Abstract
Purpose
The purpose of this study was to investigate how aerobic exercise affects oxidative stress (OS) in patients with chronic kidney disease (CKD).
Methods
Retrieval dates range from the date the database was established to 19 July 2023, without languages being restricted. A meta-analysis and sensitivity analysis were conducted using RevMan 5.3 and Stata 16.0.
Results
The meta-analysis showed that, compared to usual activity or no exercise, aerobic exercise significantly reduced the oxidative markers malondialdehyde (MDA) (mean differences (MD) − 0.96 (95% CI −1.33, − 0.59); p < 0.00001), advanced oxidation protein product (AOPP) (MD − 3.49 (95% CI − 5.05, − 1.93); p < 0.00001), F2-isoprostanes (F2-iso) (MD − 11.02 (95% CI − 17.79, − 4.25); p = 0.001). Aerobic exercise also increased the antioxidant marker superoxide dismutase (SOD) in CKD patients (standardized mean differences (SMD) 1.30 (95% CI 0.56, 2.04); p = 0.0005). Subgroup analysis showed a significant increase in glutathione peroxidase (GPX) in patients aged ≥60 years (SMD 2.11 (95% CI 1.69, 2.54); p < 0.00001). The change in total antioxidant capacity (TAC) after aerobic exercise was insignificant in patients with CKD. The trial sequential analysis supported aerobic exercise’s effectiveness in improving MDA, SOD, AOPP, and F2-iso in patients with CKD.
Conclusion
The results of this review suggest that aerobic exercise improves OS indicators (MDA, SOD, AOPP, and F2-iso) in CKD patients compared to conventional treatment or no exercise and that the effects on GPX and TAC indicators need further confirmation. For better validation of benefits and exploration of the best aerobic exercise regimen to improve OS status with CKD, further studies with high methodological quality and large sample sizes are needed.
1. Introduction
Chronic kidney disease (CKD) syndrome refers to disorders of the kidney that result in irreversible structural and functional changes. Globally, CKD prevalence is 9.1% [Citation1], even higher in China and the United States for adults over 18 years old [Citation2,Citation3]. It is also strongly associated with cardiovascular disease and disorders of lipoprotein metabolism [Citation4].
It is common for patients with CKD to experience chronic inflammation and oxidative stress (OS) [Citation5,Citation6]. Reducing antioxidants results in OS due to a rise in reactive oxygen species (ROS) in the body. ROS accumulates over time in the body, which damages many normal substances, such as proteins, lipids, and nucleic acids. In addition, it activates the pro-inflammatory factor nuclear factor-κB (NF-κB), exacerbating the inflammatory response and accelerating the progression of kidney injury [Citation7,Citation8]. Excessive production of intracellular and extracellular oxygen-derived free radicals mediates the onset of kidney injury and induces an inflammatory response. In turn, the inflammatory response to repair free radical damage stimulates the formation of additional free radicals and/or ROS [Citation9]. OS interacts with the inflammatory response to accelerate the progression of CKD [Citation10]. For this reason, controlling OS and inflammatory responses is crucial to long-term CKD management.
There have been several meta-analyses that indicate that aerobic exercise significantly improves the nutritional status and physical function of CKD patients [Citation11–17]. It also has a protective effect on cardiac and renal function in CKD patients [Citation18–20]. Therefore, our study aims to systematically summarize the evidence for aerobic exercise’s effects on OS indicators in patients with CKD based on the results of clinical trials.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [Citation21] (Table S1) and the Cochrane Handbook for Systematic Reviews [Citation22] were followed in developing this systematic review and meta-analysis. Also, the protocol of the study has been registered in the International Prospective Register of Systematic Reviews (CRD42022324191).
2. Materials and methods
2.1. Search strategy
A comprehensive search of PubMed, Web of Science, Embase, the Cochrane Library, Chinese BioMedical Database (CBM), China National Knowledge Infrastructure (CNKI), WanFang, and VIP databases from the date of creation to 19 July 2023 was undertaken for the present systematic review and meta-analysis. The search terms include ‘random,’ ‘aerobic exercise,’ ‘chronic kidney disease,’ ‘chronic renal failure,’ ‘oxidative stress,’ etc., with no language restrictions. Minor adjustments were made to suit the requirements of the different databases. The literature search used was detailed in Table S2. We also screened reference lists of selected studies and relevant review articles to identify experiments not discovered by database searches. References were stored and managed in a database created with EndNote X9. We reviewed each search result independently by two authors (MJZ and MLX) and resolved discrepancies by consulting with a third author (JL).
2.2. Selection criteria
Trials that meet the following selection criteria were included: (1) randomized controlled trials (RCTs); (2) participants: limited to patients with CKD over the age of 18 years; (3) comparison of aerobic exercise with conventional treatment (including usual activities, usual/standard care, health education, etc.) or no exercise; (4) the primary outcomes: malondialdehyde (MDA), superoxide dismutase (SOD); conversely, the secondary outcomes: glutathione peroxidase (GPX), advanced oxidation protein product (AOPP), F2-isoprostanes (F2-iso), total antioxidant capacity (TAC). We excluded trials that reported (1) duplicate publications, (2) inability to extract or calculate the appropriate data from published results, and (3) pregnant or lactating women.
