Abstract
Purpose
Since previous studies have shown a paradoxical relationship between acute kidney injury (AKI) and risk of cognitive impairment, there is an urgent need for a meta-analysis to assess the relationship between AKI and risk of cognitive impairment or dementia.
Materials and methods
From database inception to October 2023, we searched PubMed, OVID (Medline), Embase, Web of Science, and Cochrane Library. This study examined AKI and cognitive impairment or dementia observational studies. Two authors independently assessed cohort and cross-sectional study quality using the Newcastle–Ottawa Scale and AHRQ Scale. They also used ROBINS-I to assess bias. The meta-analysis used fixed effects. Sensitivity analysis verified results stability. The funnel plot, Egger test, and Begg test determined publication bias in the results.
Results
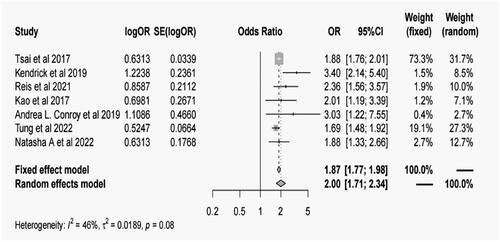
Seven studies with 423,876 patients were included in the meta-analysis. Patients with AKI were at higher risk of cognitive impairment or dementia compared to those who had not experienced AKI (OR = 1.87, 95% confidence interval [CI]: 1.77–1.98, I2=46.0%, p = 0.08). All subgroups showed a substantial connection between AKI and cognitive impairment. Compared to domestic research, the connection was stronger in overseas studies (OR = 2.18, 95% CI: 1.66–2.87). Both cognitive impairment and dementia outcomes showed a substantial connection between AKI and cognitive impairment, with OR values of 2.00 (95% CI: 1.44–2.76) and 2.04 (95% CI: 1.66–2.51).
Conclusions
We found that AKI significantly increases cognitive impairment or dementia risk. Thus, early interventions to delay cognitive impairment and prevent adverse outcomes in AKI patients are needed.
1. Introduction
Acute kidney injury (AKI) is a clinical syndrome characterized by a sudden decline in the glomerular filtration rate over a short period of time [Citation1], which is associated with an increased risk of mortality, diminished quality of life, and adverse health and economic effects [Citation2]. The prevalence of AKI reportedly ranges from 1% to 66% [Citation3], and approximately 1.7 million individuals die from AKI each year [Citation4]. In addition, there is emerging evidence that patients with AKI are at a higher risk of developing chronic kidney disease, cardiovascular diseases, and acute neurological complications such as delirium, attention deficits, and impaired visuomotor and executive tasks [Citation5–9].
Cognitive impairment refers to some degree of difficulty with cognitive functioning that occurs in a multitude of diseases and is defined as deficient thought processes that lead to memory loss and difficulties in learning, concentration, and making decisions [Citation10]. Over the last few decades, the global prevalence of cognitive impairment has ranged from 5.1% to 41%, while the incidence has ranged from 22 to 76.8 per 1000 person-year [Citation11], cognitive impairment was associated with an ∼2-fold risk of all-cause mortality [Citation12]. Thus, there is an urgent need to identify the determinants of cognitive impairment. Both AKI and cognitive impairment are important public health issues, and the occurrence of cognitive impairment in patients with AKI contributes to a high economic and humanistic burden [Citation13]. Therefore, determining the effects of AKI on cognitive function is critical.
Several studies have investigated the association of AKI with incident cognitive impairment and found that AKI is a risk factor for long-term neurocognitive impairment [Citation14–16]. The potential mechanism could be associated with the development of cognitive impairment through inflammation, which is characterized by the release of proinflammatory cytokines and chemokines [Citation17]. However, a recent retrospective cohort study indicated that there were no significant differences in cognitive function or emotional health between patients with AKI [Citation18]. Given the contradictory relationship between AKI and risk of cognitive impairment in previous studies, there is an urgent need to conduct a meta-analysis to assess the association between AKI and risk of cognitive impairment. Although a previous meta-analysis had demonstrated that AKI patients have a higher risk of dementia than those without AKI [Citation13], its reliability was limited as only four studies were included in the pooled analysis and it did not assess the effects of AKI on other categories of cognitive impairment (except dementia). Therefore, the present systematic review and meta-analysis aimed to provide information on the association between AKI and cognitive impairment according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2. Materials and methods
This systematic review and meta-analysis were performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 guidelines [Citation19]. The study protocol was registered in the International Prospective Register of Systematic Reviews Database (PROSPERO) under the registration number CRD42023471256.
