Abstract
Diabetic kidney disease (DKD) is a severe complication of diabetes mellitus (DM). The literature on DKD inflammation research has experienced substantial growth. However, there is a lack of bibliometric analyses. This study aimed to examine the existing research on inflammation in DKD by analyzing articles published in the Web of Science Core Collection (WOSCC) over the past 30 years. We conducted a visualization analysis using several software, including CiteSpace and VOSviewer. We found that the literature on inflammation research in DKD has experienced substantial growth, indicating a rising interest in this developing area of study. In this field, Navarro-Gonzalez, JF is the most frequently cited author, Kidney International is the most frequently cited journal, China had the highest number of publications in the field of DKD inflammation, and Monash University emerged as the institution with the most published research. The research area on inflammation in DKD primarily centers around the investigation of ‘Glycation end-products’, ‘chronic kidney disease’, and ‘diabetic nephropathy’. The emerging research trends in this field will focus on the ‘Gut microbiota’, ‘NLRP3 inflammasome’, ‘autophagy’, ‘pyroptosis’, ‘sglt2 inhibitor’, and ‘therapeutic target’. Future research on DKD may focus on further exploring the inflammatory response, identifying specific therapeutic targets, studying biomarkers, investigating stem cell therapy and tissue engineering, and exploring gene therapy and gene editing. In summary, this study examines the main areas of study, frontiers, and trends in DKD inflammation, which have significant implications for future research.
Introduction
DM is a chronic metabolic disorder characterized by persistent hyperglycemia [Citation1]. It is considered one of the most prevalent chronic diseases and can result in various complications, including kidney failure [Citation2]. DKD is a significant complication of diabetes mellitus and is the primary contributor to end-stage renal disease (ESRD) development [Citation3]. The pathophysiology of DKD is intricate, and recent evidence indicates renal involvement. Recent evidence indicates that the inflammatory response of the kidney is strongly associated with the onset and progression of DKD [Citation4]. This response can contribute to the development of glomerulosclerosis, tubular atrophy, and interstitial fibrosis, ultimately resulting in renal failure, representing the outcome of DKD [Citation5]. Hence, it is crucial to investigate the mechanism of renal inflammation in DKD. Several mechanisms, including the NF-κB signaling pathway, cytokines, and chemokine receptors, are closely associated with the development and advancement of DKD [Citation6]. Targeted interventions on signaling molecular pathways linked to renal inflammatory responses can delay the progression of DKD. This area shows great promise, and conducting analyses on the present state, areas of emphasis, and emerging trends in renal inflammation research will provide valuable insights.
Bibliometrics is a commonly employed approach in medical research to analyze and depict research trends effectively [Citation7,Citation8]. These analyses can facilitate researchers in acquiring a thorough comprehension of the present state and evolving research patterns within the discipline. Furthermore, they aid in identifying prospective research domains and pivotal concerns, evaluating the caliber and influence of research, comprehending the trajectory and methodologies of research, and fostering scholarly interactions and collaborations. Ultimately, these endeavors contribute to the advancement of the field. However, there is a lack of bibliometric studies on inflammation in DKD. This paper aims to systematically evaluate the current state, research focal points, and emerging trends in inflammation related to DKD over the past three decades. This will be achieved by analyzing the literature using bibliometric methods, identifying significant findings, and providing insights for future research directions.
Materials and methods
Search strategy
On January 1, 2023, online literature data were collected from the Science Citation Index Expanded (SCI-Expanded) database using a search strategy consisting of the following terms: TS=(Diabetic Nephropathy OR Diabetic Kidney Disease OR Kidney Disease; Diabetic OR Diabetic Glomerulosclerosis OR Intracapillary Glomerulosclerosis OR Nodular Glomerulosclerosis OR Glomerulosclerosis; Nodular OR Kimmelstiel-Wilson Syndrome OR Kimmelstiel-Wilson Disease) AND TS=(Inflammation OR Innate Inflammatory Response OR Innate Inflammatory Responses).
Data analysis
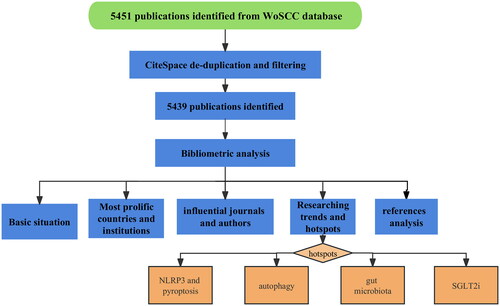
Two authors independently conducted the inclusion-exclusion process of the literature. The study did not limit the type of publications included, and duplicate studies were eliminated. The literature meeting the study’s requirements, including complete records and cited references, was exported in plain text format. CiteSpace 6.1.R6, VOSviewer1.6.18, Prism8, COOC13.4, and BibliometrixR4.1.1 were utilized to visualize and analyze the literature. CiteSpace 6.1.R6 and VOSviewer1.6.18 were employed to construct a visualization bibliometric network based on the collected literature information ().
Results
Annual literature volume and growth
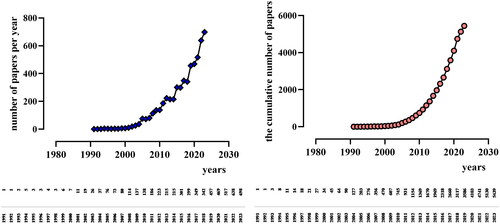
This analysis comprises 5439 papers from 1991 to 2023, The initial international literature on DKD inflammation was published in WOSCC in 1991. Notably, research in this area was in its infancy from 1991 to 2007, with few publications and slow progress. However, since 2007, the number of papers has shown a trend of rapid and continuous growth. The rapid growth of research in this field is mainly related to the increasing incidence of DKD every year, the development of disciplines such as molecular biology and immunology, and the development of biotechnology ().
