Abstract
Background
Laparoscopic techniques are being widely applied for peritoneal dialysis (PD) catheter (PDC) placement. The suture passer is a novel fixation tool that aims to reduce catheter migration. We compared the clinical value of the suture passer combined with two-hole laparoscopic PDC placement to open surgical placement by evaluating preoperative and postoperative conditions, as well as the onset of complications in both groups.
Methods
A retrospective study was conducted including 169 patients who underwent PDC placement surgery from January 2021 to May 2023. Based on the method employed, patients were divided into two groups: the suture passer combined with a two-hole laparoscopy group (SLG) and the open surgical group (SG). Comprehensive patient information, including general data, preoperative and postoperative indicators, peritoneal function after surgery, and the incidence rate of complications, were collected and analyzed.
Results
The SLG showed a statistically significant decrease in operative time, intraoperative blood loss, and 6-month postoperative drift rate compared to the SG (p < 0.05). No statistically significant differences were observed between the two groups in terms of sex, age, primary disease, hospitalization time, hospitalization costs, preoperative and postoperative examination indicators, peritonitis, and omental wrapping.
Conclusions
Suture passer combined with two-hole laparoscopic PDC placement, characterized by simplicity and facilitating secure catheter fixation, was deemed safe and effective for patients undergoing PD. It reduces the catheter migration rate and improved surgical comfort. Overall, this technique demonstrates favorable outcomes in clinical practice.
1. Introduction
Peritoneal dialysis (PD) has been present as a renal replacement therapy for nearly a century [Citation1]. It is widely acknowledged as a more liberated, safe, and effective treatment for end-stage renal disease (ESRD) compared to hemodialysis [Citation2]. However, PD is not without its challenges, including peritonitis, catheter displacement, dialysate leakage, and outflow obstruction, among other issues, which can hinder the function of PD catheters (PDCs) [Citation3,Citation4]. Therefore, the successful insertion and continuous connection of peritoneal catheters, often considered the “lifeline” for patients with PD, are critical in PDC placement [Citation5]. The introduction of the Tenckhoff catheter in 1968 is considered a significant advancement in PD for patients with ESRD [Citation1].
Reducing complications caused by PDC placement has become a vital area of research. The primary methods for PDC insertion include bedside insertion or percutaneous implantation, surgical insertion, peritoneoscopic insertion, and laparoscopic insertion [Citation6]. Compared to open surgery, laparoscopic surgery has been reported to cause fewer incidences of catheter malfunction, have shorter operative time, and lower short-term complication rates [Citation7,Citation8]. Laparoscopic technology has replaced open technology and is now the most widely used PDC placement method in the United States [Citation9], favored for its visual operation advantages. However, traditional laparoscopic surgery is relatively complex and demands high proficiency from the operator. We aimed to introduce an improved minimally invasive laparoscopic PDC internal fixation method and retrospectively compare it with catheterization using a suture passer and open surgical PDC insertion techniques, considering efficacy, economy, and complications.
2. Materials and methods
2.1. Patients
This retrospective analysis included 169 patients with ESRD who underwent PDC insertion and treatment in the nephrology department of Linyi People’s Hospital from January 2021 to May 2023. All patients were followed up after catheter insertion until 6 months later, and their clinical data were collected and retrospectively analyzed. Inclusion criteria comprised patients with ESRD who had undergone PD, were aged >18 years at the time of catheterization, and had a dialysis duration of >6 months. Exclusion criteria encompassed acute kidney injury, cardiopulmonary dysfunction, abdominal wall defects, pregnancy, mental illness or the occurrence of abdominal wall defect, severe coagulation dysfunction, severe peritonitis, and other situations where surgery is prohibited. In addition, for patients with a history of abdominal surgery, we recommend using laparoscopy for PDC placement. The characteristics of laparoscopic PDC placement using a suture passer and open surgical PDC insertion techniques were explained to the patients, the method of PDC placement is selected based on the patient’s preferences and their physical condition, whether they can accept general anesthesia, whether they are obese, and whether they have a history of abdominal surgery. All patients provided written informed consent.
This study was approved by the Ethics Committee of Linyi People’s Hospital (Approval Number: 202311-H036). Informed consent was waived in our study due to the retrospective nature of the study.