2.1. Search strategy
We retrieved potentially eligible studies on the association of AKI with the risk of cognitive impairment from five public electronic databases, including PubMed, Embase, Medline (Ovid), Web of Science databases, and Cochrane library. The search period was up to October 2023. The search strategy was customized for each database, and a combination of medical subject headings and free words was utilized to search for articles related to AKI and cognitive impairment. The detailed search strategy can be found in Supplementary search strategy. In addition, grey literature was manually searched through Google Scholar, as well as references cited in review articles and conference papers.
2.2. Study selection
Two authors (Jiang Wang and Xiao Xu) independently screened studies according to the predefined inclusion and exclusion criteria. The inclusion criteria were as follows: (1) Prospective or retrospective studies or case–control studies or cross-sectional study; (2) studies investigating the association between AKI and cognitive impairment risk; and (3) age of patients ≥18 years. The exclusion criteria were as follows: (1) case report, review, conference, or abstract; (2) studies with insufficient data; (3) studies without available full text; (4) non-English literature; and (5) Study with acute kidney injury with cognitive impairment as the initial cohort population. In case of disagreement between the two authors (Xiao Xu and Chunyan Wang), the issue was discussed until a consensus was reached by including a third author (Jiang Wang).
2.3. Data extraction and quality assessment
Two researchers (Dongmei Ye and Ruzhao Chen) independently extracted the data, including the first author, publication year, study design, country, male proportion, age, actual sample size, follow-up time, assessment of AKI, assessment of cognitive impairment, and cognitive impairment category and Covariate adjustment degree. In addition, independent reviewers Wang Chunyan and Xu Xiao used the Newcastle–Ottawa Scale (NOS) to assess the methodological quality of the included cohort studies and case–control studies. The NOS scale consists of three main domains: selection, comparability, and outcome. The maximum score of this scale is 9, and a score of ≥7 is considered as high-quality research [Citation20]. Furthermore, we evaluated the included cross-sectional studies using 11 assessment items recommended by the Agency for Healthcare Research and Quality (AHRQ). We quantified the system by assigning a score of ‘1’ if the answer was ‘yes’ and a score of ‘0’ if the answer was ‘no’ or ‘unclear’. The total score is 11, and a quality assessment score of ≥8 is considered as high-quality research [Citation21]. On this basis, we further assessed the potential risk of bias in the selected non-randomized studies using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool [Citation22]. The ROBINS-I tool consists of seven domains that help evaluate bias caused by confounding, selection of participants, exposure assessment, misclassification during follow-up, missing data, outcome assessment, and selective reporting. Reviewers systematically assess the risk associated with each domain and assign one of the following ratings: low, moderate, serious, critical, or no information. Table S3 provides a comprehensive overview of the detailed descriptions and decision parameters for each domain of the ROBINS-I tool. This ensures the transparency and replicability of the bias assessment process, supporting the robustness of our research methodology. In cases of disagreement between the two authors, a third senior researcher (Jiang Wang) discussed and arbitrated the issue until a consensus was reached.
2.4. Statistical analysis
This meta-analysis was performed using Stata Version 17.0 (Stata Corp) and R 4.2.1. The odds ratio (OR) for the association between AKI and risk of cognitive impairment in each study were used for the statistical analysis. Cochran’s Q test and I2 statistics were used to test the heterogeneity of the studies. If heterogeneity was found to be significant (p < 0.05, I2 ≥ 50%) [Citation23], the random-effects model was used. Otherwise, the fixed-effects model was used. To explore the source of heterogeneity, we conducted a subgroup analysis and meta-regression according to the study region and design, assessment of AKI, assessment of cognitive impairment, and cognitive impairment category. In addition, sensitivity analysis was performed to assess the stability of the pooled results by removing each study in turn. The funnel plot and quantitative analysis (Begg test and Egger test) were used to determine whether there was publication bias in the meta-analysis.