2.3. Data extraction and quality assessment
Two researchers (MJZ and QT) read the title, abstract and full text separately to screen the literature and identify studies for inclusion and exclusion, with two researchers cross-checking the extracted data. An extract included the author and date of publication; study population characteristics such as sample size, age, gender, and body mass index (BMI); exercise characteristics: frequency, duration, and intensity; outcomes: at least one of the following outcome indicators: (1) primary outcome indicator: MDA and SOD, (2) Secondary outcome indicators: GPX, AOPP, F2-iso, and TAC.
Cochrane risk-of-bias tool (RoB 2) [Citation23,Citation24] was used by two independent evaluators (MJZ and MLX) to assess each study’s risk of bias. Risk of Bias assessment covers seven areas of bias: randomly selecting subjects, concealing allocations, blinding personnel and subjects, blinding outcome assessments, selective reporting of outcomes, and other biases. Based on these specific evaluation criteria, included studies were classified as ‘low risk,’ ‘high risk,’ or ‘unclear’. In disagreements or inconsistencies, a third reviewer (FL) acted as an arbiter.
2.4. Data analysis
With 95% confidence intervals (CI), continuous variables were expressed as mean differences (MD) or standardized mean differences (SMD). If all outcome indicators have the same unit of measure, we combine the data into MD with 95% CI. Otherwise, SMD was calculated. I2 statistics and Cochran’s Q tests were used to measure study heterogeneity. We used fixed effects models when the data had little heterogeneity (P for Cochran Q test ≥ 0.1, I2 ≤ 50%). Random effects models are applied when there is substantial heterogeneity among studies (P for Cochran Q test < 0.1, I2 > 50%). We will explore possible reasons for heterogeneity by considering subgroup analyses. Since our meta-analysis included fewer than 10 studies, funnel plots were not performed. Two-tailed tests with p < 0.05 were considered statistically significant. Using Review Manager 5.3 software, quantitative data synthesis was conducted. The results of this meta-analysis were tested using Stata 16.0 software to assess their reliability.
2.5. Trial sequential analysis
Using the TSA 0.9.5.10 Beta software, trial sequential analysis (TSA) was carried out to confirm the meta-analysis results. The type of boundary value for the hypothesis test was set to a two-sided test. The cumulative Z-curve crossing the sequential monitoring boundary or entering the futility area indicates that the corrected results are consistent and could be taken as definitive evidence.
2.6. Certainty of evidence
In order to determine the certainty of the evidence, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [Citation25] was used. There were four grades for the evidence quality: ‘high,’ ‘moderate,’ ‘low’ and ‘very low’. The results of the studies were presented using GRADEPRE 3.6 software.
3. Results
3.1. Search results
Study selection is illustrated in with a flowchart of PRISMA statements. The above search protocol resulted in 899 studies (PubMed:103 studies, Web of Science:323 studies, Embase:84 studies, the Cochrane Library:177 studies, CBM:72 studies, CNKI:41 studies, WanFang:42 studies, and VIP database:57 studies) being retrieved. A total of 246 duplicate articles were identified using the EndNote X9. There were 653 non-repetitive articles, 536 of which were excluded due to their title/abstract and 110 were excluded by reading the full text. Seven RCTs were eventually included [Citation26–32]. Three of the included literature languages were Chinese, and the remaining four were English.
3.2. Study characteristics
The summary characteristics of the seven RCTs were shown in . Among the 573 patients, 291 were assigned to the trial group and 282 to the control group. The sample sizes for individual trials ranged from 18 to 140, and their average age was 47.06 to 68.4 years, with the majority being middle-aged and elderly. Five trials included subjects who were hemodialysis patients, and two trials included subjects who were pre-hemodialysis patients. The interventions in 5 trials were aerobic exercise vs. no exercise [Citation27,Citation28], usual activity [Citation33], usual care [Citation29], or standard care [Citation32]; 2 trials used joint exercise (aerobic exercise combined with resistance exercise) vs. health education [Citation29,Citation30]. Most studies indicated the intensity of aerobic exercise, mainly focusing on moderate intensity; others did not specify the intensity of aerobic exercise. These trials were published between 2015 and 2021. The duration of treatment also varied between trials, ranging from 1 week to 16 weeks. Of the seven RCTs, four studies reported MDA [Citation26,Citation28–30], four studies reported SOD [Citation27–30], five studies reported GPX [Citation27–31], three studies reported AOPP [Citation28–30], three studies reported F2-iso [Citation27,Citation31,Citation32], and two studies reported TAC [Citation26,Citation31].
Table 1. Characteristics of the included studies.