2.2. Operative technique
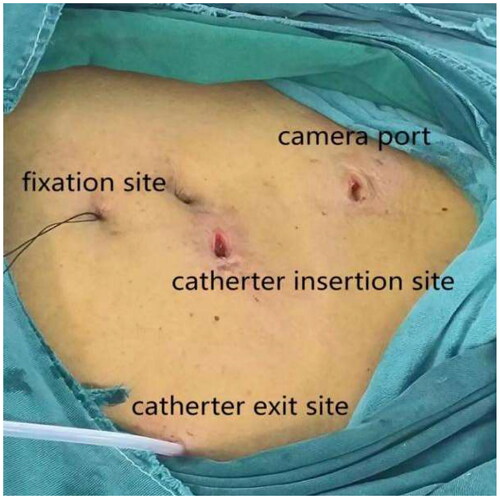
All laparoscopic surgeries were performed by the same experienced operating team, and the operator marked the preferred exit position for PDC on the abdomen of all patients prior to surgery. The bladder was emptied of any residual urine before intervention. Following successful anesthesia, patients were placed in a supine position. First, a Veress needle was used to puncture 5 cm above the navel to establish a CO2 pneumoperitoneum, with an intra-abdominal pressure set at 12 mmHg. A 10 mm trocar was then inserted into the abdominal cavity at the initial position of the Veress needle, followed by a preliminary laparoscopic examination to assess any omental length and intraperitoneal adhesions that might affect the surgery. If no abnormalities were observed, the position of the deep cuff was measured using an abdominal tube, and another 10 mm trocar was inserted at the left side of the navel under laparoscopic view. The catheter was placed at this location as the second port, and a PDC was inserted, pointing toward the pelvic cavity. It was ensured that all holes of the catheter were placed inside the peritoneum, with continuous observation via laparoscopy to prevent cuff entry into the abdominal cavity.
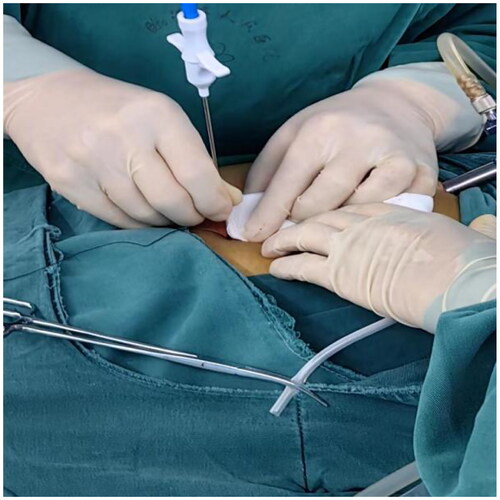
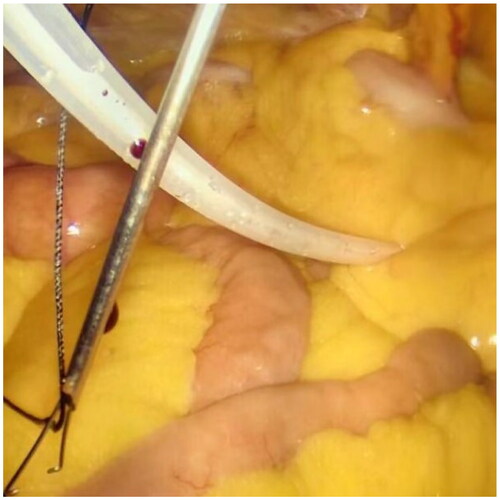
After filling the syringe with normal saline to test the patency of the catheter, a small incision of 1 mm was created approximately 2–3 cm below the Tenckhoff catheter. A suture passer with a NO. 7 suture was inserted into this incision (), the suture was released, and then inserted from the same incision. The suture was pulled out on the other side of the catheter and subcutaneously tied for fixation (). For patients with obesity or thick subcutaneous fat, Hem-o-lok was used for subcutaneous suturing as a marker, facilitating the identification of fixed stitches during later tube removal.
Figure 2. Ssuture passer with a NO. 7 suture was inserted into the 1 mm small incision (The left side is the head).
