Abstract
Objectives
Acute kidney injury (AKI) is a common global condition with high morbidity and mortality rates. Biomarkers can aid in the diagnosis, prediction, intervention, and outcome assessment of AKI. This study aimed to summarize the current research status and identify research hotspots for AKI biomarkers using bibliometric analysis.
Methods
Relevant original English language articles were retrieved from the Science Citation Index Expanded of the Web of Science Core Collection database, from inception to 31 December 2022. Full records and related cited references from all the documents were collected and analyzed.
Results
A total of 16368 authors from 3379 institutions in 83 countries/regions contributed to 2916 documents that were published in 712 academic journals. Annual publication output followed exponential growth since 2008. The United States, the University of Pittsburgh, and the American Journal of Physiology-Renal Physiology were the most productive countries, institutions, and journals in terms of research outputs, respectively. The area of interest has shifted from neutrophil gelatinase-associated lipocalin, cell cycle, and tubular damage toward sepsis and COVID-19. Apoptosis, inflammation, and chronic kidney disease have become popular in recent years, and studies on ferroptosis, machine learning, COVID-19, and renal fibrosis will be the focus of future research.
Implications
This bibliometric study suggests that future research on AKI biomarkers would focus on ferroptosis, renal fibrosis and COVID-19. Artificial intelligence, such as machine learning, maybe the most promising direction for the discovery and validation of AKI biomarkers.
1. Introduction
Acute kidney injury (AKI) is a common critical condition characterized by sudden and rapid deterioration of kidney function. Epidemiological data suggests that the global prevalence of AKI ranges from 1% to 66%, with an unacceptably high mortality rate of 9.1% to 21.9% [Citation1,Citation2]. Additionally, AKI patients are at a significantly increased risk of developing chronic kidney disease [Citation3]. Currently, AKI is diagnosed and interventions are initiated only when kidney function damage has already occurred, as indicated by rising serum creatinine or oliguria, according to the criteria of the Kidney Disease Improving Global Outcomes (KDIGO) [Citation4]. Moreover, serum creatinine level does not adequately reflect the severity of damage nor insight into the underlying specific causes. Given these limitations, there is a fervent interest in the nephrology community to identify and validate novel biomarkers that reflect the underlying pathophysiological processes and guide clinical management in various scenarios.
Biomarkers for diagnostic, therapeutic, or prognostic purposes are encouraging research hotspots in the field of AKI. In the past two decades, there has been tremendous interest in identifying and validating potential biomarkers of AKI. The available biomarkers can be broadly categorized as tubular injury markers, inflammation markers, or repair and fibrosis markers [Citation5]. Some of the previously identified biomarkers, such as cystatin C, neutrophil gelatinase-associated lipocalin, and kidney injury molecule-1, have already been translated into clinical practice in some centers.
Bibliometric analysis is an interdisciplinary science that provides insight into scientific development trends and guides the direction of future research by quantitatively analyzing bibliometric material using mathematical and statistical methods [Citation6]. Several prior studies have utilized bibliometric analysis to uncover the most productive countries, institutions, authors, and journals in the field of AKI [Citation7–10]. However, none of these studies have focused on AKI biomarkers, which have shown significant activity in recent years. In this study, we aimed to conduct a comprehensive bibliometric analysis to reveal the publication outputs and productive entities in the field of biomarkers for AKI and explore the hotspots and frontiers of research in this field.
