers. All other authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Funding
References
- Zhao L, Yang X, Zhang S, et al. Iron metabolism-related indicators as predictors of the incidence of acute kidney injury after cardiac surgery: a meta-analysis. Ren Fail. 2023;45(1):1. doi: 10.1080/0886022x.2023.2201362.
- Albert C, Haase M, Albert A, et al. Predictive value of plasma NGAL:hepcidin-25 for major adverse kidney events after cardiac surgery with cardiopulmonary bypass: a pilot study. Ann Lab Med. 2021;41(4):357–3. doi: 10.3343/alm.2021.41.4.357.
- Ho J, Reslerova M, Gali B, et al. Urinary hepcidin-25 and risk of acute kidney injury following cardiopulmonary bypass. Clin J Am Soc Nephrol. 2011;6(10):2340–2346. doi: 10.2215/cjn.01000211.
- Prowle JR, Ostland V, Calzavacca P, et al. Greater increase in urinary hepcidin predicts protection from acute kidney injury after cardiopulmonary bypass. Nephrol Dial Transplant. 2012;27(2):595–602. doi: 10.1093/ndt/gfr387.
- Albert C, Haase M, Albert A, et al. Urinary biomarkers may complement the cleveland score for prediction of adverse kidney events after cardiac surgery: a pilot study. Ann Lab Med. 2020;40(2):131–141. doi: 10.3343/alm.2020.40.2.131.
- Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115(3):610–621. doi: 10.1172/jci23056.
- Haase M, Bellomo R, Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol. 2010;55(19):2024–2033. doi: 10.1016/j.jacc.2009.12.046.
- Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase–associated lipocalin. J Am Soc Nephrol. 2007;18(2):407–413. doi: 10.1681/asn.2006080882.
- Elitok S, Kuppe H, Devarajan P, et al. Urinary neutrophil gelatinase–associated lipocalin/hepcidin-25 ratio for early identification of patients at risk for renal replacement therapy after cardiac surgery: a substudy of the BICARBONATE trial. Anesth Analg. 2021;133(6):1510–1519. doi: 10.1213/ane.0000000000005741.