Country analysis
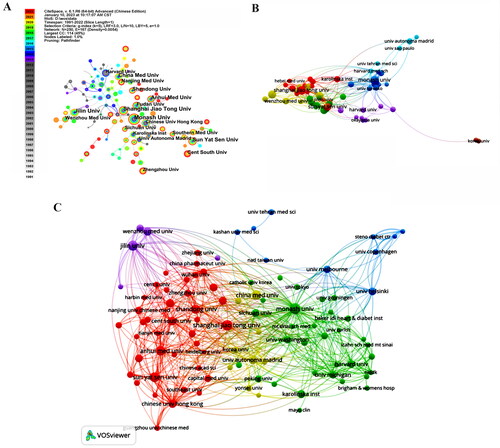
According to the results obtained from the WOSCC database search, a total of 5439 scholarly articles about the topic of inflammation in DKD have been published. These articles have been authored by researchers affiliated with 4264 distinct institutions spanning 100 nations. It is worth noting that China has the highest number of published articles, totaling 1952. Following China, the United States has 1082 published articles, Japan has 419, and Germany has 246 (). In addition, it is worth noting that a significant degree of collaboration exists among nations, with the United States displaying the highest centrality score of 0.38 (). shows the co-authorship network wherein the cooperation frequency exceeds 25 times, indicating the extent of communication among influential countries in the field. The interconnected nodes symbolize the collaborative association among nations, with the distance and thickness of the links representing the extent of cooperation. Moreover, the utilization of distinct hues visually represents various directions of cooperation. The United States is a key research hub in this sector, facilitating extensive collaboration with many countries such as China, Germany, Italy, and others. Enhanced collaboration possesses the capacity to generate more advanced outcomes in the realm of scientific study.
Figure 3. Leading countries in inflammation in DKD research. (A)Global geographic collaboration regarding the study; (B) The country co-authorship network; (C) Map of countries with publications in inflammation in DKD research; (D) Radar map of the top 10 productive countries; (E) Annual attention trend of the top 10 productive countries.
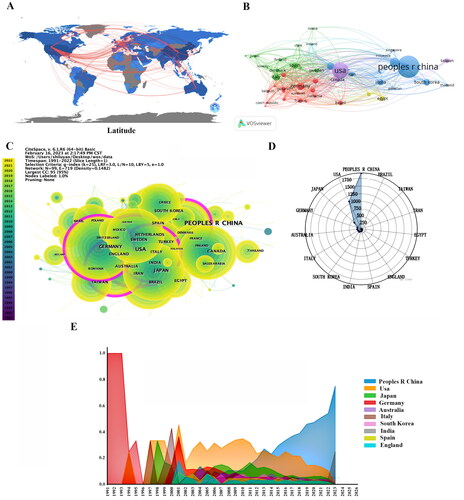
The United States is most referenced in this subject, having accumulated 54,285 citations. China follows closely behind with 38,314 citations, while Japan has garnered 14,010 citations. These two nations exhibit superior performance regarding the quantity of published scholarly articles and the overall number of citations received. DKD inflammation was examined in Germany in 1991, followed by its initiation in the United States in 1993. In contrast, the initiation of study in this field in China can be traced back to the year 2004 and has since experienced a significant surge, leading to China’s prominent position as the country with the highest degree of interest ().
Institutional analysis
In the field of DKD inflammation, a total of 4264 institutions are involved. Visual analysis indicates that Monash University secured the top position with 96 articles, followed by Jilin University (92 articles), Shanghai Jiao Tong University (91 articles), and Harvard University (88 articles), with the highest centrality observed at Harvard Med Sch (0.15) (). The level of institutional cooperation reflects the extent of communication between influential institutions in the field. The network of co-authorship organizations sharing the same color signifies their close collaboration in related fields. Notably, Shanghai Jiao Tong University, Monash University, Karolinska Institution, and Sunyata Sen University exhibit significant co-authorship with other institutions (). Additionally, the citation-organizations network, comprising 97 nodes, highlights the Australian Monash University (cited 6046 times), China University Hong Kong (cited 4969 times), and University of California, San Diego (cited 3157 times) as the most influential institutions in this domain, (). Despite the extensive publication output from various institutions, the frequency of citations does not rank highly, suggesting that there is room for improvement in the quality of specific academic papers.
Analysis of published journals
presents the top 10 journals ranked by publication and citation count, with impact factors ranging from 3.75 to 38.72. The impact factor of a scholarly journal is a metric that indicates the average annual number of citations received by the journal’s most recently published articles. It is commonly used to assess the influence of journals within their respective fields. Notably, our analysis suggests that journals with higher publication rates were more likely to accept research articles on inflammation in DKD than those with lower publication rates. The present study reveals that the ten most prestigious journals, with an impact factor of no less than 3.0, accounted for 16.1% of the total literature produced in the field.
Table 1. Top 10 journals by publication (left) and top 10 cited journals (right).
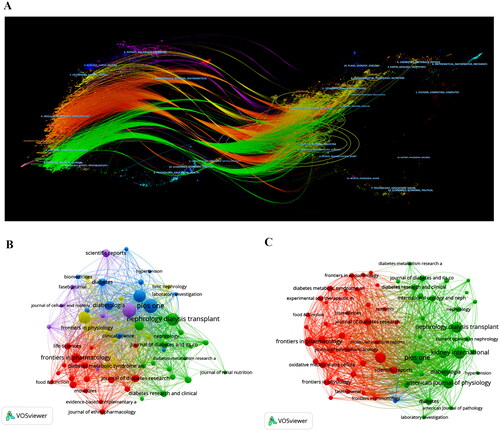
Furthermore, the citation and co-citation networks of the journals were visualized using VOSviewer (). The nodes in the same color indicate a cluster, which suggests a high degree of collaboration. The co-citation network has two main groups, with the red cluster representing the field of physiology, which includes journals such as ‘Frontiers in Pharmacology’ and ‘Oxidative Medicine.’ The blue cluster comprises various journals, including ‘PLOS One’ and ‘Kidney International,’ which pertain to general and kidney disease topics. The Dual Map Overlay of the Journals diagram () illustrates that the labels on the map denote the research area encompassed by the journal. The right side indicates the journal in which the references are cited. The links in the middle signify the cross-citation links between the references. Besides, most of the papers above have been published in journals related to molecular, biological, and immunological studies.
Figure 5. (A) The Dual map overlay of journals; (B) the citation sources network; (C) the co-citation sources network.
