Abstract
Objective
Liraglutide, a glucagon-like peptide-1 receptor agonist, has been shown to regulate blood sugar and control body weight, but its ability to treat obesity-related nephropathy has been poorly studied. Therefore, this study was designed to observe the characteristics and potential mechanism of liraglutide against obesity-related kidney disease.
Methods
Thirty-six C57BL/6J male mice were randomly divided into six groups (n = 6 per group). Obesity-related nephropathy was induced in mice by continuous feeding of high-fat diet (HFD) for 12 weeks. After 12 weeks, liraglutide (0.6 mg/kg) and AMP-activated protein kinase (AMPK) agonists bortezomib (200 μg/kg) were injected for 12 weeks, respectively. Enzyme-linked immunosorbent assay was employed to detect the levels of total cholesterol, triglycerides, low-density lipoprotein cholesterol, blood urea nitrogen, creatinine in serum, as well as urinary protein in urine. Besides, hematoxylin–eosin staining and periodic acid-Schiff staining were used to observe the pathological changes of kidney tissue; immunohistochemistry, western blot, and real-time quantitative PCR to assess the calmodulin-dependent protein kinase kinase beta (CaMKKβ)/AMPK signaling pathway activation.
Results
Liraglutide significantly reduced serum lipid loading, improved kidney function, and relieved kidney histopathological damage and glycogen deposition in the mouse model of obesity-related kidney disease induced by HFD. In addition, liraglutide also significantly inhibited the CaMKKβ/AMPK signaling pathway in kidney tissue of HFD-induced mice. However, bortezomib partially reversed the therapeutic effect of liraglutide on HDF-induced nephropathy in mice.
Conclusions
Liraglutide has a therapeutic effect on obesity-related kidney disease, and such an effect may be achieved by inhibiting the CaMKKβ/AMPK signaling pathway in kidney tissue.
Introduction
Based on the development of economy, the improvement of the standard of living for people and the prevalence of unhealthy lifestyles (such as sedentary), obesity is becoming an increasingly serious global health problem [Citation1]. Generally, obesity occurs when fat accumulates in the body due to energy intake exceeding energy consumption. According to the standards of the World Health Organization (WHO), obesity is defined as the body mass index (BMI) exceeding 30 kg/m2 [Citation2]. Referring to such standard, there are about 2.1 billion people in the world who are close to or meet the diagnostic criteria of obesity [Citation1]. It is noted that obesity is closely related to many chronic diseases. The risk of cardiovascular diseases [Citation3], diabetes [Citation4], cancer [Citation5], and kidney diseases [Citation6] in obese people is much higher than that in people with normal weight. Of them, kidney diseases caused by obesity are particularly worthy of attention. Obesity-related kidney disease is associated with abnormal changes to kidney structure. Obesity often induces pathological features such as basement membrane thickening, glomerulosclerosis, renal tubular disorder, endothelial cell dysfunction, and kidney interstitial fibrosis, thereby leading to the progressive decline of patients’ kidney function [Citation6,Citation7]. Compared with other chronic diseases induced by obesity, obesity-related kidney disease is characterized by faster progression, more hidden, and higher mortality [Citation8]. Therefore, exploring drugs that can effectively treat obesity-related kidney disease is of great significance to alleviating the pain of obese people and improving their quality of life.
As a glucagon-like peptide-1 (GLP-1) receptor agonist, liraglutide simulates the biological effect of GLP-1 by activating GLP-1 receptor. On the one hand, liraglutide can stimulate pancreatic β cells to release a large amount of insulin; on the other hand, it can inhibit the secretion of glucagon and slow down gastric emptying. When these two functions overlap, liraglutide can play a role in treating diabetes through regulating blood glucose homeostasis [Citation9]. Therefore, in 2010, liraglutide was officially approved by the Food and Drug Administration (FDA) as a diabetes drug that can be used alone or in combination with other drugs for the treatment of type 2 diabetes [Citation10]. However, in recent years, the potential of liraglutide as a weight loss drug has attracted more attention. Many animal studies have shown that liraglutide can penetrate the blood–brain barrier and affect neurotransmitter transmission and neural circuit remodeling in brain regions related to food intake and reward; then, it can induce conditioned taste aversion and loss of appetite in rodents, thereby inhibiting the eating behavior of experimental animals and achieving the effect of weight loss [Citation11,Citation12]. Clinical studies also show that whether the subjects have diabetes or not, receiving liraglutide can reduce their hunger on an empty stomach, increase their satiety after meals, and delay the rate of gastric emptying, thereby effectively reducing the energy intake and achieving the purpose of weight loss [Citation13,Citation14]. Supported by this evidence, FDA officially approved the high-dose liraglutide for weight management of obese people on 23 December 2014 [Citation10], indicating that the anti-obesity effect of liraglutide has been widely recognized. Unfortunately, there are few studies whether liraglutide can delay or reverse obesity-related kidney disease.