2. Materials and methods
2.1. Data collection
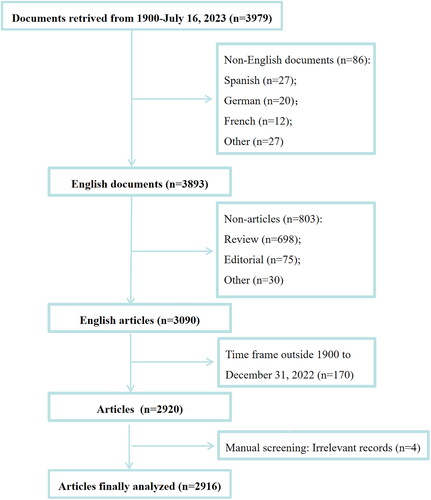
The Science Citation Index Expanded of the Web of Science Core Collection (Clarivate Analytics) was selected as the source database for document retrieval owing to its suitability and comprehensiveness [Citation11]. Similar to previous studies, the search strategy was (AK=(acute kidney injury) OR AK=(AKI) OR AK=(acute kidney failure) OR AK=(acute renal injury) OR AK=(acute renal failure)) AND (TS=(biomarker) OR TS=(biomarkers) OR TS=(marker) OR TS=(markers)), where AK refers to author keywords and TS refers to the topic [Citation12,Citation13]. The inclusion criteria encompass articles pertaining to AKI biomarkers. The exclusion criteria were non-English documents, documents type indexed other than article, and time frame outside database inception (the year 1900) to 31 December 2022. We also manually reviewed and screened the titles/abstracts of the retrieved publications to further exclude retractions and irrelevant records. The literature inclusion and exclusion process is presented in .
2.2. Data analysis
The included documents were downloaded and subsequently analyzed using common bibliometric analysis tools, such as CiteSpace 6.1.3, VOSviewer 1.6.18 (Leiden University Center for Science and Technology Studies), and the R podium Biblioshiny. Cooperation among countries/regions was analyzed using the Online Analysis Platform of Literature Metrology (http://bibliometric.com/). Cooperative relationships among institutions, authors, and journals were visually presented using VOSviewer. The timeline cluster view of the co-cited references was made using CiteSpace 6.1.3 software, and the trend topics map was generated using the Biblioshiny R package.
3. Results
3.1. Annual publication outputs
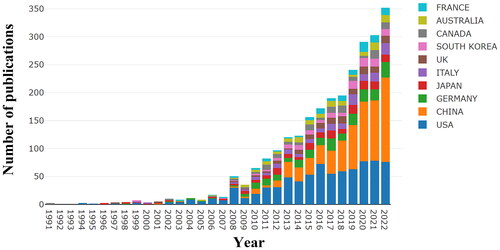
A total of 3979 documents related to AKI biomarkers were retrieved, of which 2916 were finally included and analyzed. As shown in , the annual publication output trend can be generally dichotomized into two phases according to the number of articles published. Phase I ranged between 1991 and 2007, during which the annual number of publications was very low (1-22 articles/year). In comparison, Phase II is the period between 2008 and 2022, which saw rapid and explosive growth, suggesting that this field has received extensive attention from researchers since 2008.
3.2. Analysis of contributions from countries and regions
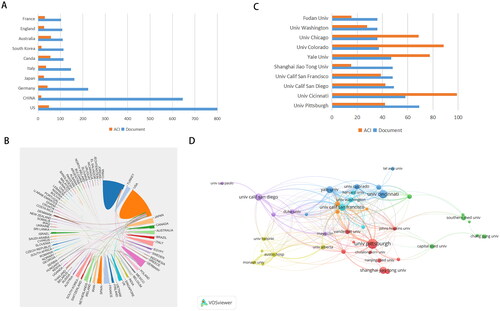
Overall, these 2916 publications were contributed by 83 countries or regions, of which the United States (n = 800), China (n = 646), Germany (n = 224), Japan (n = 162), and Italy (n = 147) were the top five countries with the largest number of publications (). Interestingly, Australia had the highest average number of citations (57.31), followed by Canada (54.90) and the United States (48.36). Despite the increasing proportion of publications contributed by Chinese scholars, China’s average number of citations is merely 14.17, suggesting that the impact of studies from China is still relatively low (, and Citation3A). Research collaboration on this subject, as shown in , has been concentrated in the United States, China, and Italy.