Additionally, the cited papers in this study were published in various fields, including molecular biology, genetics, medicine, nursing, rehabilitation, health, and others. From 1991 to 2023, a cumulative total of 5349 articles were published, encompassing 14 different literature types as outlined in . Notably, research articles comprised most of these publications (77.84%), followed by review articles (20.33%).
Table 2. Types of journal articles (The original results were collected by the WoS analysis tool).
Author analysis
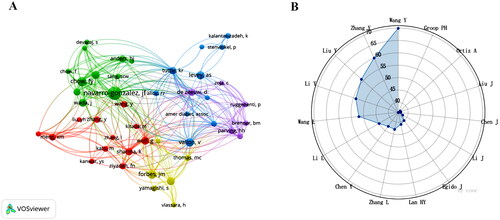
The field boasts a vast pool of 20,834 authors, among whom high-producing authors hold prominent positions as distinguished researchers and core team figures. Wang Y, with 70 articles to their credit, occupies the top spot, accounting for 1.28% of the total output, thereby exemplifying the high productivity of such authors (). The citation-author network () reflects authors who have made noteworthy contributions. Authors of the same color describe their close collaboration, with the top 10 cited more than 200 times, indicating that they are distinguished and influential researchers. Navarro-Gonzalez, JF, Chow, FY, Tesch, GH have significant co-authorship relationships, but Brownlee M and Lewis EI have the highest centrality (0.11).
Keyword co-occurrence and clustering analysis
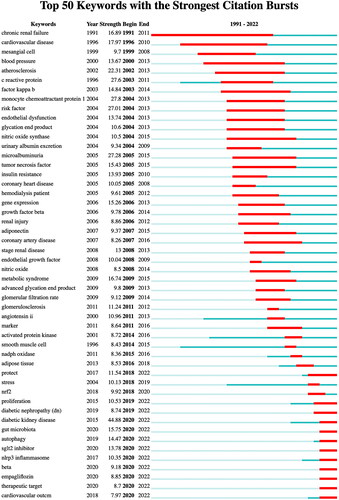
Keywords are succinct descriptors utilized in indexing or cataloging to provide a brief and precise summary of an article. Three overarching clusters of keywords can be identified based on the co-occurrence of all keywords (). The red clusters encompass ‘type 2 diabetes,’ ‘metabolic syndrome,’ ‘mortality,’ and ‘cardiovascular risk,’ which primarily pertain to complications associated with DKD. The blue clusters, on the other hand, comprise ‘expression,’ ‘mechanisms,’ ‘injury,’ and ‘pathogenesis,’ and the focus is on the protection and damage mechanisms and pathways of DKD. The targets and pathways of action of DKD inflammation are mainly the subjects of studies in the green cluster, focusing on ‘glycation end-products,’ and ‘fibrosis,’ ‘tgf-beta.’ The research in this field from 2010 to 2012 was centered around ‘Monocyte,’ ‘C-Reactive Protein,’ and ‘Chemoattractant Protein-1’, while from 2012 to 2014, the dominant areas of investigation were ‘peritoneal dialysis,’ ‘proteinuria,’ ‘Angiotensin li,’ and ‘Advanced glycation end products.’ The years 2014–2016 were characterized by a preponderance of research on ‘TNF-Alpha,’ ‘endothelial dysfunction,’ and ‘TNF- Reta,’ while the subsequent period of 2016–2018 saw a shift in focus toward ‘podocytes,’ ‘High Glucose,’ ‘Oxidative Stress,’ and ‘Fibrosis.’ The years 2018–2020 were marked by a surge in research on ‘Nrf2,’ ‘Autophagy,’ and ‘Biomarkers,’ whereas ‘Pyroptosis’ and ‘NLRP3’ emerged as the primary research subjects during 2020–2022. Additionally, ‘Meta-analysis’ studies increased significantly during this same period ().
Figure 7. (A) Co-occurrence of All keywords network; (B) map of Keyword-weighted evolution path; (C) cluster analysis of keyword co-appearance; (D) The timeline view of keywords co-appearance.
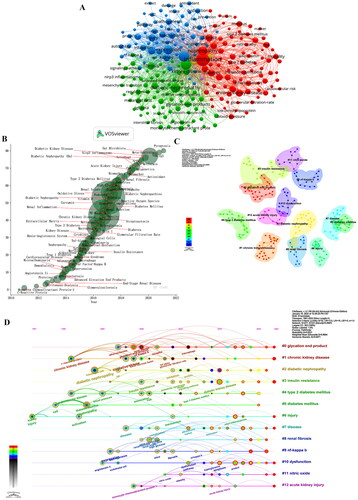
This study used the LLR algorithm to cluster the keywords, resulting in 12 clusters, Modular Q (Q value) = 0.8531 > 0.3 and mean silhouette (S value) = 0.9684 > 0.7 proved the importance of this clustering structure, and the results were convincing. ‘Glycation end product’, ‘chronic kidney disease,’ and ‘diabetic nephropathy’ are the focus of the DKD inflammation research field. ().
Keyword burst detection
In contrast to the analysis of high-frequency keywords, identifying bursts keywords (i.e., frequently cited keywords over a specified period) in the field until 1 January 2023 provides greater insight into the frontier themes and emerging trends. The top 50 keywords were selected based on their Burst intensity and arranged chronologically according to their burst time, as illustrated in . The earliest Burst keywords in chronic renal failure include ‘mesangial cell.’ Prominent early research hotspots also include ‘Factor kappa B,’ ‘insulin resistance,’ ‘Adiponectin,’ ‘Advanced glycation end product’, ‘Nitric oxide’, and others. Moreover, DKD primarily contributes to the heightened risk of cardiovascular disease (CVD) in individuals with DM. Additional areas of focus include ‘coronary heart disease,’ ‘SGLT2 inhibitor’ and ‘Empagliflozin.’ Recent research has identified ‘Gut microbiota’, ‘Autophagy’, ‘NLRP3 inflammasome’ and ‘Therapeutic target’ as prominent keywords that have experienced a surge in attention over the past two years.