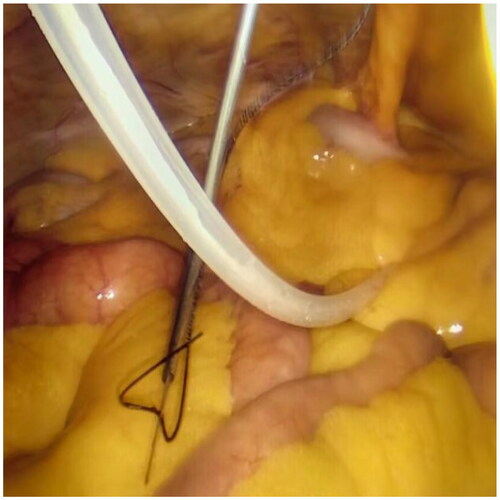
Laparoscopy was employed again to check for hematoma in the abdominal cavity, ensuring the accurate position of the catheter tip in the pelvic cavity, and confirming the absence of active bleeding at each puncture point. The camera was then removed, releasing the pneumoperitoneum. Connect the catheter to the titanium joint of the catheter and the external short tube of the abdominal dialysis tube through a subcutaneous tunnel. Finally, after removing each trocar, all wounds were layered and sutured (). The entire modified procedure, which can be performed in a straightforward manner, may reduce the operative time and simplify the operation, facilitating dissemination and promotion.
2.3. Postoperative catheter use and follow-up
On the first day after surgery, abdominal flushing was performed. On the second day after surgery, low-dose lying position and short-term peritoneal dialysis (1000 mL of 1.5% peritoneal dialysis solution was left on the abdomen for 2 h x 2 times) can be started. Unless there are any special circumstances, we require patients to undergo regular monthly outpatient check ups, adjust medication based on the results, undergo peritoneal dialysis evaluation six months later, followed up with abdominal X-rays every six months to observe for catheter displacement, and if there is poor catheter drainage, X-rays should also be taken.
2.4. Data collection
General patient information was collected before the surgery, including sex, age, body mass index (BMI), and primary disease. Subsequently, we analyzed surgical conditions, including duration of hospitalization, hospitalization costs, surgical duration, and blood loss. Preoperative and postoperative 6-month examination indicators, including renal function tests and blood routine parameters, were compared. We also evaluated peritoneal function status and complications such as catheter migration, peritonitis, and omental wrapping at 6 postoperative months.
2.5. Statistical analyses
Statistical analyses were performed using SPSS Statistics 26.0. All indicators that conform to a normal distribution are expressed as mean ± standard deviation, and categorical variables are presented as number (percentage). Inter group comparisons were analyzed using two independent sample t-tests, and Chi-squared or rank-sum tests were used for measurement data. p < 0.05 indicates a statistically significant difference.
3. Results
This study comprised a total of 169 patients, with 66 patients in the laparoscopic PDC placement group using a stapler (SLG group) and 103 patients in the traditional PDC placement group (LG group). Within the SLG group, there were two patients who underwent kidney transplantation, four patients switched from peritoneal dialysis to hemodialysis, and two patients experienced death outcomes. The LG group had four patients who underwent kidney transplantation, four patients who switched to hemodialysis, and three cases of death. Due to inability to obtain subsequent data, these patients were excluded from the statistical comparison range of basic information, surgical conditions, and preoperative and postoperative 6-month examination indicators.
3.1. Comparison of basic information between the two groups
There were no significant differences in sex, age, and primary disease between the SLG and the SG, indicating comparability. The BMI in the SLG was higher than that in the SG (p < 0.05) ().
Table 1. Basic preoperative information.
3.2. Comparison of surgical conditions between the two groups
Compared to the SG, the operative time and intraoperative blood loss in the SLG were significantly lower (p < 0.001). The surgical time we record refers to the entire process from anesthesia preparation to the final completion of the surgery, and the amount of blood loss is estimated through visual inspection, gauze cloths stained with blood, and suction device storage tanks. Hospitalization time and costs between the SLG and the SG did not significantly differ ().
Table 2. Intraoperative conditions.