Therefore, a high-fat diet (HFD) was applied in this study to interfere with C57BL/6J male mice to build an animal model of obesity-related kidney disease. Based on this animal model, the protective effect and potential mechanism of liraglutide on the kidney were observed. The results of this study not only help to provide new ideas for clinical treatment of obesity-related kidney diseases, but also contribute to supplementing and improving the pharmacological mechanism of liraglutide.
Materials and methods
Experimental animals
In this study, 36 SPF C57BL/6J male mice (Charles River, Beijing, China) aged 6 weeks and weighing 20–24 g were used as experimental animal models. The mice were kept in the animal room of our hospital, at a temperature of 22 ± 1 °C, a humidity of 50–55% and a circadian rhythm of 12 h light/12 h dark. During the experiment, the mice had free access to food and sterile water. This study was conducted in accordance with the Guide to the Care and Use of Experimental Animals and approved by the Animal Ethics Committee of our Hospital.
Grouping and processing
After adaptive feeding for one week, C57BL/6J male mice were randomly and averagely divided into six groups (six mice per group) as follows: (1) control group (C): mice were fed with a normal diet for 24 weeks; (2) model group (M): mice were fed with a HFD (6% lard, 2% cholesterol (CHO), 10% egg yolk powder, 0.2% bile salt, and 81.1% basic diet) for 12 weeks, followed by a normal diet for 12 weeks; (3) bortezomib group (BORT + M): model mice received subcutaneous injections of saline (0.6 mg/kg) daily and intraperitoneal injections of bortezomib (AMPK agonist, 200 μg/kg) [Citation15] twice a week for a continuous period of 12 weeks, during which they were fed a normal diet; (4) normal saline group (N + M): model mice were injected with normal saline (0.6 mg/kg) subcutaneously once a day and given normal diet, for 12 weeks in a row; (5) liraglutide group (L + M): Model mice were injected subcutaneously liraglutide (0.6 mg/kg) once a day, for a total of 12 weeks, according to the dosage of liraglutide used by Wang et al. in studying the effect of liraglutide on hepatic steatosis [Citation16], and the normal diet was given during the injection. (6) Liraglutide + bortezomib group (L + BORT + M): model mice received subcutaneous injections of liraglutide (0.6 mg/kg) daily and intraperitoneal injections of bortezomib (200 μg/kg) twice a week for a continuous period of 12 weeks, during which they were fed a normal diet. The experimental flow chart is shown in .
Figure 1. Experimental flowchart. C: control; M: model; BORT: bortezomib; N: normal saline; L: liraglutide.
Mice were weighed every 2 weeks from the start of their high-fat chow feeding. After the last administration (the last day of the 24th week), all mice had a free access to water but were fasted for one night. In the early morning of the next day, the venous blood samples of mice were collected from the tail vein. After centrifugation (6000 × g, 5 min, 4 °C) of the collected samples, the serum supernatant was stored in the refrigerator at −80 °C for later use. Then, the mice were anesthetized with isoflurane (1–2% volume) and killed by cervical dislocation. Subsequently, a scalpel was used to make a longitudinal incision on the midline of the mouse abdomen, and the abdominal cavity was opened. Next, the adipose tissue and other organs in the abdominal cavity were pushed aside with tweezers to expose the kidney. Then, the kidney was gently and carefully peeled off with eye scissors. Then, one kidney was quickly put and stored in 4% paraformaldehyde, and the other kidney was stored in the refrigerator at −80 °C. In addition, a disposable sterile syringe was inserted into the exposed bladder of mice to obtain urine samples. After centrifugation (6000 × g, 5 min, 4 °C) of the obtained urine samples, the urine supernatant was stored in a refrigerator at −80 °C for later use.
Determination of serum lipid profile and kidney function index
Enzyme-linked immunosorbent assay (ELISA) was used to determine the levels of three common serum lipid profiles and two common kidney function indexes. The common serum lipid profiles included total CHO, triglycerides (TGs), and low-density lipoprotein cholesterol (LDL-C). The common kidney function indexes consisted of blood urea nitrogen (BUN) and creatinine (Cr). Besides, the level of urinary protein (UP) in mouse urine supernatant was also determined by ELISA. The ELISA kits used above were purchased from Shanghai Goldenrain Biological Technology Co., Ltd. (Shanghai, China), and the tests were carried out in strict accordance with the instructions. The experiment was repeated three times for each sample, and the average value was taken as the final result.