In conclusion, Numerous studies have demonstrated the association of cytokines, chemokines, growth factors, adhesion molecules, pro-inflammatory cytokines, TNF-α, MCP-1, and adhesion molecules with DKD pathogenesis, highlighting their crucial role in DKD complications. Scientists are exploring ways to improve the prognosis of patients with DKD from multiple perspectives. In the future, with the continuous development of molecular biology technology, we are expected to see more innovative therapeutic means applied in the clinic, providing more options for treating DKD.
Co-citation clustering analysis of references and burst detection
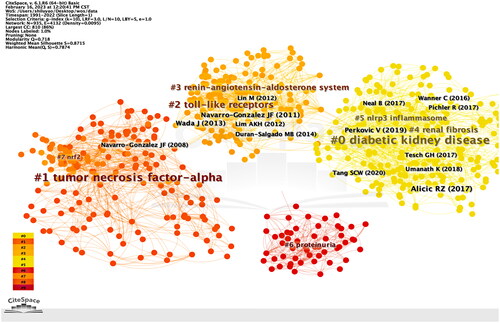
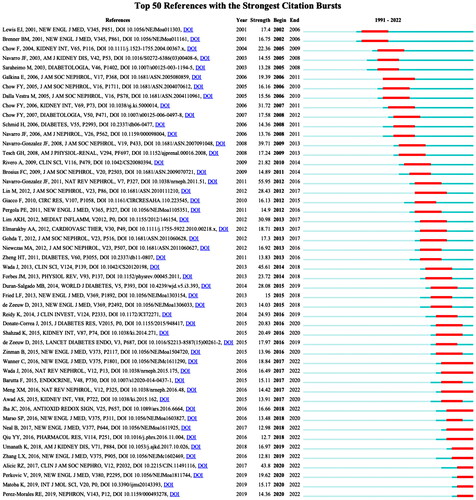
The CiteSpace tool offers a noteworthy capability in the form of reference co-citation clustering, which facilitates a comprehensible comprehension of research themes and hotspots. The mixed science map comprises the most frequently cited noun phrases in co-cited references, encompassing the most cited references and their keyword clusters. This map reveals the highly cited literature and their cited research directions (). Meanwhile, an analysis of citation bursts was conducted, as shown in . The reference that garnered the highest number of citations with the most intense burst was ‘Navarro-Gonzalez JF, 2011(55.95)’, which was published in Nature Reviews by the authors who concentrated on crucial proinflammatory molecules and pathways that are involved in the advancement and escalation of DKD [Citation9].
Furthermore, the reference with the second highest number of citations in the citation burst pertains to the work of Wada et al. They demonstrated that microinflammation and subsequent extracellular matrix expansion are common pathways for the progression of DKD and examined the various molecular mechanisms associated with DKD progression [Citation10]. The third most cited reference, authored by Alicic RZ in 2017, presents a comprehensive overview of the risk factors, diagnosis, and treatment options for diabetic nephropathy and summarizes the effects of various drugs on renal outcomes in patients with DKD [Citation11].
Discussion
General trends of inflammation in DKD
The present study aims to investigate the hot research and development trend of the regulatory mechanisms of inflammation in DKD as a crucial entry point for targeted pharmacological interventions aiming to improve the prognosis of DKD patients over the past 30 years. Renal inflammatory reactions are identified as the primary factors contributing to the occurrence and progression of DKD. Bibliometric analysis was conducted on relevant articles to gain insight into the current research status and trends of renal inflammation in DKD. It analyzed 5439 articles spanning the period from 1 January 1991 to 1 January 2023. Notably, the research field remained inactive for over a decade following the indexing of the first article by WOSCC in 1991. Since 2007, the field has experienced rapid development, which can be attributed to three reasons. Firstly, the incidence of DM has been increasing year by year, and DKD is one of the most common complications of DM [Citation12]. Practical therapeutic approaches for DKD have become an urgent need in clinical practice. Second, with the development of molecular biology and immunology disciplines, scientists have begun to delve into the molecular mechanisms of the inflammatory response [Citation13]. The in-depth investigation of molecular mechanisms provides new perspectives and ideas for understanding the occurrence and development of DKD, as well as potential targets for developing new drug therapies [Citation14]. With the development of biotechnology, more new technologies are being applied to laboratory research. For example, gene editing technology, single-cell sequencing technology, proteomics, and other technologies provide new means and methods to study the molecular mechanisms of inflammatory responses. Applying these new technologies provides more possibilities for research in this field.
The exponential expansion of the research field has significantly advanced various disciplines, fostered the emergence of multiple new research hubs, and facilitated a steady rise in inter-team collaboration across nations. Currently, 4264 institutions in 100 countries are actively engaged in this field, with China as the leading contributor of scholarly articles, followed by the United States, Japan, and Germany. Notably, the United States exhibits the highest degree of collaboration centrality. It maintains close partnerships with numerous countries, such as China, Germany, and Italy, where cross-border institutional collaborations can enhance scientific progress. Regarding publication and research quality, China boasts the highest caliber of research. However, the United States is the most frequently cited country, with China following closely behind. China has been relatively tardy in conducting research in this domain compared to other nations but has made noteworthy advancements recently. Monash University, China University Hong Kong, and the University of California, San Diego, are recognized as the most influential publishing institutions. Regarding article production, Chinese scholar Wang Y has the highest number of publications with 70, while Navarro-Gonzalez, JF is the most cited and frequently collaborated group.
Research status
We have analyzed and integrated keywords using various software tools. Through this process, we have identified several emerging research trends in the field. These trends include ‘Gut microbiota,’ ‘NLRP3 inflammasome’, ‘autophagy,’ ‘pyroptosis,’ ‘sglt2 inhibitor’, and ‘therapeutic target.’ The following sections provide a summary of these areas.