3.3. Quality assessment and risk of bias
The risk of bias evaluation results were shown in Figure S1. The seven included studies were all RCT studies. Six studies described the method of random allocation, with four studies using a random number table and two that used a computer-generated random number. Since no allocation concealment scheme was mentioned, all studies were considered ‘unclear’ risks. The interventions in the trials were all exercises, which made blinded implementation difficult. Therefore, this item was rated as high risk in all articles. All included studies did not mention blinding in the outcome assessment, contributing to the ‘unclear’ risk. Five studies had complete data results, and two clearly described what data was missing and why. There was no selective reporting in the included studies. In six trials, there was no information describing the sample size calculations, which was judged to be an ‘unclear’ risk of other biases.
3.4. Primary outcomes
3.4.1. Effect on MDA
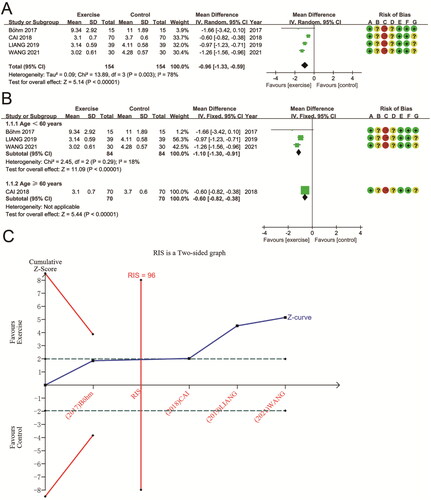
308 patients were evaluated in four RCTs to determine if aerobic exercise affected changes in MDA levels. These studies had high heterogeneity (P for Cochran Q test = 0.003, I2 = 78%). Compared to non-exercise groups and conventional treatments, the aerobic exercise group had a significant decrease in MDA based on the meta-analysis using a random effects model (MD − 0.96 (95% CI −1.33, −0.59); p < 0.00001) (). Subgroup analysis based on the age of the participants significantly reduced heterogeneity in studies with age < 60 years (P for Cochran Q test = 0.29, I2 = 18%). Regardless of age, MDA was significantly lower compared to non-exercise patients, with a 1.10 mmol/L reduction in MDA in patients aged < 60 years (MD − 1.10 (95% CI −1.30, −0.91); p < 0.00001), and in those aged ≥ 60 years, MDA was reduced by 0.6 mmol/L (MD − 0.60 (95% CI − 0.82, − 0.38); p < 0.00001) (). TSA results for MDA showed that the cumulative Z-curve crossed the futility area, and the cumulative sample size exceeded the RIS (RIS = 96 vs. actual sample size = 208), indicating the reliability of meta-analysis results support aerobic exercise’s benefits on MDA ().
3.4.2. Effect on SOD
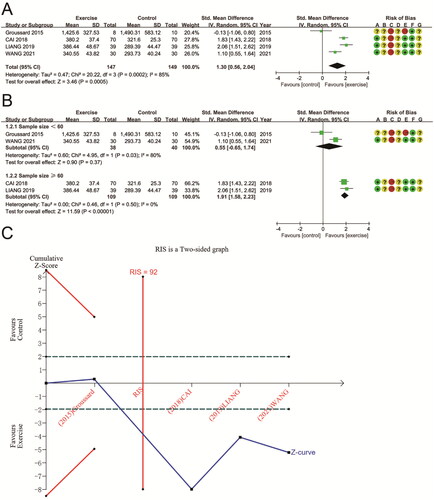
SOD data were provided for a total of 296 subjects from 4 trials. The combined results showed that SOD was significantly higher after aerobic exercise than conventional treatment or no exercise (SMD 1.30 (95% CI 0.56, 2.04); p = 0.0005), with high heterogeneity in the study (P for Cochran Q test = 0.0002, I2 = 85%) (). Study sample size appeared to be an important factor. Subgroup analysis based on sample size eliminated heterogeneity in studies with sample sizes ≥ 60 (P for Cochran Q test = 0.50, I2 = 0%) and significantly improved SOD (SMD 1.91 (95% CI 1.58, 2.23); p < 0.00001). In tests with sample sizes < 60, no significant differences were found (SMD 0.55 (95% CI −0.65, 1.74); p = 0.37; P for Cochran Q test = 0.03, I2 = 80%) (). A TSA on SOD reveals that the sample size met the RIS (RIS = 92 vs. actual sample size = 296), and the cumulative Z-curve was above futility. Therefore, sufficient evidence supports aerobic exercise’s effect on SOD in CKD ().