Histological analysis of kidney
Mouse kidney tissue was fixed in 4% paraformaldehyde solution for 24 h, then embedded in paraffin, cut into 4 µm thick coronal paraffin sections using a paraffin slicing machine (Leica, Wetzlar, Germany), and dewaxed according to the standard scheme [Citation17]. Subsequently, hematoxylin–eosin (H&E) staining was performed to assess the pathological damage of kidney; periodic acid-Schiff (PAS) staining to detect the severity of glycogen deposition in kidney tissue; and immunohistochemistry (IHC) to measure the expression level of related proteins. The IHC staining was performed in strict accordance with the steps of manufacturer’s instructions, and the primary antibodies used were listed as follows: anti-calcium/calmodulin-dependent protein kinase kinase beta (CaMKKβ) (1:1000, PA5-102029, Invitrogen, Carlsbad, CA); p-CaMKKβ (1:1000, PA5-64569, Invitrogen, Carlsbad, CA); anti-liver kinase B1 (LKB1) (1:1000, PA5-96062, Invitrogen, Carlsbad, CA); p-LKB1 (1:1000, PA5-105896, Invitrogen, Carlsbad, CA); anti-AMP-activated protein kinase (AMPK) (1:1000, PA5-105297, Invitrogen, Carlsbad, CA) and anti-p-AMPK (1:1000, PA5-104982, Invitrogen, Carlsbad, CA). The staining results were observed by Olympus optical microscope (Tokyo, Japan), photographed and recorded. Three fields of view of each section were randomly selected for observation, and the obtained images were quantitatively analyzed by ImageJ software (NIH, Bethesda, MD).
Western blot
The total protein was extracted from an appropriate amount of kidney tissue stored at −80 °C using RIPA buffer containing protease inhibitors and phosphatase inhibitors (Cell Signaling Technology, Boston, MA). After electrophoresis and membrane transfer under appropriate conditions, the protein was transferred onto the polyvinylidene fluoride (PVDF) membrane (Jintai, Qingdao, China). Next, the membrane was sealed with 5% skimmed milk for 2 h at 25 °C and then incubated with the corresponding primary antibodies at 4 °C overnight. On the next day, the membrane was washed and then incubated with HRP-conjugated Goat anti-Rabbit IgG Secondary Antibody (1:10,000, 31460, Invitrogen, Carlsbad, CA) for 1 h at 25 °C. Subsequently, the membrane was developed in a GEL imaging system (Bio-Rad, Hercules, CA), the protein bands were quantitatively analyzed using Image J software (NIH, Bethesda, MD), and the relative expression levels of the target proteins were corrected taking GAPDH as an internal control. The primary antibodies used in the western blot were displayed as follows: anti-CaMKKβ (1:1000, PA5-102029, Invitrogen, Carlsbad, CA); anti-LKB1 (1:1000, PA5-96062, Invitrogen, Carlsbad, CA); anti-AMPK (1:1000, PA5-105297, Invitrogen, Carlsbad, CA) and anti-p-AMPK (1:1000, PA5-104982, Invitrogen, Carlsbad, CA).
Extraction of RNA and real-time quantitative PCR
A proper amount of kidney tissue stored at −80 °C was homogenized with TRIzol reagent (Invitrogen, Carlsbad, CA) to extract total RNA. The extracted total RNA was reversely transcribed into cDNA using PrimeScript RT Reagent Kit (Takara Biotechnology Ltd., Kusatsu, Japan) according to the instructions. Then, the expression of related genes was quantitatively analyzed by real-time quantitative PCR (RT-qPCR) using SYBR Premix Ex Taq kit (Takara Biotechnology Ltd., Kusatsu, Japan) on ABI Step One Plus Real-Time PCR system (Applied Biosystems, Foster City, CA). The mRNA expression levels of CaMKKβ, LKB1, and AMPK were calibrated by GAPDH expression level, and were quantitatively calculated and analyzed by the 2−△△△CT method. The primers used in PCR are shown in .
Table 1. Mouse RT-qPCR primers.
Statistical analysis
Measurement data were expressed as mean ± standard deviation. The Shapiro–Wilk test was used to determine whether the data conformed to normal distribution, and the results suggested that the data of each group basically satisfied the characteristics of normal distribution. Therefore, differences among multiple groups were analyzed using one-way ANOVA, and if the results of one-way ANOVA were significant, pairwise comparisons between groups were further performed using the Tukey method. SPSS 21.0 software (IBM Corp., Armonk, NY) was adopted for statistical analyses, with p < .05 as the criterion for statistically significant differences.
Results
Liraglutide improves the serum lipid profile
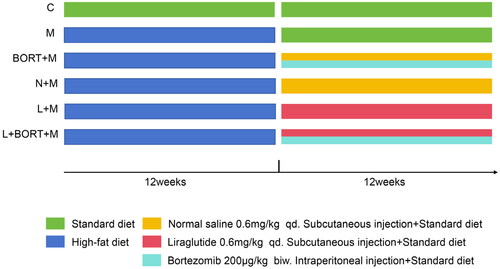
Previous clinical studies have pointed out a certain regulatory effect of liraglutide on the level of serum lipid [Citation18], so we first observed whether liraglutide played a similar role in HFD-induced obesity mouse model. The results indicate that at the 12-week mark, compared to the control group (C), the administration of a HFD significantly increased the body weight and serum levels of CHO, TG, and LDL-C in mice (p < .05). There were no statistically significant differences between the groups (p > .05). At the 24-week mark, compared to the N + M group, the liraglutide group exhibited a significant reduction in body weight and serum levels of CHO, TG, and LDL-C (p < .05) (). Furthermore, following the administration of the AMPK agonist BORT, there was a significant increase in body weight and lipid levels in the model group, reversing some of the effects of liraglutide on the model mice. CHO, TG, and LDL-C are three representative indexes of serum lipid level [Citation19], so the above results suggested that liraglutide could effectively reduce the serum lipid loading of HFD-induced mice.