Mechanisms and therapeutic perspectives of NLRP3 inflammasome and pyroptosis in regulating DKD
The NLRP3 inflammasome, which encompasses the NOD-, LRR-, and pyrin domain-containing 3 protein, has been demonstrated to have a role in several acute and chronic kidney disorders caused by microbial and non-microbial factors [Citation15–17]. Wang et al. [Citation18], Cao et al. [Citation19], Huang et al. [Citation20], and Williams et al. [Citation21]. have conducted a comprehensive review of the underlying pathogenic mechanisms, advancements, and treatment approaches to the activation of inflammatory vesicles, pyroptosis, and the associated signaling pathways in the context of renal illness, respectively. Through additional investigation, certain medicine extractions, including Fucoidan (FPS) [Citation22], Gambogenic acid [Citation23], total flavones of Abelmoschus manihot (TFA) [Citation24], ginsenosides [Citation25], Hirudin [Citation26], Punicalagin [Citation27], and anshinone IIA [Citation28], have demonstrated the ability to impede the activation of inflammatory vesicles and the occurrence of pyroptosis. Nevertheless, the precise mechanism by which these bioactive chemicals exert their effects remains uncertain. Identifying their target molecules and elucidating their molecular mechanisms of action have emerged as critical areas of investigation in contemporary research. Furthermore, recent studies have demonstrated certain compounds’ ability in DKD inflammation to hinder the activation of inflammatory vesicles and cellular juxtaposition through targeted molecular mechanisms. For instance, human umbilical cord mesenchymal stem cells (MSCs) acting on the miR-342-3p/Caspase1 signaling pathway [Citation29], interferon-gene stimulating agents (STINGs) that can drive noninfectious inflammation through suppressing NLRP3 [Citation30], the ALPK1/NF- kappa B pathway initiates the typical caspase-1- GSDMD pyroptosis pathway [Citation31] and WTAP promotes the m(6)A methylation of NLRP3 mRNA [Citation32] to upregulate NLRP3 inflammasome activation. Thorough investigations of these pathways have the potential to yield novel perspectives on the management of DKD and propose innovative approaches for medication discovery.
Potential of autophagy-related pathways and targets to modulate inflammatory responses in DKD patients
Autophagy is directly associated with the progression of DKD and can regulate the inflammatory process of DKD through numerous signaling pathways. Kimura examined the link between autophagy and renal inflammation [Citation33]. They proposed the possible application of autophagy as an inflammatory modulator in treating renal disorders. With the progress of research, scientists found that some pathways such as Risa regulate the Sirt1/GSK3 beta axis [Citation34], TNFAIP allowing TNT-mediated autophagosome and lysosome exchange [Citation35], AIF-1 regulated autophagy in HRGECs via miR-34a/ATG4B pathway [Citation36], and other mechanisms have a role in regulating autophagy levels. In addition, various plant extracts such as Paeoniflorin (PF), Jujuboside A (JuA), Complanatoside A (CA), and rutin have been identified to have modes of action related to autophagy. Much research has studied the ability of various components to control autophagy and decrease inflammatory damage in DKD. Nonetheless, research on these pathways is still in its infancy, and many of them require more examination and practical application. Therefore, exploring autophagy-related medications has been a significant and burgeoning research area in recent years.
Modulation of renal inflammation by the gut microbiota
Increasing evidence indicates a connection between DKD and intestinal dysbiosis caused by toxins originating from the gut [Citation37]. Hu et al. provided the initial evidence that acetate, produced by the gut microbiota, induces renal tubulointerstitial injury in DKD by activating GPR43 [Citation38]. Lehto conducted an analysis of the impact of gastrointestinal tract disturbances on the development of renal disorders [Citation39]. Yang, Ge et al. summarized therapeutic strategies for intestinal microecology [Citation40]. Interventions, such as Sanziguben polysaccharides (SZP) [Citation41], dietary fiber [Citation42], and probiotics [Citation43], have shown potential benefits in slowing the progression of DKD. Further investigation is warranted to explore the potential of herbal medicines, such as Bupleurum polysaccharides [Citation44], Cordyceps cicadae polysaccharides (CCP) [Citation45], Tangshen Formula (TSF) [Citation46], The Shenyang Kangfu tablet (SYKFT) [Citation47], Moutan Cortex (MC-Pa) [Citation48], Resveratrol [Citation49], and Sanziguben (SZGB) [Citation41], in modulating the inflammatory response in DKD by influencing the gut microbiota. This research should aim to deepen our understanding of the relationship between the gut microbiota and various factors, including metabolism, immunity, and the neuroendocrine system. By modulating the gut microbiota, scientists seek to improve the prognosis of DKD.
Cardiovascular (CV) and renal benefits of SGLT2i and its mechanism of action
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) represent a new class of therapeutic drugs for diabetes management [Citation50]. These medications can potentially enhance patients’ DKD prognosis by mitigating inflammation, hypoxia, oxidative stress, and renin-angiotensin system (RAS) activation. Multiple large randomized controlled studies have provided substantial evidence of the considerable advantages of SGLT2i in reducing cardiovascular mortality and decelerating the advancement of renal illness [Citation51–53]. Consequently, owing to its distinctive mode of action and diverse benefits, SGLT2i is crucial in treating kidney disease. Nevertheless, the benefits of SGLT2i cannot be adequately explained by alterations in renal or cardiovascular physiology or metabolism, as demonstrated in both experimental and clinical studies [Citation54,Citation55]. Scholars have recently shown interest in exploring novel molecular routes through which SGLT2i exert their renoprotective benefits. The distinctive pattern of cardioprotective and renoprotective effects of SGLT2i is abolished by specific inhibition or knockdown of autophagy, AMPK, and sirtuins [Citation54]. Less known effects on SGLT2i on klotho expression, capillary rarefaction, signal transducer and activator of transcription signaling, and peptidylprolyl cis/trans isomerase (Pin1) levels may partly explain the anti-fibrotic effects of SGLT2i in kidneys [Citation50]. A study has determined that the SGLT2/IGF1R/PI3K signaling pathway is crucial in regulating podocyte epithelial-mesenchymal transition (EMT). The modulation of IGF1R expression presents a potentially innovative strategy for treating DKD [Citation56]. Furthermore, it has been observed that empagliflozin exhibits a mitigating effect on harm caused by oxidative stress through the activation of the Akt/GSK-3 signaling pathway [Citation57] and the PPAR gamma/CD36 pathway [Citation58]. Dapagliflozin enhances the inflammatory response in DKD by augmentation of AMPK activity and suppressing the inflammatory response mediated by the NF-κB pathway, thereby ameliorating the long-term prognosis of individuals with DKD [Citation59].