Figure 3. A: The top 10 countries with the most publications related to biomarkers of acute kidney injury. B: Network diagrams of cooperative relationships between countries. The thickness of the line is positively proportional to the frequency of cooperation. C: the top 10 most productive institutions on biomarkers of acute kidney injury. D: Network visualization map created by VOSviewer showing the most active institutions on research of AKI biomarkers. ACI: average citations per item.
3.3. Analysis of influential institutions
Scholars from 3379 institutions contributed, of which 133 institutions contributed ≥10 papers and 42 institutions contributed ≥20. Eight out of the top ten institutions with the highest productivity in terms of publications and nine out of the top ten in terms of total citations were American institutions (). The institutional collaboration network generated according to scientific outputs by VOSviewer is presented in . The University of California in San Francisco, the University of California in San Diego, and the University of Pittsburgh were the top three institutions in terms of total link strength.
3.4. Author contributions and collaborations
A total of 16368 authors contributed to this field, of which 60 authors contributed ≥10 papers, and 13 authors contributed ≥20. Prasad Devarajan is the author with the most publications (n = 57), followed by John A. Kellum, Chirag Rohit Parikh, and Claudio Ronco. The top ten most productive authors in the field of AKI biomarkers are listed in . , which is the network visualization map for author co-authorship, shows that Chirag Rohit Parikh, John A. Kellum and Prasad Devarajan had the largest collaborations.
Figure 4. Network visualization maps of most productive authors (A) and most cited authors (B) in the field of biomarkers of acute kidney injury.
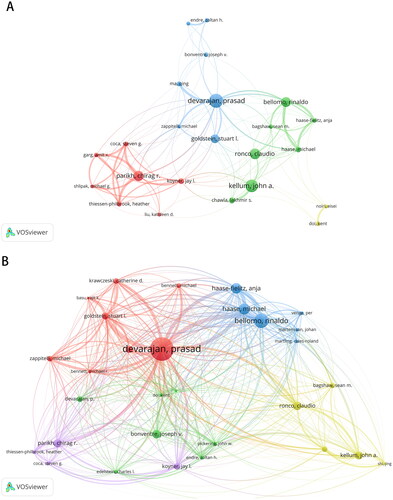
Table 1. Top 10 most productive authors in the field of biomarkers of acute kidney injury.
Citation analysis indicated that 1026 authors have been cited over 100 times, 431 over 200 times, and 93 over 500 times. As shown in , the top three authors with the largest citations were Prasad Devarajan, Rinaldo Bellomo, and Hasse Michael, which suggests their wide reputation and influence in the field of AKI biomarker research.
3.5. Journals
The results indicated that the literature was published in 712 academic journals, of which 60 journals published ≥ 10 documents and 31 journals published ≥20 papers. Journal overlay visualization map () showed that most studies were published in the American Journal of Physiology-Renal Physiology (n = 88, 3.02%), followed by Kidney International (n = 85, 2.91%), BMC Nephrology (n = 84, 2.88%), Renal Failure (n = 78, 2.67%), and Nephrology Dialysis Transplantation (n = 69, 2.37%).
Figure 5. Overlay visualization map showing the journals with most number of publications related to AKI biomarkers (A) and journals most frequently cited (B).
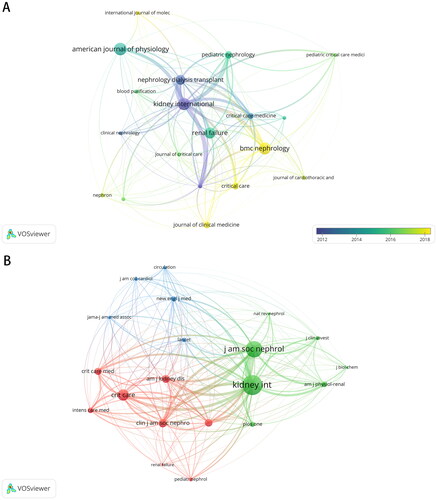
Journal citation analysis showed that Kidney International, Journal of the American Society of Nephrology, and Critical Care were the most frequently cited journals and representative centers of collaboration in the co-cited journal map (). The majority of these journals can be grouped into nephrology journals, critical care journals, and leading multi-disciplinary journals.