Figure 2. Liraglutide attenuates the serum lipid loading of mice receiving high-fat diet. (A) Body weight changes in mice from high-fat chow feeding. ELISA was used to measure the levels of CHO (B), TG (C), and LDL-C (D) in mouse serum (n = 6). **p < .01 vs. C group, ##p < .01 vs. N + M group, &&p < .01 vs. L + M group. C: control; M: model; BORT: bortezomib; N: normal saline; L: liraglutide. ELISA: enzyme-linked immunosorbent assay; CHO: total cholesterol; TG: triglycerides; LDL-C: low-density lipoprotein cholesterol.
Liraglutide improves kidney injury in high-fat diet-induced mice
Previous studies have disclosed that HFD can induce kidney injury in C57BL/6 mice [Citation20,Citation21]. Therefore, this study was recruited to explore whether liraglutide could inhibit such adverse effect.
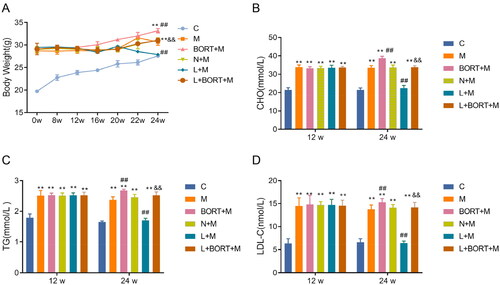
ELISA results () demonstrated that at week 12, compared to the C group, levels of BUN, Cr, and UP were significantly elevated in all other groups (p < .05), with no significant intergroup differences (p > .05). At week 24, compared to the N + M group, the liraglutide group showed a significant reduction in the levels of BUN, Cr, and UP (p < .05). Bortezomib notably increased the levels of BUN, Cr, and UP in the liraglutide group. These results suggest that liraglutide can effectively improve renal function in mice fed a HFD. Therefore, liraglutide could effectively improve the kidney function of HFD-induced mice.
Figure 3. Liraglutide improves kidney function in high-fat diet-induced mice. ELISA was used to assess the levels of BUN (A), Cr (B) in mouse serum, and UP (C) in mouse urine (n = 6). **p < .01 vs. C group, ##p < .01 vs. N + M group, &&p < .01 vs. L + M group. ELISA: enzyme-linked immunosorbent assay; BUN: blood urea nitrogen; Cr: creatinine; UP: urinary protein.
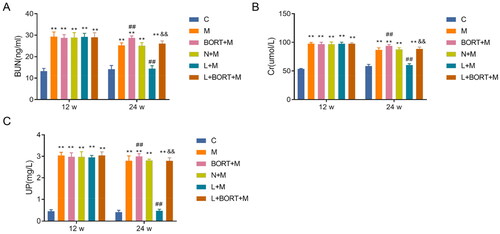
Moreover, significant morphological changes in the renal architecture of mice were observed. H&E staining results () revealed that the renal structure of the C group was relatively intact with no evident deformities. The M and N + M groups displayed interstitial clusters of inflammatory cells, increased renal erythrocytes, and notable glomerular deformation. Renal injury in the bortezomib (BORT + M) group was more severe than in the N + M group. Post-treatment with liraglutide, the pathological alterations in the kidneys were significantly ameliorated; there was a reduction in connective tissue proliferation, decreased deformation in the proximal tubules, and less structural change in the kidneys compared to the M and N + M groups. However, upon the addition of BORT, the liraglutide-treated group again showed interstitial infiltration by inflammatory cells, an abundance of renal erythrocytes, and glomerular changes. Additionally, PAS staining results () demonstrated thin and clear glomerular capillary loops in the C group; compared to the C group, the M and N + M groups exhibited glycogen deposition in glomerular lesions and significant basement membrane thickening; while the BORT + M group had even more pronounced glycogen deposition. The liraglutide group showed reduced glycogen deposition in glomerular lesions and significant thinning of the basement membrane, although BORT partially reversed this phenomenon. These findings indicate that liraglutide can effectively mitigate renal pathological damage and glycogen deposition induced by a HFD in mice.
Figure 4. Liraglutide reduces kidney pathological damage and glycogen deposition in high-fat diet-induced mice. (A) H&E staining to observe the pathological damage of mouse kidney tissues in each group; (B) PAS staining to observe the glycogen deposition of mouse kidney tissues in each group. Scale bar = 20 μm. H&E: hematoxylin–eosin; PAS: periodic acid-Schiff.