In summary, the examination of the molecular biological mechanism of SGLT2i has the potential to enhance our comprehension of the pathogenesis of DKD, refine therapeutic approaches, identify novel drug targets, advance research in associated domains, and offer theoretical backing for enhancing patients’ quality of life and prognosis.
Future perspectives
Currently, no satisfactory treatments are available for DKD, resulting in renal failure being the primary cause of mortality [Citation60]. Researchers are investigating various approaches to enhance the prognosis of individuals diagnosed with DKD [Citation61]. Investigating the molecular mechanisms of the inflammatory response is currently a prominent area of research [Citation62]. Scientists are investigating the expression and regulatory mechanisms of various factors involved in the inflammatory response, such as cytokines, signaling pathways, and regulatory factors, to enhance the prognosis of DKD by modulating the inflammatory response [Citation63,Citation64]. Furthermore, Scientists identify distinct biomarkers linked to DKD by analyzing specific molecules in urine, blood, and other biological samples [Citation65]. This research aims to monitor, diagnose, and classify the disease progression in DKD [Citation66]. Genetic factors play a role in DKD. Therefore, utilizing gene editing techniques to identify and correct genetic variants associated with DKD may offer a promising approach to enhance the prognosis of this condition [Citation67].
Future research on DKD may focus on further exploring the inflammatory response, identifying specific therapeutic targets, studying biomarkers, investigating stem cell therapy and tissue engineering, and exploring gene therapy and gene editing. These studies provide crucial information for advancing more efficient treatments in managing DKD and minimizing its physiological and lifestyle consequences.
Limitations
In this study, we used CiteSpace, VOSviewer, Prism, BibliometrixR, and COOC software to visualize and analyze the WOSCC database and may have missed papers published in other databases. As our search strategy was designed to obtain the most comprehensive data possible, the topics of the included articles may not be as relevant to DKD inflammation. In addition, none of these software program could obtain information on all authors of an article, differentiate the order of authorship, merge different formats of the same author’s name, and be unable to assess the role played by researchers in the field accurately.
Conclusion
We analyze the research hotspots, frontiers, and trends in DKD inflammation through trends emerging from keyword clusters, bursts, and timeline relationships based on a bibliometric analysis constructed using CiteSpace、VOSviewer、Prism、BibliometrixR, and COOC software. The research literature on DKD inflammation has increased significantly in recent decades, indicating a growing interest in this emerging field. Our findings provide a comprehensive summary of the current status of DKD inflammation research and have important implications for future research endeavors. Bibliometrics has a unique value compared to traditional reviews but also needs more research depth. We are very grateful to the development team of the software utilized in this research study. We hope they will further optimize the software functions to achieve a more accurate and in-depth visual analysis of the research field.
Authors contributions
SLY and LCY contributed to the study’s conception and design. SLY collected data from the database and drafted the initial manuscript draft. All authors participated in revising the manuscript, as well as perusing and endorsing the version that was submitted.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The article/supplementary material contains the novel findings of this investigation, and any additional questions may be directed to the corresponding authors.
Additional information
Funding
References
- Ayyoub S, Al-Trad B, Aljabali AAA, et al. Biosynthesis of gold nanoparticles using leaf extract of Dittrichia viscosa and in vivo assessment of its anti-diabetic efficacy. Drug Deliv Transl Res. 2022;12(12):1–16. doi:10.1007/s13346-022-01163-0.
- Kim JE, Lee JY, Kang C-H. Limosilactobacillus fermentum MG4295 improves hyperglycemia in high-fat diet-induced mice. Foods. 2022;11(2):231. doi:10.3390/foods11020231.
- Yu J, Zong G, Wu HJ, et al. Podoplanin mediates the renoprotective effect of berberine on diabetic kidney disease in mice. Acta Pharmacol Sin. 2019;40(12):1544–1554. doi:10.1038/s41401-019-0263-3.
- Huang X, Shi Y, Chen H, et al. Isoliquiritigenin prevents hyperglycemia-induced renal injuries by inhibiting inflammation and oxidative stress via SIRT1-dependent mechanism. Cell Death Dis. 2020;11(12):1040. doi:10.1038/s41419-020-03260-9.
- Song Y, Guo F, Liu Y, et al. Identification of circular RNAs and functional competing endogenous RNA networks in human proximal tubular epithelial cells treated with sodium-glucose cotransporter 2 inhibitor dapagliflozin in diabetic kidney disease. Bioengineered. 2022;13(2):3911–3929. doi:10.1080/21655979.2022.2031391.
- Yang Y, Liu M, Yang F, et al. Circular RNA expression profiles following negative pressure wound therapy in burn wounds with experimental Pseudomonas aeruginosa infection. Bioengineered. 2022;13(2):4122–4136. doi:10.1080/21655979.2021.2006965.
- Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci. 2006;57(3):359–377. doi:10.1002/asi.20317.
- Liu T, Yang L, Mao H-M, et al. Knowledge domain and emerging trends in podocyte injury research from 1994 to 2021: a bibliometric and visualized analysis. Front Pharmacol. 2021;12:772386. doi:10.3389/fphar.2021.772386.
- Navarro-González JF, Mora-Fernández C, MMd F, et al. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7(6):327–340. doi:10.1038/nrneph.2011.51.
- Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013;124(3):139–152. doi:10.1042/CS20120198.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi:10.2215/CJN.11491116.
- Xiao Y, Liang D, Li Z, et al. BMP-7 upregulates Id2 through the MAPK signaling pathway to improve diabetic tubulointerstitial fibrosis and the intervention of oxymatrine. Front Pharmacol. 2022;13:900346. doi:10.3389/fphar.2022.900346.
- Jiang Z, Tang Y, Song H, et al. miRNA-342 suppresses renal interstitial fibrosis in diabetic nephropathy by targeting SOX6. Int J Mol Med. 2020;45(1):45–52. doi:10.3892/ijmm.2019.4388.