3. Results
3.1. Study screening
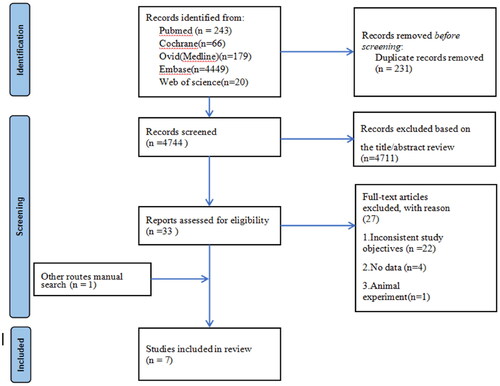
A systematic search of the five public electronic databases yielded 4,975 potential studies. After removing the duplicates, 4,744 studies remained. After screening the title and abstract, 33 studies were selected for a full-text review. According to the predefined inclusion and exclusion criteria, 6 studies were eligible for the analysis. We also obtained one extra original study by conducting a manual search. Finally, a total of 7 studies were included in the meta-analysis. The specific screening process is shown in a flow chart ().
3.2. Characteristics of the included studies
summarizes the basic characteristics of the included studies. Seven studies with 423,876 patients were included in the meta-analysis, including two retrospective cohort studies with 417,658 patients [Citation15,Citation16], two prospective cohort studies with 2,119 patients [Citation14,Citation24], and two case-control studies with 3837 patients [Citation4,Citation25]. In addition, there was one study from Portugal, three studies from the United States, and two studies from China. The mean age of the patients in the included studies ranged from 49.8 to 76.4 years.
Table 1. Baseline characteristics of included studies.
3.3. Methodological quality
The NOS scoring system consists of three ranges: 0–3, 4–6, and 7–9, corresponding to low, moderate, and high quality, respectively. In all six studies, a high-quality rating was obtained (Table S1). Table S2 provides a comprehensive description of the systematic quality assessment using the AHRQ evaluation system for the included cross-sectional study. The cross-sectional study received a score of 7, classified as low quality. Table S3 presents the results of the further risk assessment using the ROBINS-I tool for all studies. Among all the studies, two showed low bias, four showed moderate bias, and one showed high bias.
3.4. Association between AKI and cognitive impairment
Patients with AKI were at a higher risk of cognitive impairment compared to those who had not experienced AKI (OR = 1.87, 95% CI: 1.77–1.98, p < 0.001; ), Combined data show homogeneity results (I2=46.0%, p = 0.08). Subgroup analysis (, Supplementary search strategy) and meta-regression () were further conducted according to the study region, study design, Age, definition of AKI, assessment of cognitive impairment, cognitive impairment category and covariate adjustment degree. AKI was significantly associated with the risk of cognitive impairment across all sub-groups. Subgroup analysis based on different geographical regions revealed that studies conducted Foreign (OR = 2.18, 95% CI: 1.66–2.87, p < 0.001) showed a significantly higher association between AKI and cognitive impairment or dementia compared to studies conducted Native (OR = 1.88, 95% CI: 1.76–2.01, p < 0.001). Subgroup analysis based on study design demonstrated a combined OR of 2.14 (95% CI: 1.55–2.96, p < 0.001) for cohort studies, 1.88 (95% CI: 1.33–2.66, p < 0.001) for cross-sectional studies, and 2.22 (95% CI: 1.60–3.07, p < 0.001) for case–control studies. Further subgroup analysis based on age groups showed a combined OR of 1.85 (95% CI: 1.75–1.96, p < 0.001) for individuals aged ≥65 years and 2.74 (95% CI: 1.98–3.79, p < 0.001) for those aged <65 years. Regarding the definition of AKI, subgroup analysis revealed a combined OR of 2.13 (95% CI: 1.65–2.75, p < 0.001) when using the KDIGO criteria, 2.07 (95% CI: 1.46–2.94, p < 0.001) when using the ICD criteria, and 2.01 (95% CI: 1.19–3.39, p = 0.009) when using the ADQI criteria. The combined OR for cognitive impairment, assessed using the ICD criteria, was 2.23 (95% CI: 1.72–2.88, p < 0.001). Subgroup analysis based on different outcomes revealed a combined OR of 2.00 (95% CI: 1.44–2.76, p < 0.001) for cognitive impairment and 2.04 (95% CI: 1.66–2.51, p < 0.001) for dementia. Additionally, subgroup analysis based on adjustment for confounding factors demonstrated a combined OR of 2.07 (95% CI: 1.62–2.64, p < 0.001) for studies that performed adjustments and 2.06 (95% CI: 1.58–2.69, p < 0.001) for studies that did not perform adjustments.