3.3. Comparison of relevant examination indicators between the two groups before and 6 months after surgery
Preoperative blood routine and biochemical indicators including leukocyte count, hemoglobin, serum creatinine, blood urea nitrogen, blood sodium, and blood potassium did not significantly differ between the two groups (p > 0.05), indicating comparability. The levels of these indicators was still no significant difference statistically following 6 months of treatment (p > 0.05), which does not indicate a significant difference in the efficacy of the two surgical methods. ().
Table 3. Inspection indicators (preoperative).
Table 4. Inspection indicators (after 6 months of treatment).
3.4. Comparison of postoperative peritoneal function and complications between the two groups
The average 4-h creatinine D/P of patients in the SG at the 6-month follow-up was 0.67 (0.16) and 0.67 (0.23) for SLG, with no statistical differences between the two groups (p > 0.05). In the SG, 12 patients exhibited catheter displacement confirmed via imaging, whereas in the SLG, two patients exhibited catheter displacement, and there was a significant difference between the two groups (p < 0.05). There were no statistically significant differences in the incidence of peritonitis, hernia, catheter outlet infections, omental wrapping, death, renal transplant and conversion to hemodialysis between the two groups ().
Table 5. Peritoneal function and complications in patients 6 months after surgery.
4. Discussion
Since the inception of Tenckhoff catheterization technology 50 years ago [Citation10], the continually advancing PD technology has become a widely used and increasingly preferred treatment for patients with ESRD. Laparoscopy, with its advantages of safety, efficiency, and visualization, has been widely embraced over traditional open surgery [Citation11]. Regardless of the method used, catheter dysfunction remains the predominant cause of PD failure, as suggested by a cohort study that identifies mechanical PDC technology as a key factor in technique failure within the first year of PD therapy [Citation12]. Therefore, numerous refinements have been proposed based on the classic laparoscopic surgery method [Citation13–16]. The 2023 PD access guideline has also recommended the use of advanced and improved laparoscopic insertion techniques under appropriate conditions [Citation17]. To our knowledge, few studies have provided a detailed introduction to PDC placement using abdominal suture passer and two-port laparoscopy, which can simultaneously reduce surgical trauma and catheter migration.
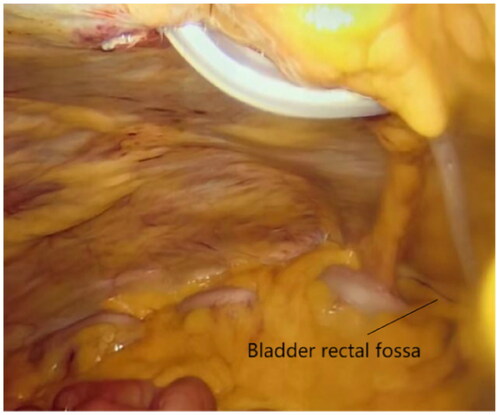
The two-hole laparoscopic PDC placement technique utilized in this study enables direct visualization of the abdominal cavity, allowing for auxiliary surgeries such as adhesiolysis, omentopexy, and catheter fixation. Simultaneously, the two surgical incisions lead to lesser abdominal damage to patients than basic laparoscopy and offer significant advantages in terms of abdominal wall leakage [Citation18] and abdominal esthetics. Another noteworthy advantage of our surgery is that the laparoscope observation hole is created 5-cm above the navel, avoiding the navel as an observation hole can prevent umbilical hernia and leakage. After removing the operating mirror, an Suture Passer was used to suture peritoneum and further prevent leakage after infusion of abdominal fluid. Due to navel weakness, laparoscopic surgery using this as the puncture site can easily result in umbilical hernia [Citation19,Citation20]. This approach also plays a substantial role in observing the deep cuff and catheter position. The intuitive visual field ensures that the position of the peritoneal dialysis tube is located in the bladder rectal fossa. The placement of the peritoneal dialysis tube is more precise than open surgery, avoiding repeated damage to the lower abdominal peritoneum caused by the tube tip due to its low position, and reducing discomfort such as pain caused by the inflow and outflow of peritoneal dialysis fluid. Prevent excessive placement of the catheter from causing incomplete discharge of peritoneal fluid or causing catheter displacement. A study comparing three cannula operations for PD reported that the incidence of umbilical hernia in the laparoscopic catheterization group (21.7%) was significantly higher than that in the percutaneous puncture catheterization (3.4%) and conventional surgical catheterization (2.8%) groups [Citation21]. Following intraperitoneal dialysate infusion in the later stage of PD, high abdominal pressure can easily result in hernia in weak regions of the abdominal wall, affecting the smooth progress of PD and even causing intestinal strangulation and necrosis [Citation22].
