Abstract
Background
Cardiovascular disease (CVD) is the leading cause of mortality in type 2 diabetes mellitus (T2DM) patients. Shrunken pore syndrome (SPS) is defined as eGFRcystatin C/eGFRcreatinine ratio <0.70 and predicts high CVD mortality. The Framingham Risk Score (FRS) is used to estimate an individual’s 10-year CVD risk. This study investigated the association between FRS and eGFRcystatin C/eGFRcreatinine ratio in T2DM patients.
Methods
Patients aged 18-80 years who were newly diagnosed with T2DM were included in this retrospective study. Ordinal logistic regression analysis was used to investigate the association between risk factors of T2DM and FRS. A Generalized Linear Model was used to calculate odds ratios (OR) and 95% confidence intervals (CI).
Results
There were 270 patients included in the study. Only 27 patients (10%) met the diagnostic criteria of SPS. Ordinal logistic regression analysis showed that SPS was not correlated with FRS risk (OR = 1.99, 95%CI = 0.94–4.23, p = 0.07), whereas eGFRcystatin C/eGFRcreatinine (OR = 0.86, 95%CI = 0.77–0.97, p = 0.01) showed a significant negative association with FRS risk. Compared with eGFRcystatin C/eGFRcreatinine>0.85, eGFRcystatin C/eGFRcreatinine≤0.85 increased FRS risk (OR = 1.95, 95%CI = 1.18–3.21, p < 0.01). After adjustment for confounding factors, increased eGFRcystatin C/eGFRcreatinine ratio was associated with decreased FRS risk when considered as a continuous variable (OR = 0.87, 95%CI = 0.77–0.99, p = 0.03). The FRS risk in patients with eGFRcystatin C/eGFRcreatinine≤0.85 is 1.86 times higher than that in patients with eGFRcystatin C/eGFRcreatinine>0.85 (OR = 1.86, 95%CI = 1.08–3.21, p = 0.03).
Conclusions
In the current study, no significant association between SPS and FRS was identified. However, lower eGFRcystatin C/eGFRcreatinine and eGFRcystatin C/eGFRcreatinine≤0.85 were associated with a significantly increased CVD risk in T2DM.
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) is increasing worldwide, and is projected to increase to 578 million by 2030 [Citation1]. T2DM is a tremendous global public health concern and financial burden. Cardiovascular disease (CVD) and chronic kidney disease (CKD) are the common comorbidities of T2DM. Approximately 32.2% of patients with T2DM sufferred from CVD, including myocardial infarction, heart failure, coronary heart disease, and atherosclerosis [Citation2]. CVD is the leading cause of mortality in patients with T2DM, accounting for 52% of all cases in the worldwide [Citation3]. CKD increases the risk of adverse clinical outcomes, particularly CVD.
The Framingham Risk Score (FRS) is a simplified and commonly used clinical algorithm for estimating an individual’s 10-year CVD risk. According to the National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP)-III guidelines, the FRS incorporates age, sex, smoking habits, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and systolic blood pressure (SBP) [Citation4].
Shrunken pore syndrome (SPS) is characterized by a difference in renal filtration between cystatin C and creatinine, leading to a decreased ratio of estimated glomerular filtration rates (eGFR) based upon cystatin C to eGFR based upon creatinine [Citation5]. Renal impairment measured by serum creatinine and cystatin C is associated with an increased risk of CVD morbidity and mortality [Citation6, Citation7]. Schöttker et al. found that the cystatin C-based CKD equation showed potentially better clinical utility for FRS than creatinine-based equations in diabetes [Citation8]. Recent studies have indicated that a lower eGFRcystatin C/eGFRcreatinine ratio is associated with increased mortality and poor prognosis in general population cohorts and several clinical settings [Citation9–13]. Whether SPS is associated with an increased risk of CVD in T2DM patients remains unclear. Based on the above, our study aimed to investigate the association between FRS and eGFRcystatin C/eGFRcreatinine ratio in patients with newly diagnosed T2DM.
Methods
Study design and population
This retrospective study was conducted in the Department of Endocrinology of the Third Affiliated Hospital of Soochow University. Patients aged between 18 and 80 years with newly diagnosed T2DM were admitted between March 2011 and December 2012. The exclusion criteria were as follows: (1) complicated with heart disease, malignancy, and severe infection; (2) missing data for cystatin C or creatinine; (3) missing data for calculation of FRS; and (4) presence of proteinuria or renal dysfunction to exclude patients with diabetic kidney disease.