Table 2. The subgroup analysis for the association of AKI and cognitive impairment.
Table 3. Univariate meta-regression of correlation between AKI and cognitive impairment.
3.5. Publication bias and sensitivity analysis
Funnel plot of publication bias showed that the study results were publication bias (Figure S8), and further Egger test (p = 0.0014) and Begg test (p = 0.0327) determined that our study results were publication bias. In addition, we performed a sensitivity analysis to assess the robustness of the combined AKI and cognitive impairment or dementia risk results by removing one study and combining the remaining studies. Sensitivity analyses showed no disruptive changes in the combined results, indicating that the combined results of the study were robust (Figure S9).
4. Discussion
4.1. Summary of main results
In this meta-analysis of seven studies with the aggregate data of 423,876 patients from different regions, we found that AKI was significantly associated with an approximately 2-fold increased risk of cognitive impairment, which provides evidence that more attention should be paid to early interventions for delaying cognitive impairment and preventing adverse outcomes in patients with AKI.
4.2. Comparison between this and other meta-analyses
The results of the current study were consistent with the results of previous studies on the association between AKI and risk of cognitive impairment. In a study of 1041 patients primarily with AKI who were matched with controls, more AKI patients developed dementia than controls (7.0% vs. 2.3%), and AKI was associated with a 3.40-fold higher risk of developing cognitive impairment [Citation15]. A recent study with 854 patients from a community-based cohort and matched controls demonstrated that participants who experienced hospitalization for AKI had a 1.69-fold increased risk of dementia based on a time-varying analysis [Citation24]. Another study that evaluated 2905 acute dialysis patients who had a full recovery revealed that those who had AKI had a 2.01-fold risk of developing dementia [Citation25].
Although the mechanisms for the link between AKI and cognitive impairment are not yet well understood, the association may be partly explained as follows. First, AKI can contribute to blood-brain barrier disruption through the increased generation of proinflammatory cytokines and reactive oxygen species, leading to an imbalance of neurotransmitters with increased neuronal activating proteins and brain transporters [Citation26–29]. Neurotransmitters can affect memory, arousal, attention, and mood [Citation30]; thus, the imbalance between inhibitory and excitatory neurotransmitters in AKI patients can further impact the cognitive function [Citation31]. S, various dysfunctions of the hippocampus and other brain regions may occur in AKI patients due to a change in the permeability of the blood-brain barrier and systemic inflammation. The hippocampus is mainly responsible for learning and memory; therefore, hippocampal dysfunction can lead to cognitive impairment in AKI patients [Citation32,Citation33]. Third, AKI patients have an increased risk of progression to CKD (chronic kidney disease) and ESKD (end-stage kidney disease) [Citation34,Citation35]. CKD is widely regarded as an important predictor of the development of cognitive impairment due to accumulation of uremic toxins [Citation36,Citation37]; thus, CKD following AKI might be partly responsible for the subsequent risk of cognitive impairment in AKI patients [Citation13]. Furthermore, AKI patients have a higher rate of co-morbidity (e.g. diabetes, hypertension, depression, chronic obstructive pulmonary disease, coronary artery disease, congestive heart failure, atrial fibrillation, malnutrition, and inflammation), which may also be associated with an increased risk of cognitive impairment.
4.3. Strengths and limitations of the study
The strengths of this study are: First, this is the current study on the association between AKI and risk of cognitive impairment the largest and most comprehensive assessment of this neighborhood association to date. The broad inclusion of a large number of patients, while the individuals included are likely to reflect individuals with AKI observed in routine clinical practice accurately, allows a strong assessment of the association between AKI and cognitive impairment. The second aspect lies in the homogeneity of the combined results of the included studies, which greatly increases the reliability and interpretability of our findings, while providing a more reliable basis for clinical decision-making. Third, the studies included in the meta-analysis presented overall high quality according to the quality evaluation, which verifies that our study conclusions are reliable. However, it is important to recognize the limitations of the methodology of this study. It mainly includes: First, due to the observational design of the included studies, our meta-analysis could not establish a causal relationship between acute kidney injury (AKI) and risk of cognitive impairment. Second, the included studies covered only English-language publications such as the United States, China, Turkey, and Canada, potentially weakening our ability to assess the association of malnutrition with AKI risk in other countries and potentially introducing language bias. Future studies are therefore warranted to conduct more well-designed cohort studies in populations of different ethnic or geographic regions to determine whether this relationship increases the risk of cognitive impairment in AKI patients elsewhere. Third, the follow-up time of the original study in AKI patients may have been insufficient to capture long-term outcomes, limiting the comprehensiveness of our results. Fourth, different definitions of AKI were used in the included studies, which may lead to variability in the results. Fifth, Egger test and Begg test determined that our study results were subject to publication bias. Due to the limited number of studies, the results of publication bias were not interpreted accurately enough. Finally, regarding the mechanisms of association between AKI and cognitive impairment as well as potential mediators, at present, more experimental studies and their clinical studies are needed to further investigate to elucidate their pathway association more accurately.