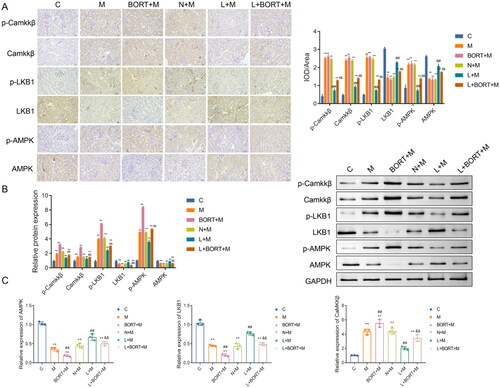
Liraglutide inhibits the CaMKKβ/AMPK signaling pathway in kidney tissue of high-fat diet-induced mice
There is a complex and close correlation between the CaMKKβ/AMPK signaling pathway and kidney injury [Citation22–24]. Therefore, the relationship between the pharmacological properties of liraglutide against kidney injury and the CaMKKβ/AMPK signaling pathway was further investigated in this study. IHC analysis () revealed that, in comparison with the group C, the expression levels of CaMKKβ, p-CaMKKβ, p-LKB1, and p-AMPK were significantly elevated (p < .05) in the renal tissues of mice fed a HFD, while the expression of LKB1 and AMPK was significantly reduced (p < .05). Compared with the N + M group, the liraglutide group exhibited a significant decrease in the expression of CaMKKβ, p-CaMKKβ, p-LKB1, and p-AMPK (p < .05), and a significant increase in the expression of LKB1 and AMPK (p < .05). Post-bortezomib (BORT) administration, there was a significant increase in the expression of CaMKKβ, p-CaMKKβ, p-LKB1, and p-AMPK, and a significant decrease in LKB1 and AMPK expression compared to the liraglutide group (p < .05). Western blot results () showed a similar trend to the IHC findings. Furthermore, RT-qPCR results () indicated that, relative to the C group, the mRNA expression levels of CaMKKβ were significantly increased (p < .05), and the mRNA expression levels of LKB1 and AMPK were significantly decreased (p < .05) in the renal tissues of the other groups; post-liraglutide treatment, CaMKKβ mRNA expression was significantly reduced (p < .05), and the mRNA expression of LKB1 and AMPK was significantly increased compared with the N + M group. Bortezomib was able to partially reverse the effects of liraglutide. Collectively, these results suggest that liraglutide can significantly inhibit renal injury in mice fed a HFD by modulating the CaMKKβ/AMPK signaling pathway.
Figure 5. Liraglutide suppresses the CaMKKβ/AMPK signaling pathway in kidney tissue of high-fat diet-induced mice. The protein levels of p-CaMKKβ/CaMKKβ, p-LKB1/LKB1, and p-AMPK/AMPK in mouse kidney tissues were detected by IHC (A) and western blot (B), respectively (n = 3). (C) The mRNA levels of CaMKKβ, LKB1, and AMPK in mouse kidney tissues were detected by RT-qPCR (n = 6). **p < .01 vs. C group, ##p < .01 vs. N + M group, &&p < .01 vs. L + M group. Scale bar = 20 μm. CaMKKβ: calmodulin-dependent protein kinase kinase beta; LKB1: anti-liver kinase B1; AMPK: AMP-activated protein kinase; IHC: immunohistochemistry; RT-qPCR: real-time quantitative PCR.
Discussion
The results of this study proved that liraglutide could effectively reduce the serum lipid level and improve kidney injury in the HFD-induced obesity mouse model. Mechanically, the pharmacological effect of liraglutide on protecting kidney was achieved by inhibiting the CaMKKβ/AMPK signaling pathway. As far as we know, this study first discovered the regulatory effect of liraglutide on the CaMKKβ/AMPK signaling pathway in kidney tissue, which provided a new theoretical basis for the clinical application of liraglutide in the treatment of obesity-related kidney diseases.
In this research, the model of mouse with obesity-related kidney diseases was established through HFD intervention, which is supported by a sufficient theoretical basis. Clinically, long-term exogenous HFD and overnutrition are the main causes of kidney dysfunction in obese people [Citation25]. Animal experiments by Mai et al. [Citation20] and Declèves et al. [Citation21] all found that C57BL/6 mice suffered from obvious pathological changes and function loss related to kidney injury after receiving HFD for 12 weeks. Our results showed that the CHO, TG, and LDL-C of mice fed with HFD were significantly higher than those fed with normal diet, indicating that the model of obese mouse was successfully established. In addition, HFD-induced mice showed the characteristics of kidney dysfunction (increased levels of BUN, Cr, and UP) and a series of typical pathological features of kidney injury such as inflammatory cell aggregation, glomerular morphological changes, glomerular glycogen deposition, and obvious thickening of basement membrane. Therefore, we believe that obesity-related kidney diseases were successfully induced in C57BL/6 mice through HFD intervention, and such result is basically consistent with previous studies [Citation20,Citation21].