Data collection
Demographic data, medical history, and laboratory examinations were obtained from the electronic medical records. SBP, diastolic blood pressure (DBP), hemoglobin, albumin, globulin, fasting plasma glucose (FPG), serum creatinine, blood urea nitrogen (BUN), cystatin C, uric acid, glycated hemoglobin (HbA1c), TC, triglyceride, HDL-C, low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (apo-A1), and apolipoprotein B (apo-B) were included. Body mass index (BMI) (kg/m2) was equal to body weight divided by the square of height. The serum cystatin C and creatinine levels were measured by a laboratory technician blinded to patients’ information. The latex-enhanced immunoturbidimetric method and the Cystatin C Kit (Meikang Biotech, China) were used to measure serum cystatin C. The serum creatinine levels were measured by enzymatic creatinine-2 reagents (Siemens Healthcare Diagnostics Inc., Germany) using the enzyme method. The eGFR was calculated from creatine and cystatin C levels using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [Citation14] (). SPS is a condition when eGFRcystatin C is less than or equal to 70% of eGFRcreatinine [Citation9]. Absolute 10-year CVD risk percentage was calculated according to the FRS scores. Individuals with FRS scores of <10% are considered to have a low 10-year risk of CVD, while those with FRS scores of 10% −20% and >20% are considered to have intermediate and high CVD risks, respectively [Citation4].
Table 1. Formulas for estimating glomerular filtration rate.
Statistical analyses
Normality tests of continuous variables were performed using the Kolmogorov–Smirnov test. Normally distributed variables presented as mean ± standard deviation (SD) were compared using an unpaired t-test, while non-normally distributed variables presented as median (interquartile range, IQR) were compared using the Mann–Whitney U-test. Categorical variables, presented as frequencies (percentages), were compared using Pearson’s chi-square test. The association between risk factors of T2DM and the FRS risk was to be analyzed by ordinal logistic regression if the proportional odds assumption was satisfied. A Generalized Linear Model was used to calculate odds ratios (OR) and 95% confidence intervals (CI). All statistical analyses were performed using SPSS 24.0 software. A 2-sided P value less than 0.05 was regarded as statistically significant.
Results
This study included 270 patients, most of whom were male (60.7%). The clinical characteristics at baseline are shown in . Only 27 patients (10%) met the diagnostic criteria of SPS. Patients were divided into 2 groups: Group A, eGFRcystatin C/eGFRcreatinine ≤0.85 (89 patients, 33% of patients, lowest tertile), and Group B, eGFRcystatin C/eGFRcreatinine >0.85 (181 patients, 67% of patients, upper 2 tertiles). The median age of onset was 54 years. The average age of the patients in group A was higher than that of those in group B (p < 0.01). One-third of the patients had a family history of DM. There was a higher proportion of patients complicated with hypertension in group A (p < 0.01). However, there were no significant differences in SBP and DBP between the 2 groups. Compared with group B, a significantly higher BMI (p < 0.01), uric acid (p < 0.01), and triglycerides (p = 0.03) were found in group A. Patients in group A showed a higher level of cystatin C (p < 0.01) and a lower level of eGFRcystatin C (p < 0.01), while no significant difference was detected in creatinine (p = 0.43) and eGFRcreatinine (p = 0.75). More patients in group A were at intermediate (36.0%) and high risk (14.6%) of CVD in the light of the FRS categorization than in group B (p = 0.03).
Table 2. Baseline characteristics of patients with type 2 diabetes.
Ordinal logistic regression analysis showed that hypertension (OR = 2.86, 95%CI = 1.75–4.67, p < 0.01), drinking habit (OR = 2.57, 95%CI = 1.33–4.98, p < 0.01), BUN (OR = 1.50, 95%CI = 1.23–1.83, p < 0.01), serum creatinine (OR = 1.04, 95%CI = 1.02–1.06, p < 0.01), cystatin C (OR = 13.77, 95%CI = 4.71–40.28, p < 0.01), eGFRcystatin C (OR = 0.97, 95%CI = 0.96–0.98, p < 0.01), eGFRcreatinine (OR = 0.97, 95%CI = 0.96–0.98, p < 0.01), LDL-C (OR = 1.60, 95%CI = 1.14–2.25, p < 0.01), and HbA1c (OR = 1.12, 95%CI = 1.01–1.24, p = 0.03) were associated with FRS risk (). Although SPS was not correlated with FRS risk (OR = 1.99, 95%CI = 0.94–4.23, p = 0.07), eGFRcystatin C/eGFRcreatinine (per 0.1unit increase, OR = 0.86, 95%CI = 0.77–0.97, p = 0.01) showed a significant negative association with FRS risk. Compared with eGFRcystatin C/eGFRcreatinine>0.85, eGFRcystatin C/eGFRcreatinine≤0.85 increased FRS risk (OR = 1.95, 95%CI = 1.18–3.21, p < 0.01). We further stratified patients into 4 groups by eGFRcystatin C/eGFRcreatinine ratio: ≤0.70, 0.71–0.85, 0.86-0.99, and ≥1.00 (). Compared with patients with eGFRcystatin C/eGFRcreatinine ≥1.00, patients with eGFRcystatin C/eGFRcreatinine ratio of ≤0.70 showed the highest FRS risk (OR = 2.88, 95%CI= 1.28–6.44, p = 0.01), those with eGFRcystatin C/eGFRcreatinine ratio of 0.71–0.85 showed a still significant but not as high FRS risk (OR = 2.21, 95%CI = 1.20–4.07, p = 0.01), and those with eGFRcystatin C/eGFRcreatinine ratio of 0.86-0.99 showed a borderline FRS risk (OR = 1.79, 95%CI = 0.95–3.38, p = 0.07).