3.6. Cited articles and co-cited references
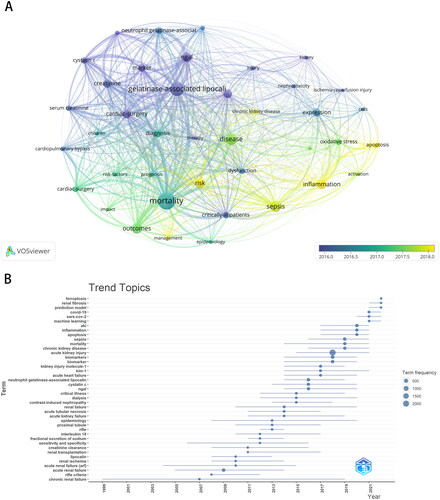
In total, 330 articles were cited ≥ 50 times, and 123 articles were cited ≥ 100 times. and list the top 10 most-cited articles for Phase I and Phase II, respectively. The highest and lowest number of citations of an article were 1275 and 265, respectively. The article with the highest number of citations for Phase I is about the identification of kidney injury molecule-1 as a biomarker for acute tubular injury, while it is about neutrophil gelatinase-associated lipocalin for AKI diagnosis and prognostication in Phase II. Co-citation analysis showed that 57832 references were cited in this field’s publications, with an average of 19.8 citations per article. The top ten co-cited references for Phase I and Phase II are listed in and Citation5, respectively. The co-cited reference timeline map () showed that neutrophil gelatinase-associated lipocalin, cell cycle, and tubular damage were the primary research focus, whereas current research hotspots are shifting toward sepsis and COVID-19.
Figure 6. Timeline view of co-citation references. The density of nodes at different time periods can reflect the dynamic changes of the corresponding clusters on the time axis.
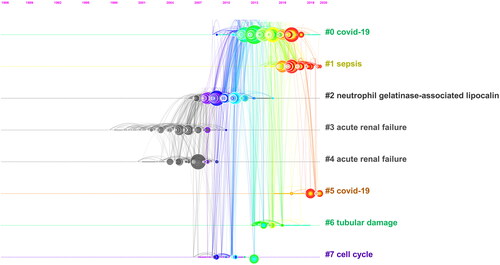
Table 2. Top 10 most cited articles in the field of biomarkers of acute kidney injury between 1991 and 2007.
Table 3. Top 10 most cited articles in the field of biomarkers of acute kidney injury between 2008 and 2022.
Table 4. Top 10 most co-cited references in the field of biomarkers of acute kidney injury between 1991 and 2007.
Table 5. Top 10 most co-cited references in the field of biomarkers of acute kidney injury between 2008 and 2022.
3.7. Keywords, hotspots and trend topics
A total of 8114 keywords were extracted from these 2916 publications using VOSviewer. After excluding keywords such as acute kidney injury, biomarkers/biomarkers, acute renal failure, and AKI, the overlay visualization map () with different colors applied for each keyword according to the average appearing year in articles showed that keywords such as neutrophil gelatinase-associated lipocalin (n = 205), sepsis (n = 220), cardiac surgery (n = 178), cystatin C (n = 151), and inflammation (n = 142) were the most frequent, whereas keywords such as apoptosis, inflammation, and chronic kidney disease have emerged recently. As shown in which depicts the chronological progression and evolution of specific research themes, current research in the field of AKI biomarkers is centered on ferroptosis, renal fibrosis, prediction models, COVID-19, and machine learning.
4. Discussion
In the current study, we performed a bibliometric analysis to summarize the current research status and future trends in the field of AKI biomarkers. We observed that the number of publications exploded after 2008, which might be attributed to the fact that the Acute Kidney Injury network classification, a modified version of the initial Risk, Injury, Failure, Loss, ESRD criteria, was released in 2007 [Citation14]. Subsequently, following the publication of the KDIGO criteria for AKI in 2012, the field of AKI biomarkers continues to grow exponentially, suggesting that these guidelines and expert consensus may propel clinical trials by establishing diagnostic criteria.