3.5. Secondary outcomes
3.5.1. Effect on GPX
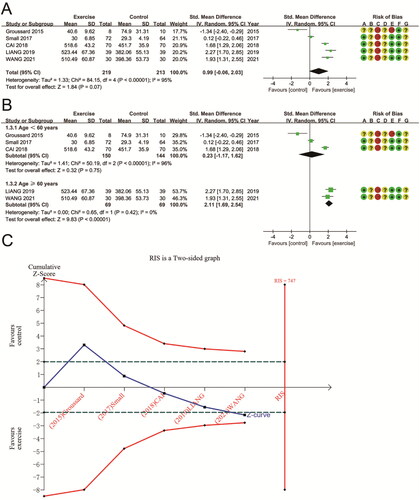
The effects of aerobic exercise on GPX were pooled from five RCTs, which involved 432 patients. As compared to conventional treatment or no exercise, aerobic exercise had no statistically significant difference in GPX (SMD 0.99 (95% CI − 0.06, 2.03); p = 0.07) (). Because the heterogeneity between studies was significant (P for Cochran Q test < 0.00001, I2 = 95%), subgroup analyses were conducted to investigate possible sources of heterogeneity. Subgroup analysis using participant age showed that GPX was significantly higher after aerobic exercise in studies aged ≥ 60 years (SMD 2.11 (95% CI 1.69, 2.54); p < 0.00001) and removed between-study heterogeneity (P for Cochran Q test = 0.42, I2 = 0%). In contrast, there was no statistically significant difference in studies aged < 60 years (SMD 0.23 (95% CI − 1.17, 1.62); p = 0.75; P for Cochran Q test < 0.00001, I2 = 96%) (). TSA results for GPX indicate that the cumulative Z-curve crosses the futility area, suggesting a potential advantage of aerobic exercise in improving GPX in patients with CKD. However, the cumulative sample size did not reach the RIS (RIS = 747 vs. actual sample size = 432). It is necessary to conduct more studies to validate the effects of aerobic exercise on GPX in patients with CKD ().
3.5.2. Effect on AOPP
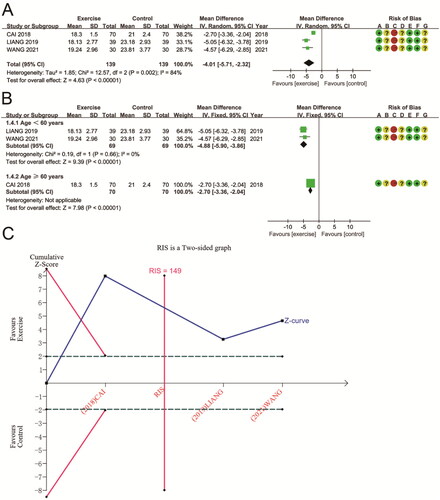
Three trials, including 278 participants, reported AOPP as an outcome. Overall, the meta-analysis showed a significant decrease in AOPP following aerobic exercise interventions compared to treatment as usual or no exercise (MD − 4.01 (95% CI − 5.71, − 2.32); p < 0.00001) (). Among the included studies, there was significant heterogeneity (P for Cochran Q test = 0.002, I2 = 84%). Subgroup analysis based on participant age eliminated heterogeneity in studies with age < 60 years (P for Cochran Q test = 0.66, I2 = 0%) and did not affect the reduced effect of either subgroup on AOPP. In subjects aged < 60 years, AOPP was reduced by 4.88 μmol/L (MD − 4.88 (95% CI − 5.90, − 3.86); p < 0.00001). In patients aged ≥ 60 years, AOPP was reduced by 2.70 μmol/L (MD − 2.70 (95% CI − 3.36, − 2.04); p < 0.00001) (). According to TSA results for AOPP, the cumulative Z-curve crossed both boundaries of the trial sequential monitoring and futility. Furthermore, the cumulative sample size reached the RIS (RIS = 149 vs. actual sample size = 278), suggesting the meta-analysis results are reliable and support aerobic exercise’s benefits for AOPP ().
3.5.3. Effect on F2-iso
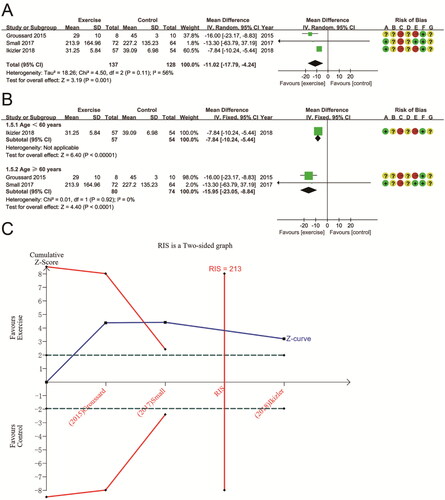
Three RCTs involving 265 patients assessed the aerobic exercise’s effect on changes in F2-iso. There was high heterogeneity between these studies (P for Cochran Q test = 0.11, I2 = 56%). Meta-analysis showed a significant reduction in F2-iso after aerobic exercise compared to conventional treatment or no exercise (MD − 11.02 (95% CI − 17.79, − 4.24); p = 0.001) (). Subgroup analysis based on participant age showed a significant reduction in F2-iso after aerobic exercise in both subgroups (p < 0.0001). Subgroup analysis eliminated heterogeneity in the study for age ≥60 years (MD − 15.95 (95% CI − 23.05, − 8.84); P for Cochran Q test = 0.92, I2 = 0%) (). Moreover, TSA showed that the F2-iso’s Z-curve crossed the sequential monitoring boundary and the futility area and that the cumulative sample size reached RIS (RIS = 213 vs. actual sample size = 265). This result suggests that the benefits observed in the current information set are shown to be decisive ().