- Guo J, Lei M, Cheng F, et al. RNA-binding proteins tristetraprolin and human antigen R are novel modulators of podocyte injury in diabetic kidney disease. Cell Death Dis. 2020;11(6):413. doi:10.1038/s41419-020-2630-x.
- Komada T, Muruve DA. The role of inflammasomes in kidney disease. Nat Rev Nephrol. 2019;15(8):501–520. doi:10.1038/s41581-019-0158-z.
- Xiang H, Zhu F, Xu Z, et al. Role of inflammasomes in kidney diseases via both canonical and non-canonical pathways. Front Cell Dev Biol. 2020;8:106. doi:10.3389/fcell.2020.00106.
- Shahzad K, Fatima S, Khawaja H, et al. Podocyte-specific Nlrp3 inflammasome activation promotes diabetic kidney disease. Kidney Int. 2022;102(4):766–779. doi:10.1016/j.kint.2022.06.010.
- Wang Y, Li Y, Xu Y. Pyroptosis in kidney disease. J Mol Biol. 2022;434(4):167290. doi:10.1016/j.jmb.2021.167290.
- Cao Z-h, Huang D, Tang C, et al. Pyroptosis in diabetes and diabetic nephropathy. Clin Chim Acta. 2022;531:188–196. doi:10.1016/j.cca.2022.04.011.
- Huang G, Zhang Y, Zhang Y, et al. Chronic kidney disease and NLRP3 inflammasome: pathogenesis, development and targeted therapeutic strategies. Biochem Biophys Rep. 2023;33:101417. doi:10.1016/j.bbrep.2022.101417.
- Williams BM, Cliff CL, Lee K, et al. The role of the NLRP3 inflammasome in mediating glomerular and tubular injury in diabetic nephropathy. Front Physiol. 2022;13:907504. doi:10.3389/fphys.2022.907504.
- Wang M-Z, Wang J, Cao D-W, et al. Fucoidan alleviates renal fibrosis in diabetic kidney disease via inhibition of NLRP3 Inflammasome-Mediated podocyte pyroptosis. Front Pharmacol. 2022;13:790937. doi:10.3389/fphar.2022.790937.
- Li N-X, Wen X-y, Tang M-K, et al. Gambogenic acid protects against high glucose-induced damage of renal tubular epithelial cells by inhibiting pyroptosis through regulating the AMPK–TXNIP pathway. Qual Assur Saf Crops Foods. 2022;14:40–46.
- Liu B, Tu Y-h, Ni G-X, et al. Total flavones of abelmoschus manihot ameliorates podocyte pyroptosis and injury in high glucose conditions by targeting METTL3-Dependent m6A Modification-Mediated NLRP3-Inflammasome activation and PTEN/PI3K/akt signaling. Front Pharmacol. 2021;12:667644. doi:10.3389/fphar.2021.667644.
- Chen X-M, Lin G-X, Wang X, et al. Beneficial effects of ginsenosides on diabetic nephropathy: a systematical review and meta-analysis of preclinical evidence. J Ethnopharmacol. 2023;302(Pt A):115860. doi:10.1016/j.jep.2022.115860.
- Han J, Zuo Z, Shi X, et al. Hirudin ameliorates diabetic nephropathy by inhibiting gsdmd-mediated pyroptosis. Cell Biol Toxicol. 2023;39(3):573–589. doi:10.1007/s10565-021-09622-z.
- An X, Zhang Y, Cao Y-y, et al. Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients. 2020;12(5):1516. doi:10.3390/nu12051516.
- Li Y, Deng X, Zhuang W, et al. Tanshinone IIA down-regulates -transforming growth factor beta 1 to relieve renal tubular epithelial cell inflammation and pyroptosis caused by high glucose. Bioengineered. 2022;13(5):12224–12236. doi:10.1080/21655979.2022.2074619.
- Zheng S, Zhang K, Zhang Y, et al. Human umbilical cord mesenchymal stem cells inhibit pyroptosis of renal tubular epithelial cells through miR-342-3p/Caspase1 signaling pathway in diabetic nephropathy. Stem Cells Int. 2023;2023:5584894–5584812. doi:10.1155/2023/5584894.
- Yang X, Chen Z, Luo Z, et al. STING deletion alleviates podocyte injury through suppressing inflammation by targeting NLRP3 in diabetic kidney disease. Cell Signal. 2023;109:110777. doi:10.1016/j.cellsig.2023.110777.
- Cui X, Li Y, Yuan S, et al. Alpha-kinase1 promotes tubular injury and interstitial inflammation in diabetic nephropathy by canonical pyroptosis pathway. Biol Res. 2023;56(1):5. doi:10.1186/s40659-023-00416-7.
- Lan J-h, Xu B, Shi X, et al. WTAP-mediated N6-methyladenosine modification of NLRP3 mRNA in kidney injury of diabetic nephropathy. Cell Mol Biol Lett. 2022;27(1):51. doi:10.1186/s11658-022-00350-8.
- Kimura T, Isaka Y, Yoshimori T. Autophagy and kidney inflammation. Autophagy. 2017;13(6):997–1003. doi:10.1080/15548627.2017.1309485.
- Su P, Liu D, Zhou S, et al. Down-regulation of Risa improves podocyte injury by enhancing autophagy in diabetic nephropathy. Mil Med Res. 2021;9:23.
- Barutta F, Bellini S, Kimura S, et al. Protective effect of the tunneling nanotube-TNFAIP2/M-sec system on podocyte autophagy in diabetic nephropathy. Autophagy. 2023;19(2):505–524. doi:10.1080/15548627.2022.2080382.
- Jianbing H, Xiaotian L, Jie T, et al. The effect of allograft inflammatory factor-1 on inflammation, oxidative stress, and autophagy via miR-34a/ATG4B pathway in diabetic kidney disease. Oxid Med Cell Longev. 2022;2022:1668000–1668022. doi:10.1155/2022/1668000.
- Miao L, Zhang J, Zhang Z, et al. A bibliometric and knowledge-map analysis of CAR-T cells from 2009 to 2021. Front Immunol. 2022;13:840956. doi:10.3389/fimmu.2022.840956.
- Hu ZB, Lu J, Chen P-P, et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics. 2020;10(6):2803–2816. doi:10.7150/thno.40571.