First, the effect of liraglutide on the serum lipid level of mice was discussed. Through the comparison between the normal saline group and the liraglutide group, we discovered that liraglutide could effectively reduce the level of serum lipid markers in obese mice. Studies have pointed out that GLP-1 receptor agonists can not only reduce fat accumulation through inhibiting the conversion of glucose to fat in vivo, but also promote the oxidative metabolism of fat and enhance fat consumption in vivo [Citation26]. Hence, liraglutide has a significant regulatory effect on the level of serum lipid markers. The experimental results of this part are also consistent with some previous animal experiments designed to explore the effect of liraglutide on serum lipid markers [Citation27,Citation28].
Subsequently, the reversal effect of liraglutide on kidney injury was probed. In this study, the detection of BUN and Cr levels in serum samples and UP levels in urine samples was relied on to reflect the severity of kidney injury; also, H&E staining and PAS staining were employed to directly observe the pathological changes of kidney. Notably, there are many factors that affect serum BUN and Cr, especially HFD, which may cause acute liver injury (mainly because HFD can lead to fat deposition and induce oxidative stress and inflammatory response in the liver) [Citation29]. Under this premise, the ability of the liver to convert creatine into Cr is impaired, which may lead to the fact that serum BUN and Cr levels cannot truly reflect the kidney’s renal function. Therefore, to make the results of this study more reliable, H&E staining and PAS staining were applied additionally to evaluate the severity of pathological changes in the mouse kidney. In this study, the levels of kidney function markers as well as the results of H&E staining and PAS staining were basically matched. Such finding indicated that liraglutide could effectively improve kidney function and reduce kidney pathological damage in mice, thereby confirming the pharmacological effect of liraglutide on obesity-related kidney diseases. Besides, our results are consistent with the findings of Liang et al. [Citation30].
After revealing the therapeutic effect of liraglutide on obesity-related kidney diseases, the specific molecular mechanism of liraglutide was further explored. In this study, the CaMKKβ/AMPK signaling pathway was focused on. The expression of CaMKKβ, p-CaMKKβ, p-LKB1, and p-AMPK was significantly elevated in the kidney tissues of mice given HFD feeding, while the expression of LKB1 and AMPK was significantly reduced. This suggests that the CaMKKβ/AMPK signaling pathway is activated in obesity-associated nephropathy. The CaMKKβ/AMPK pathway is an important signaling pathway in cells. As one of the two known AMPK upstream kinases, CaMKKβ is activated by the increase of intracellular calcium level or the stimulation of other exogenous signals. Subsequently, the activated CaMKKβ is further phosphorylated to activate AMPK, thereby playing a key role in regulating physiological processes such as cell energy metabolism, cell proliferation, inflammation, and immunity through influencing different downstream molecules [Citation31]. The function of CaMKKβ/AMPK pathway in obesity-related kidney diseases seems to be a ‘double-edged sword’. On the one hand, in the early stage of obesity-related kidney diseases, a large number of p-AMPK produced after activation of the CaMKKβ/AMPK pathway can regulate the abnormal lipid metabolism in obese mouse models by promoting fat oxidation and inhibiting lipogenesis, thereby reducing excessive kidney fat accumulation and relieving kidney injury [Citation22,Citation23]. On the other hand, with the development of obesity-related kidney diseases, the abnormal activation of CaMKKβ/AMPK signaling pathway also leads to the hyperactivity of AMPK-dependent autophagy, which may affect the normal structure and function of kidney cells, and then lead to the loss of kidney function [Citation24]. In this article, the levels of CaMKKβ and p-AMPK in the kidney tissue of mice treated with liraglutide decreased significantly, while the level of AMPK increased significantly. Such result strongly indicated that liraglutide inhibited the CaMKKβ/AMPK pathway, thereby preventing the phosphorylation process of AMPK. However, the AMPK agonist Bortezomib was able to partially reverse the effects of liraglutide, abrogating the lipid inhibitory effects of liraglutide in HFD-induced mice and the protective effects against renal injury. Bortezomib is an anticancer drug that works by inhibiting proteasomal degradation [Citation32]. However, some studies have demonstrated that bortezomib induces phosphorylation of AMPK and reduces inflammation and insulin resistance [Citation15]. Additionally, HFD intervention lasted as long as 12 weeks in this paper, and therefore, the mice receiving HFD could be considered to enter the progressive stage of obesity-related kidney diseases. Hence, we suspected that liraglutide inhibited the abnormal autophagy of kidney cells by down-regulating the CaMKKβ/AMPK signaling pathway, and then exerted its therapeutic effect on progressive obesity-related kidney diseases. Notably, in vitro studies by Krasner et al. showed that liraglutide can increase intracellular Ca2+ content and several Ca2+ sensitive molecules (including CaMKKβ, CaMKI, AMPK, eNOS, and CREB) in cultured human aortic endothelial cells. Further experiments showed that liraglutide enhanced the ability to activate CAMKKβ, which in turn activated CAMKKβ to activate AMPK, thereby exerting an anti-inflammatory effect [Citation33]. This seemingly contradictory result may be due to the differences in the regulation and response of different types of cells or tissues in signaling pathways. In addition, Li et al.'s research also showed that liraglutide can significantly improve impaired glucose homeostasis, weight, and lipid levels in obese mice, simultaneously reducing urinary albumin excretion and glomerular hypertrophy in obese mice. Their research further confirms that liraglutide activates the AMPK-eNOS pathway in the glomeruli of obese mice, upregulates renal heme oxygenase-1 activity, reduces renal malondialdehyde levels in obese mice, and reduces the ability of HFD induced uncoupling of the glomerular vascular endothelial growth factor nitrogen oxide (VEGF-NO) axis in obese mice to reduce urinary albumin excretion [Citation34]. It is suggested that liraglutide in the treatment of obesity related kidney disease with CaMKKβ/AMPK/eNOS pathway plays a role, but the exact mechanism still needs further experimental verification.