The US is unequivocally the leader in the field of AKI biomarkers in terms of annual publication output, cooperation with other countries, and the number of leading institutions. Notably, although China ranked 2nd in terms of publication volume, the average number of citations per document is very low. Furthermore, only two of the top ten most productive institutions were located in China. These results indicate that China should focus on improving the quality of its articles and global impacts.
Author analysis indicated that the top three most productive authors and the three most cited authors were American scholars, reinforcing the notion of American leadership in the field of AKI biomarkers. Journal and journal co-citation analyses showed that most of these studies were published in nephrology and critical care journals. Therefore, researchers could focus on these authors and journals to keep up with the latest developments in this field.
A comparison of the top 10 most-cited articles in Phase I and Phase II indicates a shift in hot spots. For example, in Phase I, widely investigated AKI biomarkers were neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and cystatin C [Citation15–21]. In contrast, novel biomarkers such as Klotho, liver fatty acid-binding protein, cell-cycle arrest markers, and exosomes/microvesicles are emerging gradually in Phase II [Citation22–25]. This highlights that the existing biomarkers for AKI are currently limited and do not fully meet clinical requirements. Thus, novel biomarkers that could assist in early identifying AKI and predicting patient outcomes require exploration and rigorous testing.
The timeline view of co-citation references revealed that citations between 2010 and 2018 were related to cell cycle markers and tubular damage. In fact, G1 cell cycle arrest markers such as tissue inhibitors of metalloproteinases and insulin-like growth factor binding protein 7 have been implicated during the early stages of cellular stress and are thus predictive of imminent AKI [Citation26,Citation27]. It is being increasingly known that the tubular cells undergo cell cycle arrest as a protective measure during AKI. However, sustained cell cycle arrest of kidney tubular cells leads to maladaptive repair and eventually fibrosis, thereby increasing the risk of chronic kidney disease [Citation28]. A paradigm shift was then observed, in which the hotspots of AKI biomarkers have shifted toward COVID-19, which coincides with the outbreak of this viral condition that frequently causes AKI in critical patients [Citation29]. This hotspot transition is compatible with the release of the 25th Acute Disease Quality Initiative Workgroup consensus report that laid down the diagnosis, management, prognostication and future research gaps for COVID-19-associated AKI [Citation30].
Apoptosis, inflammation, and chronic kidney disease have recently emerged as keywords in the field of AKI biomarkers. Apoptosis is a form of programmed cell death in addition to necroptosis, autophagy, and ferroptosis. Excessive apoptosis has been demonstrated to be a cardinal feature of renal tubular cells in the presence of an acute inflammatory milieu and contributes to the development of chronic kidney disease by tubular atrophy and the progression of interstitial fibrosis [Citation31]. The trend topic map showed that frontiers of AKI biomarkers were concentrated on ferroptosis, renal fibrosis, COVID-19, and machine-learning-based prediction models. Congruently, a recent bibliometric analysis of ferroptosis in AKI showed that the study of ferroptosis in AKI is still in its infancy, and the crosstalk between ferroptosis and other types of programmed cell death would be a hotspot for future research [Citation32]. Moreover, ferroptosis has also been implicated in the transition from AKI to chronic kidney disease, and regulating ferroptosis would be a promising strategy to halt this transition [Citation33]. In addition, with the COVID-19 pandemic, AKI has become a common clinical conundrum, especially in critically ill patients. Recently, there has been a growing interest in applying machine learning and other artificial intelligence algorithms for early detection, risk stratification, and outcome assessment in patients with AKI [Citation34,Citation35]. Notably, the Acute Disease Quality Initiative Workgroup in 2015 recognized AKI as an ideal disease entity to apply artificial intelligence due to its standardized definitions and major burden on healthcare systems [Citation36]. Thereafter, there are growing studies that explore machine learning or other artificial intelligence techniques to derive composite biomarkers for the early diagnosis and prognostication of AKI [Citation37,Citation38]. However, before the AKI biomarkers identified can be successfully translated into clinical practice, extensive validations are required.