The displacement of the catheter tip or omental wrapping in laparoscopic catheterization is often the primary cause of early catheter dysfunction [Citation23], as observed in the study by Alabi et al. [Citation24]. Various techniques have been suggested to enhance the long-term survival of the catheter. For instance, Kao et al. [Citation25]. achieved PDC placement with only one port and intra-abdominal fixation, providing good cosmesis, but the study lacked clear results for late complications such as hernia and catheter leakage. Shen et al. [Citation5] described a method of fixing catheters with a suture, albeit with multiple ports, potentially causing significant abdominal damage. In the latest multicenter prospective study followed up for 5 years, a surgical method for achieving 0% catheter displacement was introduced. However, the three-cuff PDC is more complex to operate compared to conventional catheters, there is no routine clinical application currently. In addition, the three-cuff PDC and the right size dilator are difficult to obtain in many hospitals [Citation26]. In our method, the use of a suture passer aimed to reduce additional trocar drilling, minimizing damage to the abdominal wall. This method, performed under microscopic observation, proved relatively simple and safe. In addition, for patients with obesity or thick subcutaneous fat, after fixing the catheter with a suture passer, Hem-o-lok was used at the 2 mm small hole of the suture passer to mark the fixing thread, which is used to find the fixing thread for easy removal of the peritoneal dialysis catheter when it is difficult to remove it. However, due to the fact that Hem-o-lok is buried beneath the skin and feels slightly lumpy to the touch, we generally do not use this material. Currently, patients using Hem-o-lok do not feel uncomfortable or affect their daily life. Regarding mechanical complications, only two patients in the laparoscopic group experienced catheter displacement compared to 12 patients in the open surgery group, indicating the efficacy of this catheter fixation method. The catheter displacement does not mean the failure of PD treatment. Successful reduction can be achieved through movement, manipulation, and wire guiding methods, in some patients, catheter reduction is performed under laparoscopy. Among the patients with catheter displacement, we did not find any cases of catheter removal or replacement. Although there is no significant difference in the statistical data of peritonitis in patients receiving two surgical methods, the incidence rate of SLG is significantly lower than that of SG. For omental wrapping, we don’t take the prophylactic laparoscopic omentopexy or epiploectomy to prevent catheter malfunction caused by catheter omental wrapping as a routine method. We performed omentopexy when a redundant, thin, and filmy omentum was encountered. The omentopexy can be done without any additional port through the suture passer. Within 6 months after treatment, no patients with hernia were found in both groups, which may be due to insufficient follow-up time. In further research, we will continue to follow up on the relevant situation.
With regard to the two outcomes of death and hemodialysis, after follow-up, we found that three patients in SLG died of intrapulmonary infection at the time of the outbreak of the COVID-19 epidemic, it is not ruled out that COVID-19 died of infection due to low immunity. One patient died of a sudden heart attack, and one patient was transferred to hemodialysis at a local hospital due to peritonitis, another patient who underwent hemodialysis was unable to support peritoneal dialysis due to severe edema accompanied by ultrafiltration failure. In the SG group, one patient died of ANCA related vasculitis combined with recurrent gastrointestinal bleeding, another two patients died respectively due to multiple organ failure and sudden myocardial infarction, two patients switched to hemodialysis treatment at a local hospital due to peritonitis, and two patients respectively switched to hemodialysis treatment due to ultrafiltration failure and pulmonary infection. We can conclude that there is no statistically significant difference in mortality and conversion to hemodialysis between the two groups of patients, and there is no significant difference in the impact of the two methods of PDC placement on these two outcomes.