3.5.4. Effect on TAC
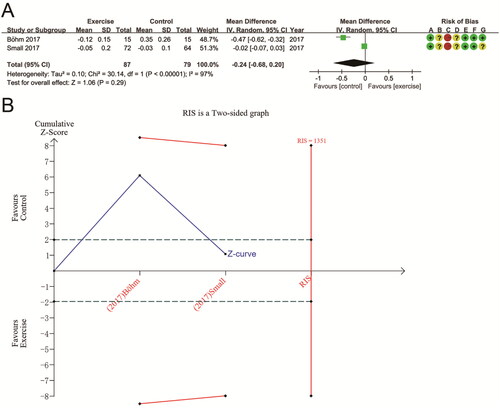
The two studies were analyzed using a random effects model. The pooled results indicated that aerobic exercise had no significant effect on TAC compared to usual treatment or no exercise (MD − 0.24 (95% CI − 0.68, 0.20); p = 0.29), with significant heterogeneity across studies (P for Cochran Q test <0.00001, I2 = 97%) (). Since there were only two studies, we did not perform subgroup analyses. Given the differences in the characteristics of the populations included in the two studies, we considered that the presence of heterogeneity might be related to patient age and dialysis status. In TSA analysis, the Z-curve for TAC did not cross either the futility area or the trial sequential monitoring boundary, nor did it enter the RIS (RIS = 1351 vs. actual sample size = 166). This result suggests that more research is necessary to validate it ().
3.6. Adverse events
This review found only one study that reported adverse outcomes associated with exercise training, including rapid atrial fibrillation after hospitalization (1 patient), hypotension due to weight loss (2 patients), chest pain during exercise (1 patient), Achilles tendon pain (1 patient), and joint pain during exercise (1 patient); the one adverse event potentially relevant to the study was knee pain during exercise.
3.7. Sensitivity analysis
We conducted sensitivity analyses separately for MDA, SOD, GPX, AOPP, F2-iso, and TAC in order to verify their robustness. After removing each study, sensitivity analyses for the six groups showed strong and stable overall results (Figure S2).
3.8. Certainty of evidence
In Table S3, the quality of evidence scores for AOPP is moderate, and the quality of evidence for MDA and F2-iso is low. There was a very low level of evidence for the remaining outcomes. This was largely due to the high risk of bias in the included studies, inconsistency and publication bias.
4. Discussion
This study aimed to evaluate the effect of aerobic exercise on the changes in OS indicators in patients with CKD. We included seven studies involving 573 subjects. According to pooled analyses, aerobic exercise decreased MDA, F2-iso, and AOPP significantly and increased levels of the antioxidant SOD compared with conventional treatment or no exercise. Here, the results of the TSA are consistent with those of the meta-analysis, suggesting that no further evidence is required. However, changes in GPX and TAC were not significant. Our subgroup analysis by age showed that aerobic exercise significantly increased GPX in older patients aged ≥ 60 years, while TAC remained insignificantly improved. Similarly, the TSA further confirms the results for GPX and TAC. The TSA analysis shows that the two indicators are insufficient to obtain definitive results and that more high-quality, extensive sample-size studies are needed.
According to a recent meta-analysis [Citation33], aerobic exercise in older adults aged ≥60 years significantly reduced oxidative markers MDA, F2-iso, glutathione/oxidized glutathione (GSH/GSSG), and increased antioxidant markers SOD and TAC. This is consistent with our findings. Furthermore, aerobic exercise showed a significant age-related effect in improving OS levels. This may be related to the higher basal OS levels in the elderly compared to the young [Citation34]. Therefore, older people showed a more substantial ameliorative effect when performing aerobic exercise at the same intensity.
After aerobic exercise, TAC was significantly higher in CKD patients, according to Sovatzidis et al. [Citation35] and Forsse et al. [Citation36]. In TAC, the availability of free radical-scavenging compounds is measured rather than innate antioxidant capacity, and nutrition plays a significant role [Citation37]. Studies have confirmed that TAC in CKD patients is both calorie-limited and associated with inadequate antioxidant intake [Citation38]. Hemodialysis treatment itself can also exacerbate OS damage [Citation39, Citation40]. Therefore, differences in studies of TAC in CKD may be related to multiple factors such as dialysis, comorbidities, and inadequate intake of dietary restrictions antioxidants.
As CKD is a chronic inflammatory disease, OS is crucial in causing inflammation [Citation41]. According to a recent systematic review and meta-analysis [Citation42], exercise significantly reduced inflammatory factor levels, improved renal function, and delayed kidney disease progression, which was more pronounced in aerobic exercise. Despite this, none of the recent meta-analyses has systematically examined how aerobic exercise affects OS in chronic kidney disease. It is important to consider that reduced levels of organismal inflammatory factors are insufficient to eliminate the clinical outcome of CKD [Citation43–45]. It has been found that organismal OS indicators are at high levels in CKD, leaving patients with chronic OS [Citation10]. Therefore, reducing the level of OS in the body of CKD patients is essential to control and mitigate the development of CKD. The results of a meta-analysis showed that exercise reduced pro-oxidant parameters and increased human antioxidant capacity [Citation46]. However, the impact of aerobic exercise on OS in CKD seems inconsistent. Meléndez-Oliva et al. [Citation47] found that aerobic exercise did not significantly improve the OS level of hemodialysis patients. Fatouro et al. [Citation48] showed that acute exercise exacerbated OS in hemodialysis patients. The results of aerobic exercise on OS in CKD are contradictory. Therefore, this study summarized all RCTs and found that aerobic exercise improved OS indicators and, to some extent, OS status in CKD.