- Lehto M, Groop P-H. The Gut-Kidney axis: putative interconnections between gastrointestinal and renal disorders. Front Endocrinol. 2018;9:553. doi:10.3389/fendo.2018.00553.
- Yang G, Wei J, Liu P-y, et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. 2021;117:154712. doi:10.1016/j.metabol.2021.154712.
- Wang F, Liu C, Ren L, et al. Sanziguben polysaccharides improve diabetic nephropathy in mice by regulating gut microbiota to inhibit the TLR4/NF-κB/NLRP3 signalling pathway. Pharm Biol. 2023;61(1):427–436. doi:10.1080/13880209.2023.2174145.
- Li YJ, Chen X, Kwan TK, et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid-mediated activation of G protein-coupled receptors GPR43 and GPR109A. J Am Soc Nephrol. 2020;31(6):1267–1281. doi:10.1681/ASN.2019101029.
- Lau WL, Chang Y, Vaziri ND. The consequences of altered microbiota in immune-related chronic kidney disease. Nephrol Dial Transplant. 2020;36:1791–1798.
- Feng Y, Weng H, Ling L-J, et al. Modulating the gut microbiota and inflammation is involved in the effect of bupleurum polysaccharides against diabetic nephropathy in mice. Int J Biol Macromol. 2019;132:1001–1011. doi:10.1016/j.ijbiomac.2019.03.242.
- Yang J, Dong H, Wang Y, et al. Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. Int J Biol Macromol. 2020;163:442–456. doi:10.1016/j.ijbiomac.2020.06.153.
- Zhao T, Zhang H, Yin X, et al. Tangshen formula modulates gut microbiota and reduces gut-derived toxins in diabetic nephropathy rats. Biomed Pharmacother. 2020;129:110325. doi:10.1016/j.biopha.2020.110325.
- Chen Q, Ren D, Wu J, et al. Shenyan kangfu tablet alleviates diabetic kidney disease through attenuating inflammation and modulating the gut microbiota. J Nat Med. 2021;75(1):84–98. doi:10.1007/s11418-020-01452-3.
- Zhang M, Yang L, Zhu M-M, et al. Moutan cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. Int J Biol Macromol. 2022;206:849–860. doi:10.1016/j.ijbiomac.2022.03.077.
- Cai T, Ye X-L, Li R, et al. Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front Pharmacol. 2020;11:1249. doi:10.3389/fphar.2020.01249.
- Afsar B, Afsar RE. Sodium–glucose cotransporter inhibitors and kidney fibrosis: review of the current evidence and related mechanisms. Pharmacol Rep. 2023;75(1):44–68. doi:10.1007/s43440-022-00442-4.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi:10.1056/NEJMoa1812389.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi:10.1056/NEJMoa1811744.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi:10.1056/NEJMoa1504720.
- Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation. 2022;146(18):1383–1405. doi:10.1161/CIRCULATIONAHA.122.061732.
- Park CH, Lee B, Han M, et al. Canagliflozin protects against cisplatin-induced acute kidney injury by AMPK-mediated autophagy in renal proximal tubular cells. Cell Death Discov. 2022;8(1):12. doi:10.1038/s41420-021-00801-9.
- Guo R, Wang P, Zheng X, et al. SGLT2 inhibitors suppress epithelial–mesenchymal transition in podocytes under diabetic conditions via downregulating the IGF1R/PI3K pathway. Front Pharmacol. 2022;13:897167. doi:10.3389/fphar.2022.897167.
- Mihaljević V, Zjalic M, Kizivat T, et al. Molecular mechanisms linking empagliflozin to renal protection in the LLC-PK1 model of diabetic nephropathy. Biomedicines. 2022;10(11):2983. doi:10.3390/biomedicines10112983.
- Huang C-C, Chou C-A, Chen WY, et al. Empagliflozin ameliorates free fatty acid induced-lipotoxicity in renal proximal tubular cells via the PPARγ/CD36 pathway in obese mice. Int J Mol Sci. 2021;22(22):12408. doi:10.3390/ijms222212408.
- Xu J, Kitada M, Ogura Y, et al. Dapagliflozin restores impaired autophagy and suppresses inflammation in high glucose-treated HK-2 cells. Cells. 2021;10(6):1457. doi:10.3390/cells10061457.
- Jiang Z-Z, Liu Y-M, Niu X, et al. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7(1):24. doi:10.1186/s13287-016-0287-2.
- Wang J, Liu F, Kong R, et al. Association between globulin and diabetic nephropathy in Type2 diabetes mellitus patients: a cross-sectional study. Front Endocrinol. 2022;13:890273. doi:10.3389/fendo.2022.890273.
- Gu C-M, Liu S, Wang H, et al. Role of the thioredoxin interacting protein in diabetic nephropathy and the mechanism of regulating NOD-like receptor protein 3 inflammatory corpuscle. Int J Mol Med. 2019;43:2440–2450.
- Li G, Fang Y, Ma Y, et al. Screening and isolation of potential anti-inflammatory compounds from Saxifraga atrata via affinity ultrafiltration-HPLC and multi-target molecular docking analyses. Nutrients. 2022;14(12):2405. doi:10.3390/nu14122405.
- Sachan R, Kundu A, Dey P, et al. Dendropanax morbifera protects against renal fibrosis in Streptozotocin-Induced diabetic rats. Antioxidants. 2020;9(1):84. doi:10.3390/antiox9010084.
- Kwan BK, Fuhrer T, Zhang J, et al. Metabolomic markers of kidney function decline in patients with diabetes: evidence from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2020;76(4):511–520. doi:10.1053/j.ajkd.2020.01.019.
- Qiu C, Hanson RL, Fufaa GD, et al. Cytosine methylation predicts renal function decline in American Indians. Kidney Int. 2018;93(6):1417–1431. doi:10.1016/j.kint.2018.01.036.
- Wang Y-T, Zhang J, Zhao Y, et al. COL4A3 gene variants and diabetic kidney disease in MODY. Clin J Am Soc Nephrol. 2018;13(8):1162–1171. doi:10.2215/CJN.09100817.