5. Conclusion
The present meta-analysis demonstrated that AKI was significantly associated with an increased risk of cognitive impairment. Therefore, more attention should be paid to early interventions for delaying cognitive impairment and prevention of adverse outcomes in patients after AKI. However, further well-designed large-sample prospective cohort studies with ethnically or geographically diverse populations are needed to verify this association.
Author contributions
Lan Chen and Huping Gong conceived and designed this study. Jiang Wang and Xiao Xu performed the search, data analysis, examination, quality assessment and writing work. Chunyan Wang and Dongmei Ye participated in data extraction and article modification. Ruzhao Chen, Pai Peng, Huadong Huang,
Yuxiang Yan, Ying Chen, Shixuan Wang participated in literature screening, writing work, and quality assessment. All the authors have approved the final version of the manuscript.
Supplemental Material
Download PDF (980.7 KB)Supplemental Material
Download PDF (629.3 KB)Acknowledgments
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Wald R, Beaubien-Souligny W, Chanchlani R, et al. Delivering optimal renal replacement therapy to critically ill patients with acute kidney injury. Intensive Care Med. 2022;48(10):1–8. doi: 10.1007/s00134-022-06851-6.
- Vaara ST, Bhatraju PK, Stanski NL, et al. Subphenotypes in acute kidney injury: a narrative review. Crit Care. 2022;26(1):251. doi: 10.1186/s13054-022-04121-x.
- Hoste EAJ, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–625. doi: 10.1038/s41581-018-0052-0.
- Mehta RL, Cerdá J, Burdmann EA, et al. International society of nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987):2616–2643. doi: 10.1016/S0140-6736(15)60126-X.
- James MT, Ghali WA, Knudtson ML, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123(4):409–416. doi: 10.1161/CIRCULATIONAHA.110.970160.
- Nagasaki T, Maeda H, Taguchi K, et al. A bioinspired carbon monoxide delivery system prevents acute kidney injury and the progression to chronic kidney disease. Redox Biol. 2022;54:102371. doi: 10.1016/j.redox.2022.102371.
- Pang H, Kumar S, Ely EW, et al. Acute kidney injury-associated delirium: a review of clinical and pathophysiological mechanisms. Crit Care. 2022;26(1):258. doi: 10.1186/s13054-022-04131-9.
- Vanderlinden JA, Semrau JS, Silver SA, et al. Acute kidney injury is associated with subtle but quantifiable neurocognitive impairments. Nephrol Dial Transplant. 2022;37(2):285–297. doi: 10.1093/ndt/gfab161.
- Li Z, Weng M, Lin L, et al. Acute kidney injury in patients with idiopathic membranous nephropathy: influencing factors and prognosis. Ren Fail. 2023;45:2194451.
- Rost NS, Brodtmann A, Pase MP, et al. Post-stroke cognitive impairment and dementia. Circ Res. 2022;130(8):1252–1271. doi: 10.1161/CIRCRESAHA.122.319951.
- Pais R, Ruano L, P Carvalho O, et al. Global cognitive impairment prevalence and incidence in community dwelling older Adults-a systematic review. Geriatrics (Basel). 2020;5(4):84. doi: 10.3390/geriatrics5040084.
- Zhang H, Jie Y, Sun Y, et al. Association of cognitive impairment with mortality and readmission in patients with heart failure: a meta-analysis. Curr Probl Cardiol. 2022;47(12):101354. doi: 10.1016/j.cpcardiol.2022.101354.
- Hussain S, Singh A, Antony B, et al. Association of acute kidney injury with the risk of dementia: a meta-analysis. J Clin Med. 2021;10(19):4390. doi: 10.3390/jcm10194390.