Nuclear factor erythroid 2-related factor 2 (Nrf2) may have an essential role in regulating OS and inflammation. Nrf2 activation and downstream gene products are significantly downregulated in the CKD setting [Citation45]. It may be related to the impaired antioxidant capacity of CKD patients. Therefore, Nrf2 could be an important therapeutic target to counteract OS in CKD [Citation49]. Some studies have confirmed [Citation50,Citation51] that exercise positively affects the expression of Nrf2. During aerobic exercise, Nrf2 activates the antioxidant enzymes GPX and SOD [Citation52,Citation53], inhibiting peroxidative tissue damage. Therefore, aerobic exercise is considered a non-pharmacological treatment to improve the OS status of CKD patients.
In only one study [Citation32], adverse events associated with exercise training were reported in patients with CKD stages 3 and 4, most of whom had an underlying condition, such as hypertension or diabetes. In addition, about half of the patients had a history of smoking. We hypothesize that adverse events during exercise training may be influenced by many factors, including the patient’s underlying disease, CKD stage, and personal history. Therefore, it is essential to consider demographic factors when targeting aerobic exercise interventions for CKD patients’ OS. Individualized aerobic exercise training programs should be developed based on the characteristics of CKD patients.
The GRADE system uses a highly structured approach to classify the levels of evidence. It presents the evaluation items in an item-by-item listing so that clinicians can understand the effectiveness and feasibility of the interventions and make clinical decisions [Citation54]. Of the indicators in this study, AOPP was moderate quality evidence, MDA and F2-iso were low-quality evidence, and SOD, GPX, and TAC were all very low-quality evidence. This suggests there may be a gap between the efficacy predicted in this study and the actual effectiveness. There is still a need to include higher-quality RCTs to improve the level of evidence in the future.
The current study has several limitations. First, complete data were unavailable, and despite our efforts to contact the authors, data were missing from some trials. Second, the varied data collection and measurement methods across studies could cause significant heterogeneity. Third, the study subjects were not completely homogeneous, with factors like age, dialysis, co-morbidities, obesity, and CKD stage potentially affecting outcomes. Despite these issues, we collected a comprehensive range of literature. We also conducted subgroup analyses and a secondary assessment via TSA to enhance the reliability and credibility of our findings. Therefore, this study may offer objective evidence on the link between aerobic exercise and OS in CKD patients.
5. Conclusions
In conclusion, our study showed that aerobic exercise significantly increased the antioxidant marker SOD and decreased the oxidative markers MDA, F2-iso, and AOPP in CKD compared to conventional treatment or no exercise. However, the effects on GPX and TAC need further confirmation. This may be partly related to patient age, dialysis, comorbidities, low antioxidant intake, and the few included articles. The current data are only preliminary results, and in the future, it will be necessary to develop uniform exercise training standards for patients according to the different stages of chronic kidney disease. More RCTs with large sample sizes, multicenter, long-term follow-ups, and testing of a broader range of plasma oxidative and antioxidant markers should be conducted to confirm the effect of aerobic exercise on the treatment of OS and prevention of CKD progression at all stages of CKD.
Supplemental Material
Download PDF (168.5 KB)Supplemental Material
Download PDF (234.5 KB)Supplemental Material
Download PDF (91 KB)Disclosure statement
No conflict of interest exists in the publication of this study.
Data availability statement
All data supporting the conclusions of the article have been included in the manuscript.
Additional information
Funding
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):1–14. doi:10.1016/S0140-6736(20)30045-3.
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi:10.1016/S0140-6736(12)60033-6.
- Saran R, Robinson B, Abbott KC, et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 Suppl 1):A7. doi:10.1053/j.ajkd.2018.01.002.
- Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24(5):822–830. doi:10.1681/ASN.2012070702.
- Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33(suppl_3):iii35–iii40. doi:10.1093/ndt/gfy175.
- Himmelfarb J, Stenvinkel P, Ikizler TA, et al. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62(5):1524–1538. doi:10.1046/j.1523-1755.2002.00600.x.
- Ratliff BB, Abdulmahdi W, Pawar R, et al. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal. 2016;25(3):119–146. doi:10.1089/ars.2016.6665.
- Cachofeiro V, Goicochea M, de Vinuesa SG, et al. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;74(111):S4–S9. doi:10.1038/ki.2008.516.
- Closa D, Folch-Puy E. Oxygen free radicals and the systemic inflammatory response. IUBMB Life. 2004;56(4):185–191. doi:10.1080/15216540410001701642.