Figure 1. The association between FRS and eGFRcystatin C/eGFRcreatinine ratio at 4 ranges in univariate analysis.
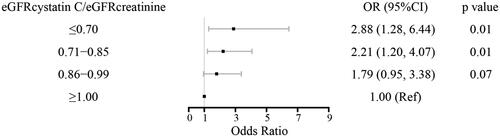
Table 3. Association between baseline characteristics of T2DM and Framingham risk score (univariate Ordinal logistic regression analysis).
As shown in and , hypertension, drinking habit, LDL-C, HbA1c, and BUN were associated with FRS risk in the multivariate ordinal logistic regression analysis. After adjusting for these confounding factors, increased eGFRcystatin C/eGFRcreatinine ratio was associated with decreased FRS risk when considered as a continuous variable (per 0.1unit, OR = 0.87, 95%CI = 0.77–0.99, p = 0.03). The FRS risk in patients with eGFRcystatin C/eGFRcreatinine≤0.85 is 1.86 times higher than that in patients with eGFRcystatin C/eGFRcreatinine>0.85 (OR = 1.86, 95%CI = 1.08–3.21, p = 0.03).
Table 4. Association between continuous eGFRcystatin C/eGFRcreatinine and Framingham risk score (multivariate Ordinal logistic regression analysis).
Table 5. Association between eGFRcystatin C/eGFRcreatinine≤0.85 and Framingham risk score (multivariate Ordinal logistic regression analysis).
Discussion
In the present study, a significant relationship between renal function and different FRS categories among patients with T2DM was identified. Moreover, lower eGFRcystatin C/eGFRcreatinine makes patients more susceptible to intermediate and high risks of CVD. The eGFRcystatin C/eGFRcreatinine ratio could provide a predictive value for CVD risk in Chinese T2DM patients.
Our study demonstrated an inverse adjusted association between eGFR and FRS according to logistic regression analysis (eGFRcystatin C: OR = 0.97, 95%CI = 0.96–0.98, p < 0.01; eGFRcreatinine: OR = 0.97, 95%CI = 0.96–0.98, p < 0.01). Previous studies have indicated an association between renal function and FRS in healthy and general population [Citation15–17]. Consistent with our study, Khaloo et al. also confirmed an inverse association in patients with T2DM. However, a loss of correlation between eGFR and FRS was observed in patients with moderate to severe CKD [Citation18]. These findings indicate that slowing the progression of diabetic kidney disease may reduce the chances of developing CVD events. A collaborative meta-analysis revealed that the inclusion of creatinine-based eGFR and albuminuria significantly improved the discrimination of CVD events in the general population, especially in individuals with diabetes and CKD [Citation19]. Nevertheless, the addition of eGFR and albuminuria to the FRS formula did not substantially improve CVD risk prediction in patients with T2DM or CKD [Citation18,Citation20]. Future studies should investigate risk equations that include unique comorbidities associated with CKD to predict CVD events in T2DM patients.