In this study, only male C57BL/6J mice were selected for construction of the animal model of obesity-related kidney diseases, because higher estrogen in women or female animals will change the fat distribution. Previous studies have stated that higher estrogen levels can help fat deposit in superficial parts of buttocks, thighs or subcutaneous tissues, thereby reducing fat deposition in abdominal organs [Citation35]. In other words, estrogen has a certain protective effect on kidney injury, and female mice are less likely to develop obesity-related kidney diseases than male mice. Therefore, the female mouse model was not enrolled in this study.
However, there are some shortcomings in this study. First of all, this study only clarified the inhibitory effect of liraglutide on the CaMKKβ/AMPK pathway in kidney tissue, and did not further discuss whether liraglutide inhibited the autophagy-related pathway downstream of this signaling pathway. Second, the mechanism of liraglutide in the treatment of obesity-related kidney diseases may be more than the single way of regulating CaMKKβ/AMPK signaling pathway. In the future research, the pharmacological effects of liraglutide are necessary to be comprehensively explored to draw more perfect and credible conclusions.
Conclusions
To sum up, this study revealed that liraglutide, a GLP-1 receptor agonist commonly used in the treatment of diabetes and obesity, can effectively improve kidney function and alleviate kidney pathological damage in the mouse model of obesity-related kidney diseases induced by HFD. Mechanically, the therapeutic effect of liraglutide on obesity-related kidney diseases is probably achieved by inhibiting the CaMKKβ/AMPK signaling pathway. Overall, this study has not only clarified the new pharmacological effects of liraglutide, but also provided a new idea for clinical treatment of kidney diseases caused by obesity.
Author contributions
Yingli Xuan designed this study. Ting-ting Ding, Xiao-lei Mao, Shiqing Pang, Ruibin He, and Li Qin collated the data, carried out the data analyses, and produced the initial draft of the manuscript. Jiang zi Yuan drafted the manuscript. All authors read and approved the final submitted manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Additional information
Funding
References
- Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl.):S176–S185.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004.
- Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–1770. doi: 10.1161/CIRCRESAHA.115.306883.
- Rubio-Almanza M, Cámara-Gómez R, Merino-Torres JF. Obesity and type 2 diabetes: also linked in therapeutic options. Endocrinol Diabetes Nutr (Engl Ed). 2019;66(3):140–149. doi: 10.1016/j.endien.2018.11.006.
- Karczewski J, Begier-Krasińska B, Staszewski R, et al. Obesity and the risk of gastrointestinal cancers. Dig Dis Sci. 2019;64(10):2740–2749. doi: 10.1007/s10620-019-05603-9.
- Jiang Z, Wang Y, Zhao X, et al. Obesity and chronic kidney disease. Am J Physiol Endocrinol Metab. 2023;324(1):E24–E41. doi: 10.1152/ajpendo.00179.2022.
- Xu T, Sheng Z, Yao L. Obesity-related glomerulopathy: pathogenesis, pathologic, clinical characteristics and treatment. Front Med. 2017;11(3):340–348. doi: 10.1007/s11684-017-0570-3.
- Praga M, Morales E. The fatty kidney: obesity and renal disease. Nephron. 2017;136(4):273–276. doi: 10.1159/000447674.
- Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl. 1):5–21. doi: 10.1111/dom.13129.
- Ladenheim EE. Liraglutide and obesity: a review of the data so far. Drug Des Devel Ther. 2015;9:1867–1875.
- Talsania T, Anini Y, Siu S, et al. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146(9):3748–3756. doi: 10.1210/en.2005-0473.
- Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150(4):1680–1687. doi: 10.1210/en.2008-1045.