- Dounousi E, Papavasiliou E, Makedou A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48(5):752–760. doi:10.1053/j.ajkd.2006.08.015.
- Vanden Wyngaert K, Van Craenenbroeck AH, Van Biesen W, et al. The effects of aerobic exercise on eGFR, blood pressure and VO2peak in patients with chronic kidney disease stages 3-4: a systematic review and meta-analysis. PLoS One. 2018;13(9):e0203662. doi:10.1371/journal.pone.0203662.
- Nicolodi GV, Della Méa Plentz R, Righi NC, et al. Effects of aerobic exercise on patients with pre-dialysis chronic kidney disease: a systematic review of randomized controlled trials. Disabil Rehabil. 2022;44(16):4179–4188. doi:10.1080/09638288.2021.1900929.
- Hargrove N, El Tobgy N, Zhou O, et al. Effect of aerobic exercise on dialysis-related symptoms in individuals undergoing maintenance hemodialysis: a systematic review and meta-analysis of clinical trials. Clin J Am Soc Nephrol. 2021;16(4):560–574. doi:10.2215/CJN.15080920.
- Pei G, Tang Y, Tan L, et al. Aerobic exercise in adults with chronic kidney disease (CKD): a meta-analysis. Int Urol Nephrol. 2019;51(10):1787–1795. doi:10.1007/s11255-019-02234-x.
- Junqué-Jiménez A, Morera-Mas A, Pérez-Ventana-Ortiz C, et al. Home-based exercise programs in patients with chronic kidney disease: a systematic review and META-analysis. Worldviews Evid Based Nurs. 2022;19(4):322–337. doi:10.1111/wvn.12579.
- Araujo AM, Orcy RB, Feter N, et al. Effects of intradialytic exercise on functional capacity in patients with end-stage chronic kidney disease: a systematic review and meta-analysis. Res Sports Med. 2022. doi:10.1080/15438627.2022.2079983.
- Cai X, Zeng D, Deng J. A systematic review and meta-analysis of the efficacy of aerobic exercise combined with resistance training on maintenance hemodialysis patients. Ann Palliat Med. 2022;11(4):1360–1368. doi:10.21037/apm-22-226.
- Yamamoto R, Ito T, Nagasawa Y, et al. Efficacy of aerobic exercise on the cardiometabolic and renal outcomes in patients with chronic kidney disease: a systematic review of randomized controlled trials. J Nephrol. 2021;34(1):155–164. doi:10.1007/s40620-020-00865-3.
- Wang H, Xie D, Wu L, et al. Association of exercise with vascular function in patients with CKD: a meta-analysis of randomized controlled trials. Front Med (Lausanne). 2022;9:904299. doi:10.3389/fmed.2022.904299.
- Wu X, Yang L, Wang Y, et al. Effects of combined aerobic and resistance exercise on renal function in adult patients with chronic kidney disease: a systematic review and meta-analysis. Clin Rehabil. 2020;34(7):851–865. doi:10.1177/0269215520924459.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions., 2nd edn. John Wiley & Sons, Chichester (UK). 2019.
- Eldridge S, Campbell MK, Campbell MJ, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2) – additional considerations for cluster-randomized trials (RoB 2 CRT). 2021.
- Higgins JPT, Savović J, Page MJ, et al. Assessing risk of bias in a randomized trial. In: Welch VA (ed) Cochrane handbook for systematic reviews of interventions. 2nd edn. John Wiley & Sons, Chichester (UK), 2019, pp 205–228.
- Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE working group. BMC Health Serv Res. 2004;4(1):38. doi:10.1186/1472-6963-4-38.
- Böhm J, Monteiro MB, Andrade FP, et al. Acute effects of intradialytic aerobic exercise on solute removal, blood gases and oxidative stress in patients with chronic kidney disease. J Bras Nefrol. 2017;39(2):172–180.
- Groussard C, Rouchon-Isnard M, Coutard C, et al. Beneficial effects of an intradialytic cycling training program in patients with end-stage kidney disease. Appl Physiol Nutr Metab. 2015;40(6):550–556. doi:10.1139/apnm-2014-0357.
- Cai SL, Jia HG, Zhang SY, et al. Influence of aerobic exercise on oxidative stress and life quality in hemodialysis patients with diabetic nephropathy. J North China Unive Scid Technol. 2018;20(01):21–24.
- Wang CR, Liang CH, Chang ST. Effect of planned aerobic exercise resistance exercise intervention on oxidative stress in maintenance hemodialysis patients. Smart Healthcare. 2021;7(31):157–159.
- Liang YP, Zheng JL, Zhang YJ. Effects of aerobic-resistance exercise intervention on somatic function and oxidative stress in maintenance hemodialysis patients. Chinese General Pract Nurs. 2019;17(27):3358–3361.
- Small DM, Beetham KS, Howden EJ, et al. Effects of exercise and lifestyle intervention on oxidative stress in chronic kidney disease. Redox Rep. 2017;22(3):127–136. doi:10.1080/13510002.2016.1276314.