This is the first study to explore the association between SPS and FRS in patients with T2DM. Increased levels of cystatin C and/or decreased levels of creatinine result in a lower eGFRcystatin C/eGFRcreatinine ratio. The result indicated the eGFRcystatin C/eGFRcreatinine ratio was valuable in predicting future CVD events. Serum creatinine is the most widely used endogenous filtration marker and is freely filtered across glomeruli [Citation21,Citation22]. It is influenced by muscle mass, dietary protein, and secretion by renal tubular epithelial cells, and extrarenal clearance [Citation21,Citation23]. Cystatin C is produced by nucleated cells, freely filtered across glomeruli, completely reabsorbed and metabolized by renal tubular epithelial cells [Citation24]. Compared with creatinine, cystatin C is less affected by non-renal confounders; therefore, the eGFR calculated by cystatin C alone or in combination with creatinine is more dependable [Citation25]. Possible mechanisms of SPS include decreased pore size in the filtration barrier, dysfunction of endothelial cell fenestrae, and thickness of the glomerular basement membrane [Citation9,Citation26,Citation27]. An inverse correlation between glomerular basement membrane thickness and the eGFRcystatin C/eGFRcreatinine ratio in kidney biopsies of diabetic kidney disease provides a basis for the latter mechanism [Citation27]. SPS indicates renal function impairment and is associated with high mortality because of the accumulation of atherosclerosis-promoting proteins [Citation11]. A high increase in morbidity and mortality has been observed in patients with SPS during long-term follow-up [Citation28]. In 2781 patients measured GFR using iohexolclearance, patients with SPS showed a significantly higher all-cause mortality than those with cancer, CVD, diabetes, and CKD [Citation29]. Furthermore, in individuals with normal measured GFR, there was a marked increase in all-cause mortality in individuals with SPS [Citation29]. Although we did not detect a significant association between SPS and FRS, we found that a lower eGFRcystatin C/eGFRcreatinine ratio was significantly related to intermediate and high CVD risk. Moreover, the increase in FRS categorization was inversely proportional to the eGFRcystatin C/eGFRcreatinine starting at a ratio of 0.85 (OR = 1.86, 95%CI = 1.08–3.21, p = 0.03). We speculated that patients with a lower eGFRcystatin C/eGFRcreatinine ratio might have a poor prognosis that supported by statistical association. These results will be useful for enabling early interventional strategies to prevent CVD and improve prognosis. The cutoff value of eGFRcystatin C/eGFRcreatinine≤0.7 regarded as the definition of SPS, was obtained from patients undergoing cardiac surgery [Citation28] and coronary artery bypass grafting [Citation30]. Different eGFRcystatin C/eGFRcreatinine ratio cutoff values are needed in various clinical settings to identify CVD risk. Thus, future studies with larger sample sizes are needed to determine suitable cutoff values for different patient populations.
Our study has some limitations. First, the FRS is only an estimation algorithm to predict CVD risk and is an imprecise tool in the young population. Second, other potential CVD risk factors may have been ignored. Finally, this was a cross-sectional study with a small sample size, the applicability of our results to other T2DM clinics may not be guaranteed. We were unable to clarify the causal relationship between the eGFRcystatin C/eGFRcreatinine ratio and CVD events. A prospective multicenter study with long-term follow-up is needed to address the direction of causation.
Conclusions
This study evaluated the association between eGFRcystatin C/eGFRcreatinine and FRS to estimate the 10-year CVD risk in patients with T2DM in China for the first time. Although we found no significant association between SPS and FRS, we concluded that lower eGFRcystatin C/eGFRcreatinine and eGFRcystatin C/eGFRcreatinine≤0.85 were associated with significantly increased CVD risk. The results provide valuable information for designing interventional strategies to prevent CVD in patients with T2DM.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University, China (registration number:27/2013), and conformed to the principles of the Declaration of Helsinki. Written informed consent was obtained from all the patients.
Authors’ contributions
Yan Yang: Methodology, Formal Analysis, Writing-Original Draft Preparation, Funding Acquisition; Bixia Yang: Resources, Data Curation, Formal Analysis, Investigation; Shizhu Zhao: Resources, Data Curation, Writing–Review & Editing; Shusu Liu: Software, Investigation, Data Curation; Hua Zhou: Validation, Visualization; Min Yang: Conceptualization, Funding Acquisition, Project Administration; Ning Xu: Conceptualization, Supervision.
Consent for publication
Not applicable.
Acknowledgements
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and materials
Data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9 edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843.
- Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6.
- Morrish NJ, Wang SL, Stevens LK, et al. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44 Suppl 2(S2):S14–S21. doi: 10.1007/pl00002934.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421.
- Grubb A, Lindström V, Jonsson M, et al. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: ‘Shrunken pore syndrome. Scand J Clin Lab Invest. 2015;75(4):333–340. doi: 10.3109/00365513.2015.1025427.
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–S9. doi: 10.1053/ajkd.1998.v32.pm9820470.
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060. doi: 10.1056/NEJMoa043161.
- Schöttker B, Herder C, Müller H, et al. Clinical utility of creatinine- and cystatin C-based definition of renal function for risk prediction of primary cardiovascular events in patients with diabetes. Diabetes Care. 2012;35(4):879–886. doi: 10.2337/dc11-1998.