While there was no statistically significant difference in basic information, various indicators before and after surgery, and unplanned readmissions between the two groups, patients who received abdominal wall stapler combined with two-port laparoscopic catheterization exhibited shorter operative time, less bleeding, and shorter hospitalization time compared to traditional open surgery. Although laparoscopic catheterization surgery is more expensive due to anesthesia and laparoscopy use, it presents a lower incidence of short-term complications, such as catheter drift and peritonitis, shorter hospitalization time, and fewer unplanned readmissions. Overall hospitalization costs did not significantly differ between the two surgical methods.
In conclusion, our study introduces a novel internal fixation method for placing PDCs under laparoscopy, showcasing advantages such as reducing the risk of catheter displacement, improving the accuracy of catheter tip position and minimizing abdominal injury, with only two ports. In addition, compared with traditional surgery, the use of laparoscopy improves the patient’s surgical comfortand reduces abdominal injuries. However, due to the novelty of this technology, more information on long-term complications is needed, warranting further monitoring.
5. Conclusions
The presented technique of suturing the Tenckhoff catheter to the anterior abdominal wall using a suture passer during laparoscopic insertion with the two-hole method not only addresses many early complications but also enhances esthetics to a certain extent. It has proven to be a safe, easy-to-operate, and minimally invasive technology worthy of dissemination and promotion in clinical practice.
Ethics approval and consent to participate
This research was approved by the Ethics Committee of Linyi People’s Hospital (Approval Number: 202311-H036).
Authors’ contributions
Yinan Zhu and PeiQin Xin collected all data, conducted statistical analysis and finish the initial manuscript, LIna Sun and Yulin Man critically reviewed and revised the manuscript, Xiaoming Zhang is responsible for the surgical operation. All authors reviewed and approved the final manuscript.
Acknowledgments
The authors thank all the participants for their contributions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Misra M, Phadke GM. Historical milestones in peritoneal dialysis. Contrib Nephrol. 2019;197:1–8. doi: 10.1159/000496301.
- Janez J. Laparoscopically assisted insertion of peritoneal dialysis catheter. J Minim Access Surg. 2019; 15(1):80–83. doi: 10.4103/jmas.JMAS_196_17.
- Abdijalil G, Shuijuan S. Laparoscopic versus open-surgery catheter placement in peritoneal dialysis patients: a meta-analysis of outcomes. Indian J Nephrol. 2022; 32(1):8–15. doi: 10.4103/ijn.IJN_482_20.
- Htay H, Johnson DW, Craig JC, et al. Catheter type, placement and insertion techniques for preventing catheter-related infections in chronic peritoneal dialysis patients. Cochrane Database Syst Rev. 2019; 5(5):CD004680. doi: 10.1002/14651858.CD004680.pub3.
- Shen Q, Jiang X, Shen X, et al. Modified laparoscopic placement of peritoneal dialysis catheter with intra-abdominal fixation. Int Urol Nephrol. 2017; 49(8):1481–1488. doi: 10.1007/s11255-017-1593-z.
- Bircan HY, Kulah E. Effects of a novel peritoneal dialysis: the open versus laparoscopic preperitoneal tunneling technique. Ther Apher Dial. 2016; 20(1):66–72. doi: 10.1111/1744-9987.12377.
- Gao X, Peng Z, Li E, et al. Modified minimally invasive laparoscopic peritoneal dialysis catheter insertion with internal fixation. Ren Fail. 2023; 45(1):2162416. doi: 10.1080/0886022X.2022.2162416.
- Jwo SC, Chen KS, Lee CC, et al. Prospective randomized study for comparison of open surgery with laparoscopic-assisted placement of tenckhoff peritoneal dialysis catheter–a single center experience and literature review. J Surg Res. 2010; 159(1):489–496. doi: 10.1016/j.jss.2008.09.008.
- Wilkie MZ, Zhao J, Bieber B, et al. Sp503international variation in peritoneal dialysis (Pd) catheter practices: preliminary results from the peritoneal dialysis outcomes and practice patterns study (pdopps). Nephrol Dial Transplant. 2017;32(suppl_3):iii297–iii299. doi: 10.1093/ndt/gfx151.SP503.