- Ikizler TA, Robinson-Cohen C, Ellis C, et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol. 2018;29(1):250–259. doi:10.1681/ASN.2017010020.
- Ye Y, Lin H, Wan M, et al. The effects of aerobic exercise on oxidative stress in older adults: a systematic review and meta-analysis. Front Physiol. 2021;12:701151. doi:10.3389/fphys.2021.701151.
- Konopka AR, Sreekumaran Nair K. Mitochondrial and skeletal muscle health with advancing age. Mol Cell Endocrinol. 2013;379(1-2):19–29. doi:10.1016/j.mce.2013.05.008.
- Sovatzidis A, Chatzinikolaou A, Fatouros IG, et al. Intradialytic cardiovascular exercise training alters redox status, reduces inflammation and improves physical performance in patients with chronic kidney disease. Antioxidants. 2020;9(9):868. doi:10.3390/antiox9090868.
- Forsse JS, Papadakis Z, Peterson MN, et al. The influence of an acute bout of aerobic exercise on vascular endothelial function in moderate stages of chronic kidney disease. Life. 2022;12(1):91. doi:10.3390/life12010091.
- Poulianiti KP, Kaltsatou A, Mitrou GI, et al. Systemic redox imbalance in chronic kidney disease: a systematic review. Oxid Med Cell Longev. 2016;2016:8598253. doi:10.1155/2016/8598253.
- Canaud B, Cristol J, Morena M, et al. Imbalance of oxidants and antioxidants in haemodialysis patients. Blood Purif. 1999;17(2-3):99–106. 2016doi:10.1159/000014381.
- Morena M, Cristol JP, Canaud B. Why hemodialysis patients are in a prooxidant state? What could be done to correct the pro/antioxidant imbalance. Blood Purif. 2000;18(3):191–199. doi:10.1159/000014418.
- Liakopoulos V, Roumeliotis S, Zarogiannis S, et al. Oxidative stress in hemodialysis: causative mechanisms, clinical implications, and possible therapeutic interventions. Semin Dial. 2019;32(1):58–71. doi:10.1111/sdi.12745.
- Himmelfarb J. Oxidative stress in hemodialysis. Contrib Nephrol. 2008;161:132–137.
- Meléndez Oliva E, Villafañe JH, Alonso Pérez JL, et al. Effect of exercise on inflammation in hemodialysis patients: a systematic review. J Pers Med. 2022;12(7):1188. doi:10.3390/jpm12071188.
- Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737–1745. doi:10.1001/jama.293.14.1737.
- Stenvinkel P, Chertow GM, Devarajan P, et al. Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Int Rep. 2021;6(7):1775–1787. doi:10.1016/j.ekir.2021.04.023.
- Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298(3):F662–F671. doi:10.1152/ajprenal.00421.2009.
- de Sousa CV, Sales MM, Rosa TS, et al. The antioxidant effect of exercise: a systematic review and meta-analysis. Sports Med. 2017;47(2):277–293. doi:10.1007/s40279-016-0566-1.
- Meléndez-Oliva E, Sánchez-Vera Gómez-Trelles I, Segura-Orti E, et al. Effect of an aerobic and strength exercise combined program on oxidative stress and inflammatory biomarkers in patients undergoing hemodialysis: a single blind randomized controlled trial. Int Urol Nephrol. 2022;54(9):2393–2405. doi:10.1007/s11255-022-03146-z.
- Fatouros IG, Pasadakis P, Sovatzidis A, et al. Acute exercise may exacerbate oxidative stress response in hemodialysis patients. Nephron Clin Pract. 2008;109(2):c55–64. doi:10.1159/000139990.
- Ito M, Tanaka T, Nangaku M. Nuclear factor erythroid 2-related factor 2 as a treatment target of kidney diseases. Curr Opin Nephrol Hypertens. 2020;29(1):128–135. doi:10.1097/MNH.0000000000000556.
- Abreu CC, Cardozo LF, Mafra D. Could physical exercises modulate Nrf2-Keap1 pathway in chronic kidney disease? Med Hypotheses. 2015;84(1):44–46. doi:10.1016/j.mehy.2014.11.013.
- Abreu CC, Cardozo LFMF, Stockler-Pinto MB, et al. Does resistance exercise performed during dialysis modulate Nrf2 and NF-κB in patients with chronic kidney disease? Life Sci. 2017;188:192–197. 2017doi:10.1016/j.lfs.2017.09.007.
- Wang HT, Yang WQ, Liu YQ. Effects of aerobic exercise on Nrf2/GPX4/ferroptosis pathway in myocardial injury in high-fat diet mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2022;38(2):143–148.
- Liu YQ, Zhang J, Gao LN, et al. Effects of aerobic exercise on Nrf2-SOD pathway in the gastrocnemius of rats with high-glucose and high-fat diet. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2020;36(5):481–485.
- Søndergaard E, Ertmann RK, Reventlow S, et al. Using a modified nominal group technique to develop general practice. BMC Fam Pract. 2018;19(1):117. doi:10.1186/s12875-018-0811-9.