- Grubb A. Shrunken pore syndrome - a common kidney disorder with high mortality. Diagnosis, prevalence, pathophysiology and treatment options. Clin Biochem. 2020;83:12–20. doi: 10.1016/j.clinbiochem.2020.06.002.
- Larsson AO, Hultström M, Frithiof R, et al. Shrunken pore syndrome is frequently occurring in severe COVID-19. Int J Mol Sci. 2022;23(24):15687. doi: 10.3390/ijms232415687.
- Almén MS, Björk J, Nyman U, et al. Shrunken pore syndrome is associated with increased levels of atherosclerosis-Promoting proteins. Kidney Int Rep. 2019;4(1):67–79. doi: 10.1016/j.ekir.2018.09.002.
- Zhang LW, Luo MQ, Xie XW, et al. Shrunken pore syndrome: a new and more powerful phenotype of renal dysfunction than chronic kidney disease for predicting Contrast-Associated acute kidney injury. J Am Heart Assoc. 2023;12:e027980.
- Xhakollari L, Grubb A, Jujic A, et al. The shrunken pore syndrome is associated with poor prognosis and lower quality of life in heart failure patients: the HARVEST-Malmö study. ESC Heart Fail. 2021;8(5):3577–3586. doi: 10.1002/ehf2.13485.
- Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248.
- Jin B, Bai X, Han L, et al. Association between kidney function and framingham global cardiovascular disease risk score: a chinese longitudinal study. PLoS One. 2014;9(1):e86082. doi: 10.1371/journal.pone.0086082.
- Lee K, Kim J. Estimated glomerular filtration rate and albuminuria in korean population evaluated for cardiovascular risk. Int Urol Nephrol. 2016;48(5):759–764. doi: 10.1007/s11255-016-1244-9.
- Bai X, Han L, Liu J, et al. The relationship between age-related kidney dysfunction and framingham risk score in healthy people in China. Curr Aging Sci. 2010;3(3):188–197. doi: 10.2174/1874609811003030188.
- Khaloo P, Alemi H, Mansournia MA, et al. Loss of inverse association between framingham risk score and estimated glomerular filtration rate in moderate to severe diabetic kidney disease. Arch Iran Med. 2019;22:91–98.
- Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6.
- Chang A, Kramer H. Should eGFR and albuminuria be added to the framingham risk score? Chronic kidney disease and cardiovascular disease risk prediction. Nephron Clin Pract. 2011;119(2):c171–c178. discussion c177-8. doi: 10.1159/000325669.
- Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol. 2008;21(6):797–807.
- Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–1953.
- Yoo J-J, Kim SG, Kim YS, et al. Estimation of renal function in patients with liver cirrhosis: impact of muscle mass and sex. J Hepatol. 2019;70(5):847–854. doi: 10.1016/j.jhep.2018.12.030.
- Shlipak MG, Inker LA, Coresh J. Serum cystatin C for estimation of GFR. JAMA. 2022;328(9):883–884. doi: 10.1001/jama.2022.12407.
- Grubb A, Björk J, Nyman U, et al. Cystatin C, a marker for successful aging and glomerular filtration rate, is not influenced by inflammation. Scand J Clin Lab Invest. 2011;71(2):145–149. doi: 10.3109/00365513.2010.546879.
- Li Z, Wang S, Huo X, et al. Cystatin C expression is promoted by VEGFA blocking. With Inhibitory Effects on Endothelial Cell Angiogenic Functions Including Proliferation, Migration, and Chorioallantoic Membrane Angiogenesis. J Am Heart Assoc. 2018;7:e009167.
- Öberg CM, Lindström M, Grubb A, et al. Potential relationship between eGFRcystatin C/eGFRcreatinine-ratio and glomerular basement membrane thickness in diabetic kidney disease. Physiol Rep. 2021;9:e14939.
- Herou E, Dardashti A, Nozohoor S, et al. The mortality increase in cardiac surgery patients associated with shrunken pore syndrome correlates with the eGFRcystatin C/eGFRcreatinine-ratio. Scand J Clin Lab Invest. 2019;79(3):167–173. doi: 10.1080/00365513.2019.1576101.
- Åkesson A, Lindström V, Nyman U, et al. Shrunken pore syndrome and mortality: a cohort study of patients with measured GFR and known comorbidities. Scand J Clin Lab Invest. 2020;80(5):412–422. doi: 10.1080/00365513.2020.1759139.
- Dardashti A, Nozohoor S, Grubb A, et al. Shrunken pore syndrome is associated with a sharp rise in mortality in patients undergoing elective coronary artery bypass grafting. Scand J Clin Lab Invest. 2016;76(1):74–81. doi: 10.3109/00365513.2015.1099724.