- Lean MEJ, Carraro R, Finer N, et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes. 2014;38(5):689–697. doi: 10.1038/ijo.2013.149.
- Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36(6):843–854. doi: 10.1038/ijo.2011.158.
- Kwak HJ, Choi H-E, Jang J, et al. Bortezomib attenuates palmitic acid-induced ER stress, inflammation and insulin resistance in myotubes via AMPK dependent mechanism. Cell Signal. 2016;28(8):788–797. doi: 10.1016/j.cellsig.2016.03.015.
- Wang H, Wang L, Li Y, et al. The HIF-2alpha/PPARalpha pathway is essential for liraglutide-alleviated, lipid-induced hepatic steatosis. Biomed Pharmacother. 2021;140:111778. doi: 10.1016/j.biopha.2021.111778.
- Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014;2014(6):655–658. doi: 10.1101/pdb.prot073411.
- Gorgojo‐MartíNez JJ, Basagoiti‐Carreño B, Sanz‐Velasco A, et al. Effectiveness and tolerability of orlistat and liraglutide in patients with obesity in a real-world setting: the XENSOR study. Int J Clin Pract. 2019;73(11):e13399. doi: 10.1111/ijcp.13399.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143.
- Mai X, Yin X, Chen P, et al. Salvianolic acid B protects against fatty acid-induced renal tubular injury via inhibition of endoplasmic reticulum stress. Front Pharmacol. 2020;11:574229. doi: 10.3389/fphar.2020.574229.
- Declèves A-E, Mathew AV, Cunard R, et al. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol. 2011;22(10):1846–1855. doi: 10.1681/ASN.2011010026.
- Zhou D, Zhou M, Wang Z, et al. Progranulin alleviates podocyte injury via regulating CAMKK/AMPK-mediated autophagy under diabetic conditions. J Mol Med. 2019;97(11):1507–1520. doi: 10.1007/s00109-019-01828-3.
- Zhou Y, Tian S, Wang Q, et al. DHA-enriched phosphatidylserine ameliorates high-fat diet-induced kidney injury in mice possibly by regulating TLR4/NF-kappaB and AMPK pathways. J Food Sci. 2022;87(9):4233–4249. doi: 10.1111/1750-3841.16284.
- Lai L-L, Lu H-Q, Li W-N, et al. Protective effects of quercetin and crocin in the kidneys and liver of obese Sprague-Dawley rats with type 2 diabetes: effects of quercetin and crocin on T2DM rats. Hum Exp Toxicol. 2021;40(4):661–672. doi: 10.1177/0960327120954521.
- Castro BBA, Foresto-Neto O, Saraiva-Camara NO, et al. Renal lipotoxicity: insights from experimental models. Clin Exp Pharmacol Physiol. 2021;48(12):1579–1588. doi: 10.1111/1440-1681.13556.
- Lutz TA, Osto E. Glucagon-like peptide-1, glucagon-like peptide-2, and lipid metabolism. Curr Opin Lipidol. 2016;27(3):257–263. doi: 10.1097/MOL.0000000000000293.
- Rakipovski G, Rolin B, Nøhr J, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE(−/−) and LDLr(−/−) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3(6):844–857. doi: 10.1016/j.jacbts.2018.09.004.
- O'Harte FPM, Parthsarathy V, Hogg C, et al. Long-term treatment with acylated analogues of apelin-13 amide ameliorates diabetes and improves lipid profile of high-fat fed mice. PLOS One. 2018;13(8):e0202350.
- Parlati L, Régnier M, Guillou H, et al. New targets for NAFLD. JHEP Rep. 2021;3(6):100346. doi: 10.1016/j.jhepr.2021.100346.
- Liang R, Wang M, Fu C, et al. Liraglutide protects against high-fat diet-induced kidney injury by ameliorating apoptosis. Endocr Connect. 2020;9(9):946–954. doi: 10.1530/EC-20-0294.
- Kennedy G, Gibson O, O'Hare DT, et al. The role of CaMKK2 in Golgi-associated vesicle trafficking. Biochem Soc Trans. 2023;51(1):331–342. doi: 10.1042/BST20220833.
- Tang J-H, Yang L, Chen J-X, et al. Bortezomib inhibits growth and sensitizes glioma to temozolomide (TMZ) via down-regulating the FOXM1–Survivin axis. Cancer Commun. 2019;39(1):81. doi: 10.1186/s40880-019-0424-2.
- Krasner NM, Ido Y, Ruderman NB, et al. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLOS One. 2014;9(5):e97554. doi: 10.1371/journal.pone.0097554.
- Li K, Sun J, Huang N, et al. Liraglutide improves obesity-induced renal injury by alleviating uncoupling of the glomerular VEGF-NO axis in obese mice. Clin Exp Pharmacol Physiol. 2020;47(12):1978–1984. doi: 10.1111/1440-1681.13391.
- Mattsson C, Olsson T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem. 2007;14(27):2918–2924. doi: 10.2174/092986707782359972.