- Conroy AL, Opoka RO, Bangirana P, et al. Acute kidney injury is associated with impaired cognition and chronic kidney disease in a prospective cohort of children with severe malaria. BMC Med. 2019;17(1):98. doi: 10.1186/s12916-019-1332-7.
- Kendrick J, Holmen J, Srinivas T, et al. Acute kidney injury is associated with an increased risk of dementia. Kidney Int Rep. 2019;4(10):1491–1493. doi: 10.1016/j.ekir.2019.07.012.
- Tsai HH, Yen RF, Lin CL, et al. Increased risk of dementia in patients hospitalized with acute kidney injury: a nationwide population-based cohort study. PLoS One. 2017;12(2):e0171671. doi: 10.1371/journal.pone.0171671.
- Ouma BJ, Ssenkusu JM, Shabani E, et al. Endothelial activation, acute kidney injury, and cognitive impairment in pediatric severe malaria. Crit Care Med. 2020;48(9):e734–e43. doi: 10.1097/CCM.0000000000004469.
- Mayer KP, Ortiz-Soriano VM, Kalantar A, et al. Acute kidney injury contributes to worse physical and quality of life outcomes in survivors of critical illness. BMC Nephrol. 2022;23(1):137. doi: 10.1186/s12882-022-02749-z.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z.
- Scanlon MC, Harris JM, 2nd, Levy F, et al. Evaluation of the agency for healthcare research and quality pediatric quality indicators. Pediatrics. 2008;121(6):e1723-31–e1731. doi: 10.1542/peds.2007-3247.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919.
- Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851–861. doi: 10.1016/S2468-1253(22)00165-0.
- Tung S, Kendrick J, Surapaneni A, et al. Association between acute kidney injury and dementia in the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis. 2022;80(4):495–501. doi: 10.1053/j.ajkd.2022.02.015.
- Kao CC, Wu CH, Lai CF, et al. Long-term risk of dementia following acute kidney injury: a population-based study. Ci Ji Yi Xue Za Zhi. 2017;29:201–207.
- Bernardo-Castro S, Sousa JA, Brás A, et al. Pathophysiology of Blood-Brain barrier permeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front Neurol. 2020;11:594672. doi: 10.3389/fneur.2020.594672.
- Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the at(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29(3):640–647. doi: 10.1038/jcbfm.2008.158.
- Malek M. Brain consequences of acute kidney injury: focusing on the hippocampus. Kidney Res Clin Pract. 2018;37(4):315–322. doi: 10.23876/j.krcp.18.0056.
- Sun Q, Zheng J, Zhang Y, et al. Altered spontaneous brain activities in maintenance hemodialysis patients with cognitive impairment and the construction of cognitive function prediction models. Ren Fail. 2023;45:2217276.
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68(1):1–14. doi: 10.1097/NEN.0b013e3181919a48.
- Nongnuch A, Panorchan K, Davenport A. Brain-kidney crosstalk. Crit Care. 2014;18(3):225. doi: 10.1186/cc13907.
- Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19(7):1360–1370. doi: 10.1681/ASN.2007080901.
- Zhao L, Cao X, Li L, et al. Acute kidney injury sensitizes the brain vasculature to ang II (angiotensin II) constriction via FGFBP1 (fibroblast growth factor binding protein 1). Hypertension. 2020;76(6):1924–1934. doi: 10.1161/HYPERTENSIONAHA.120.15582.
- See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160–172. doi: 10.1016/j.kint.2018.08.036.
- Guo D, Wang H, Lai X, et al. Development and validation of a nomogram for predicting acute kidney injury after orthotopic liver transplantation. Ren Fail. 2021;43(1):1588–1600. doi: 10.1080/0886022X.2021.2009863.
- Kelly DM, Pendlebury ST, Rothwell PM. Associations of chronic kidney disease with dementia before and after TIA and stroke: population-Based cohort study. Neurology. 2022;98(7):e711–e20. doi: 10.1212/WNL.0000000000013205.
- Singh-Manoux A, Oumarou-Ibrahim A, Machado-Fragua MD, et al. Association between kidney function and incidence of dementia: 10-year follow-up of the whitehall II cohort study. Age Ageing. 2022;51(1):1–7. doi: 10.1093/ageing/afab259.