5. Limitations
Although bibliometric analysis is superior to traditional narrative reviews in terms of providing better insight into the research focus and future trends, it has several inherent limitations. First, only the Science Citation Index Expanded of the Web of Science Core Collection was used as the database for bibliometric analysis. Incorporating other databases, such as PubMed and Scopus, would enhance literature coverage and improve study accuracy. Second, only articles published in English were included, increasing potential selection bias that prominent articles published in other languages may be overlooked. Thus, American leadership in AKI biomarker research may be influenced by the language selection bias present in this article. Third, bibliometric studies focus only on quantitative metrics regardless of study quality. Fourth, the number of citations may not accurately reflect study quality as highly cited articles could simply be widely known rather than of high quality. Lastly, tools for bibliometric analysis, including CiteSpace, VOSviewer and Biblioshiny, all have inherent limitations and biases that can potentially influence analytic results.
6. Conclusions
In conclusion, the current bibliometric analysis comprehensively investigated the current research status and guided future research trends in the field of AKI biomarkers. This area is gaining momentum and an increasing number of publications are expected. Future directions in AKI biomarker research will aim to investigate ferroptosis and AKI transition to chronic kidney disease. Additionally, artificial intelligence, such as machine learning, will be explored to derive more sensitive and specific composite biomarkers for AKI.
Authors contributions
Peifeng Xu: Conceived and designed the experiments;
Fan Fan: Analyzed and interpreted the data; Wrote the paper.
Acknowledgement
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are available upon reasonable request to the corresponding author.
Additional information
Funding
References
- Hoste EAJ, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):1–11. doi: 10.1038/s41581-018-0052-0.
- Brown JR, Rezaee ME, Marshall EJ, et al. Hospital mortality in the United States following acute kidney injury. Biomed Res Int. 2016;2016:4278579–4278576. doi: 10.1155/2016/4278579.
- Heung M, Steffick DE, Zivin K, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis. 2016;67(5):742–752. doi: 10.1053/j.ajkd.2015.10.019.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789.
- Wen Y, Parikh CR. Current concepts and advances in biomarkers of acute kidney injury. Crit Rev Clin Lab Sci. 2021;58(5):354–368. doi: 10.1080/10408363.2021.1879000.
- Aykaç S, Eliaçık S. What are the trends in the treatment of multiple sclerosis in recent studies? - a bibliometric analysis with global productivity during 1980–2021. Mult Scler Relat Disord. 2022;68:104185. doi: 10.1016/j.msard.2022.104185.
- Li C, Li M, Ji P. Research trends and hotspots on cardiac surgery-associated acute kidney injury: a bibliometric analysis. Asian J Surg. 2023;46(10):4500–4502. doi: 10.1016/j.asjsur.2023.04.129.
- Li J, Gong X. Bibliometric and visualization analysis of kidney repair associated with acute kidney injury from 2002 to 2022. Front Pharmacol. 2023;14:1101036. doi: 10.3389/fphar.2023.1101036.
- Yu X, Wu R, Ji Y, et al. Bibliometric and visual analysis of machine learning-based research in acute kidney injury worldwide. Front Public Health. 2023;11:1136939. doi: 10.3389/fpubh.2023.1136939.
- Wang H, Gao T, Zhang R, et al. The intellectual base and global trends in contrast-induced acute kidney injury: a bibliometric analysis. Ren Fail. 2023;45(1):2188967. doi: 10.1080/0886022X.2023.2188967.
- Wu H, Cheng K, Li C. Ferroptosis, necroptosis, pyroptosis, and cuproptosis in cancer: a comparative bibliometric analysis. Cell Death Discov. 2023;9(1):238. doi: 10.1038/s41420-023-01542-7.