- Tenckhoff H, Curtis FK. Experience with maintenance peritoneal dialysis in the home. Trans Am Soc Artif Intern Organs. 1970;16:90–95. PMID: 4318265.
- Rege SA, Churiwala J, Takalkar Y, et al. Laparoscopic peritoneal dialysis catheter insertion: an oasis harnessed-experience and results at a tertiary care Centre in India. Surg Endosc. 2023; 37(8):6491–6494. doi: 10.1007/s00464-023-10154-2.
- See EJ, Johnson DW, Hawley CM, et al. Risk predictors and causes of technique failure within the first year of peritoneal dialysis: an Australia and New Zealand dialysis and transplant registry (ANZDATA) study. Am J Kidney Dis. 2018; 72(2):188–197. doi: 10.1053/j.ajkd.2017.10.019.
- Guilbert A, Benoit O, Lupinacci RM. Laparoscopic peritoneal dialysis catheter insertion. J Visc Surg. 2023; 160(1):60–64. doi: 10.1016/j.jviscsurg.2022.07.006.
- Khan SF, Rosner MH. Optimizing peritoneal dialysis catheter placement. Front Nephrol. 2023; 3:1056574. doi: 10.3389/fneph.2023.1056574.
- Krezalek MA, Bonamici N, Lapin B, et al. Laparoscopic peritoneal dialysis catheter insertion using rectus sheath tunnel and selective omentopexy significantly reduces catheter dysfunction and increases peritoneal dialysis longevity. Surgery. 2016; 160(4):924–935. doi: 10.1016/j.surg.2016.06.005.
- Musbahi A, Kanakala V. A modified laparoscopic peritoneal dialysis insertion technique, the ‘one scar technique’ can minimise short and long term complications: a retrospective cohort study. J Vasc Access. 2021;22(5):744–748. doi: 10.1177/1129729820961970.
- Haggerty SP, Kumar SS, Collings AT, et al. SAGES peritoneal dialysis access guideline update 2023. Surg Endosc. 2023;38 (1):1–23. doi: 10.1007/s00464-023-10550-8.
- Peng J, Lin H, Cai C, et al. New method of internal fixation in laparoscopic tenckhoff catheter placement. Semin Dial. 2022;35(6):498–503. doi: 10.1111/sdi.13087.
- Barutcu AG, Klein D, Kilian M, et al. Long-term follow-up after single-incision laparoscopic surgery. Surg Endosc. 2020; 34(1):126–132. doi: 10.1007/s00464-019-06739-5.
- Tanaka H, Kitazawa M, Miyagawa Y, et al. Risk factors for umbilical incisional hernia after laparoscopic colorectal surgery. ANZ J Surg. 2022; 92(12):3219–3223. doi: 10.1111/ans.17979.
- Chen B, Sun C, Zhou J, et al. The study of comparing three different cannula operations for peritoneal dialysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021; 33(9):1084–1087. doi: 10.3760/cma.j.cn121430-20210425-00608.
- Betancourt L, Pico S, Rojas E, et al. Relationship between intraperitoneal pressure and the development of hernias in peritoneal dialysis: confirmation for the first time of a widely accepted concept. Int Urol Nephrol. 2023;56. (2):759–765. doi: 10.1007/s11255-023-03663-5.
- Crabtree JH, Fishman A. A laparoscopic method for optimal peritoneal dialysis access. Am Surg. 2005; 71(2):135–143. doi: 10.1177/000313480507100209.
- Alabi A, Dholakia S, Ablorsu E. The role of laparoscopic surgery in the management of a malfunctioning peritoneal catheter. Ann R Coll Surg Engl. 2014;96(8):593–596. doi: 10.1308/003588414X14055925058319.
- Kao CY, Chuang JH, Lee SY. A new simplified one-port laparoscopic technique for peritoneal dialysis catheter placement. Perit Dial Int. 2014; 34(1):109–113. doi: 10.3747/pdi.2012.00130.
- Al-Hwiesh AK, Abdul-Rahman IS, Divino-Filho JC, et al. A nephrologist dream of peritoneal dialysis catheter with zero migration: a multicenter prospective study. Ther Apher Dial. 2024;28(1):89–95. doi: 10.1111/1744-9987.14045.