- Liu YH, Wang SQ, Xue JH, et al. Hundred top-cited articles focusing on acute kidney injury: a bibliometric analysis. BMJ Open. 2016;6(7):e011630. doi: 10.1136/bmjopen-2016-011630.
- Wan Y, Shen J, Hong Y, et al. Mapping knowledge landscapes and emerging trends of the biomarkers in melanoma: a bibliometric analysis from 2004 to 2022. Front Oncol. 2023;13:1181164. doi: 10.3389/fonc.2023.1181164.
- Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713.
- Mishra J, Mori K, Ma Q, et al. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24(3):307–315. doi: 10.1159/000078452.
- Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22(12):2089–2095. doi: 10.1007/s00467-007-0601-4.
- Han WK, Bailly V, Abichandani R, et al. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x.
- Ichimura T, Hung CC, Yang SA, et al. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286(3):F552–F563. doi: 10.1152/ajprenal.00285.2002.
- Vaidya VS, Ramirez V, Ichimura T, et al. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290(2):F517–F529. doi: 10.1152/ajprenal.00291.2005.
- Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66(3):1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x.
- Koyner JL, Bennett MR, Worcester EM, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74(8):1059–1069. doi: 10.1038/ki.2008.341.
- Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78(12):1240–1251. doi: 10.1038/ki.2010.328.
- Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73(4):465–472. doi: 10.1038/sj.ki.5002721.
- Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189(8):932–939. doi: 10.1164/rccm.201401-0077OC.
- Miranda KC, Bond DT, McKee M, et al. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010;78(2):191–199. doi: 10.1038/ki.2010.106.
- Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLOS One. 2014;9(3):e93460. doi: 10.1371/journal.pone.0093460.
- Koyner JL, Shaw AD, Chawla LS, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)⋅IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol. 2015;26(7):1747–1754. doi: 10.1681/ASN.2014060556.
- Canaud G, Bonventre JV. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant. 2015;30(4):575–583. doi: 10.1093/ndt/gfu230.
- Chen YT, Shao SC, Hsu CK, et al. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24(1):346. doi: 10.1186/s13054-020-03009-y.
- Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat Rev Nephrol. 2020;16(12):747–764. doi: 10.1038/s41581-020-00356-5.
- Li Z, Liu Z, Luo M, et al. The pathological role of damaged organelles in renal tubular epithelial cells in the progression of acute kidney injury. Cell Death Discov. 2022;8(1):239. doi: 10.1038/s41420-022-01034-0.
- Liu C, Zhou W, Mao Z, et al. Bibliometric analysis of ferroptosis in acute kidney injury from 2014 to 2022. Int Urol Nephrol. 2023;55(6):1509–1521. doi: 10.1007/s11255-022-03456-2.
- Fiorentino M, Grandaliano G, Gesualdo L, et al. Acute kidney injury to chronic kidney disease transition. Contrib Nephrol. 2018;193:45–54. doi: 10.1159/000484962.
- Le S, Allen A, Calvert J, et al. Convolutional neural network model for intensive care unit acute kidney injury prediction. Kidney Int Rep. 2021;6(5):1289–1298. doi: 10.1016/j.ekir.2021.02.031.
- Churpek MM, Carey KA, Edelson DP, et al. Internal and external validation of a machine learning risk score for acute kidney injury. JAMA Netw Open. 2020;3(8):e2012892. doi: 10.1001/jamanetworkopen.2020.12892.
- Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi: 10.1038/nrneph.2017.2.
- Ibrahim NE, McCarthy CP, Shrestha S, et al. A clinical, proteomics, and artificial intelligence-driven model to predict acute kidney injury in patients undergoing coronary angiography. Clin Cardiol. 2019;42(2):292–298. doi: 10.1002/clc.23143.
- Van Biesen W, Vanmassenhove J, Decruyenaere J. Prediction of acute kidney injury using artificial intelligence: are we there yet? Nephrol Dial Transplant. 2020;35(2):204–205. doi: 10.1093/ndt/gfz226.